2014 Volume 233 Issue 2 Pages 141-148
2014 Volume 233 Issue 2 Pages 141-148
The process of hematopoiesis is associated with hematopoietic stem cells (HSCs) and the hematopoietic microenvironment. Osteoblasts, derived from mesenchymal stem cells (MSCs), are one of the most important components in the hematopoietic microenvironment. Osteoblasts secrete a variety of cytokines including interleukin-6 (IL-6) and granulocyte-macrophage colony-stimulating factor (GM-CSF), thereby regulating the biological activities of HSCs. It has been shown that hematopoiesis dysfunction can be induced by estrogen-deficiency through the exhaustion of HSCs. However, the effect of estrogen on the proliferation of HSCs is not fully understood. The aim of this study was to investigate the role of estradiol in the process of hematopoiesis, especially regarding the proliferation of HSCs in vitro. Bone marrow-derived MSCs and HSCs were isolated from 3-month-old female Sprague-Dawley rats. Mineralization ability and osteocalcin assays demonstrated that treatment with 17β-estradiol (E2) significantly enhanced the osteogenic differentiation of MSCs. HSCs and MSCs were then cocultured with or without E2 treatment. Colony forming assays demonstrated that E2 increased the number of colony forming units-granulocyte/macrophage in a dose-dependent manner when HSCs were co-cultured with MSCs in Osteogenic Medium that is suitable for the in vitro osteogenic differentiation. Further, increased concentrations of GM-CSF and IL-6 were detected by enzyme linked immunosorbent assay (ELISA). These results indicate that E2 induces the proliferation of HSCs, which depends on the promotion of osteogenic differentiation of MSCs, and that process is mediated by both GM-CSF and IL-6.
Estradiol is the most active type of estrogen in vivo, and is responsible for the development of secondary sex characteristics and a series of metabolic processes. In recent years, the relationship between estrogen and hematopoiesis has attracted extensive attention worldwide. Early in the 1960s, observations confirmed the growth-promoting role of estrogen on granulopoiesis. It was then reported that a high dose of estrogen promoted cell proliferation and the differentiation of megakaryocytes in mice and postmenopausal women (Bord et al. 2000; Perry et al. 2000). Moreover, estrogen was shown to play an important role in the differentiation and maturation of early hematopoietic progenitor cells (Shim et al. 2003). In our previous studies, we found that estrogen-deficiency caused by ovariectomy leads to hematopoiesis dysfunction in rats, which is related with the reduction of hematopoietic stem cells (HSCs) in the bone marrow (Qiu et al. 2012). However, the mechanism whereby estrogen stimulates the proliferation of HSCs remains unclear.
Mesenchymal stem cells (MSCs) are a cluster of precursor cells with the potentiality of self-renewal and multi-directional differentiation, which can generate various types of bone marrow stromal cells (BMSCs) to establish the hematopoietic microenvironment. Osteoblasts are derived from MSCs and are one of the most important components in the hematopoietic microenvironment. Importantly, osteoblasts secrete a variety of cytokines including interleukin-1 (IL-1), IL-6, IL-7 and granulocyte-macrophage colony-stimulating factor (GM-CSF), which maintain and regulate the biological activities of HSCs (Taichman and Hauschka 1992; Taichman and Emerson 1996; Taichman et al. 1997; Calvi et al. 2003; Zhang et al. 2003; Visnjic et al. 2004; Shiozawa et al. 2008). Over the years, the effects of estrogen on MSCs have been demonstrated. Benayahu et al. (2000) found that MSCs were more likely to differentiate into adipocytes as a result of estrogen-deficiency, which leads to a decrease in the number of osteoblasts. Further studies suggested that estrogen supports and promotes the osteogenic differentiation of MSCs due to the increased expression of bone calcium and alkaline phosphatase (ALP) (Leskela et al. 2006). Given those findings, we hypothesized that estrogen may induce the proliferation of HSCs via its effects on osteoblasts. In this study, we investigated the role of estradiol on HSCs in vitro and its mechanism of action.
Three-month-old female Sprague-Dawley rats were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China) and were maintained at Zhejiang Chinese Medical University Laboratory Animal Research Center. Each rat weighed about 250 ± 5 g and was housed in a room with a 12 h-12 h light-dark cycle and had unlimited access to food and water. All experiments in this study were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and received the approval of the Institutional Animal Care and Use Committee of Zhejiang Chinese Medical University.
Cell culture and isolation of MSCs and HSCsEach rat was sacrificed by cervical dislocation, after which femurs and tibias without accessory tissues were excised. A syringe needle was inserted into the bone marrow cavities of femurs and tibias several times to flush out all cells using a 5 mL syringe with Iscove modified Dulbecco medium (IMDM, GIBCO, USA) containing 10% fetal bovine serum (Siji Qing Biotech, China). Mononuclear cells were then harvested using rat lymphocyte separation liquid (Sigma, USA) by density gradient centrifugation at 3,500 rpm for 20 min.
The mononuclear cell suspensions were plated in 25 cm2 culture flasks at a density of 1 × 106/mL and were incubated at 37°C with 5% CO2 under a saturated humidity environment. Non-adherent cells were harvested after 24 h. The remaining cells which were rich in MSCs, were cultured until they reached 80% confluence. According to the standard procedure of the manufacturer’s protocol, MSCs from the second passage (P2) were collected, washed with phosphate buffered-saline (PBS) for three times and were then resuspended. Each cell suspension (5 × 103 cells/100 μL) was incubated with lineage-specific monoclonal antibodies, such as FITC mouse anti-rat CD29, FITC mouse anti-rat CD90, PE mouse anti-rat CD34, PE mouse anti-rat CD45 (BD Pharmingen, USA), FITC mouse anti-rat CD44 (SeroTec, USA) and nonspecific mouse IgG (isotype control) for 30 min at 4°C. Data were analyzed using a flow cytometer (BD Biosciences, San Jose, CA) and positive expression was determined as fluorescence intensity greater than 95% of the corresponding isotype control. Each sample was analyzed three times.
HSCs were obtained by using immunomagnetic beads (Miltenyi Biotec, Germany) from non-adherent cells prepared as described above. Briefly, CD90+ cells were segregated and harvested with a magnetic particle separator according to the manufacturers’ instructions. CD45RC was then used for magnetic activated cell sorting again in order to remove CD90+CD45RC+ cells. CD90+CD45RC− cells (McCarthy et al. 1987; Pu and Yang 2002; Qiu et al. 2012) were obtained and seeded into 75 cm2 culture flasks. More than 95% cell viability was achieved as assessed by trypan blue staining.
Preparation of estradiol and osteogenic medium17β-estradiol (E2) (MW 272.38, purity ≥ 98%, Sigma-Aldrich Co., USA) was dissolved in absolute ethyl alcohol at a concentration of 0.07 mM for storage. Osteogenic Medium (OM) consisted of Dulbecco Modified Eagle Medium in high glucose (DMEM, GIBCO, USA) with 10% fetal bovine serum, 20 mM vitamin C (Sigma, USA), 1 M β-glycerophosphate (Sigma, USA) and 1 mM dexamethasone (Sigma, USA).
Detection of calcified nodules and osteocalcin assayAdherent cells were harvested, resuspended in IMDM and OM, respectively, and plated at a density of 1 × 105/mL in 35 mm culture dishes. E2 was added at concentrations of 10−7 and 10−6 mM. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2, and the medium was changed every 3 days. After incubation for 21 days, staining with alizarin red (Sangon Biotech Co., Ltd., Shanghai, China) was used to identify the mineralization of osteoblasts. Calcified nodules were formed and dyed red. The optical density of each sample was determined with a plate reader (Bio-TEK, USA). Moreover, osteocalcin expression in the culture supernatants was detected by an enzyme linked immunosorbent assay (ELISA) kit (Boster Biotech, China). All operations were performed in accordance with the manufacturers’ instructions and each assay was performed in triplicate.
Colony forming assayHSCs were obtained using immunomagnetic beads, washed and resuspended in IMDM. Cells were plated at a density of 1 × 105/mL in 35 mm culture dishes with E2 at concentrations of 10−7 and 10−6 mM. Moreover, MSCs at a density of 1 × 105/mL were co-cultured with HSCs at a density of 1 × 105/mL in 35 mm culture dishes with E2 at concentrations of 10−7 and 10−6 mM. The medium was then replaced with OM and the same culture conditions were performed as mentioned before. All cells were incubated for 21 days at 37°C in a humidified atmosphere of 5% CO2 and the medium was changed every 3 days.
After incubation for 21 days, according to the manufacturers’ instructions, 1.5 × 104 cells in suspension with 1.1 mL of methylcellulose medium (Stem Cell Technologies, Canada) in 35 mm culture dishes were incubated for 14 days at 37°C with 5% CO2 in a saturated humidity environment. Colony numbers were then assessed using an inverted microscope. Each assay was performed in triplicate.
Hematopoietic growth factors assayHematopoietic growth factors including GM-CSF and IL-6 in culture supernatants were detected by ELISA kits (KeyGEN Biotech, China) according to the manufacturers’ protocols.
Statistical analysisAll data were analyzed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and are expressed as means ± standard deviation (s.d.) of independent experiments. Student’s t test was used to analyze significant differences between groups. Linear regression was used to analyze the variable relevance. Values of p < 0.05 are considered to be statistically significant.
All cells derived from the bone marrow cavities of rats remained in a state of suspension at their early stage. Cells were usually rounded with some having irregular shapes. After incubation for 24-48 h, most cells adhered to the surface of the flasks for growth. After 96 h, those cells became flat, irregularly rounded and some were even fusiform, spindle-shaped or radiated out in all directions (Fig. 1Aa). About 5-7 days later, primary adherent cells reached 80% confluence and needed to be subcultured (Fig. 1Ab).
The surface markers of these adherent cells were analyzed by flow cytometry, including CD34, CD44, CD45, CD90 and CD105. As shown in Fig. 1B, these cells had high expression of CD44 (93.83%), CD90 (93.17%) and CD105 (96.17%), but had low expression of CD34 (0.82%) and CD45 (0.53%), which are typical characteristics of surface markers on MSCs.

Morphological characteristics and cell surface markers of rat MSCs.
A. Morphological characteristics of rat MSCs. (a) After incubation for 96 h, most primary MSCs adhered to the surface of the culture dish and were round, although some were irregular and fusiform in shape (arrows); (b) primary adherent cells tended to become fusiform, spindle-shaped (arrows) and more numerous after incubation for 5-7 days. B. Cell surface markers of rat MSCs. The cell surface markers are positive for CD44, CD90 and CD105, but negative for CD34 and CD45.
Osteogenic differentiation of MSCs was identified by alizarin red staining of calcified nodules. As shown in Fig. 2Aa, no calcified nodules were induced when MSCs were incubated with IMDM alone or were exposed to E2. However, after incubation in OM for 21 days, MSCs changed from fusiform to square or cubic shapes with a few calcified nodules dyed red (Fig. 2Ab). Further, the amount of calcified nodules significantly increased when the medium was supplemented with E2 at concentrations of 10−7 and 10−6 mM (Fig. 2Ac, d). These results imply that MSCs cultured in OM successfully differentiate into osteoblasts, and E2 promotes this process.
Ultraviolet spectrophotometry was applied to quantitatively detect the amount of calcium nodules induced by the osteogenic differentiation of MSCs. As shown in Fig. 2B, the OD value increased significantly when MSCs were cultured in OM in the presence of E2. It was highest (1.16 ± 0.18) when the E2 concentration was increased to 10−6 mM. Similar to the results with alizarin red staining, the OD value could not be detected when MSCs were cultured with IMDM alone or were exposed to E2.
Osteocalcin, which is synthesized and secreted by osteoblasts, could further verify the osteogenic differentiation of MSCs. Osteocalcin levels in the culture supernatants were measured by ELISA. As shown in Fig. 2C, osteocalcin can hardly be detected when MSCs were cultured with IMDM alone or were exposed to E2. However, the level of osteocalcin rose to 2.67 ± 0.73 μg/L in MSCs cultured with OM alone. Osteocalcin increased significantly from 5.85 ± 0.41 μg/L to 7.47 ± 0.59 μg/L when the concentration of E2 was increased from 10−7 to 10−6 mM.

E2 promotes the osteogenic differentiation of MSCs.
A. Calcified nodules were dyed red by alizarin red staining when MSCs were incubated with IMDM or OM at different concentrations of E2. (a) No calcified nodules were found in MSCs cultured in IMDM at an E2 concentration of 10−7 mM; (b) a few calcified nodules were induced in MSCs cultured in OM without E2; (c) many more calcified nodules were evident in MSCs cultured in OM at an E2 concentration of 10−7 mM compared with MSCs cultured without E2; (d) many calcified nodules were dyed red and even merged into each other in MSCs cultured in OM at an E2 concentration of 10−6 mM. B. Optical density (OD) of Alizarin red staining in osteoblasts when MSCs were incubated in IMDM or OM at different concentrations of E2. With or without E2, the OD value was too low to detect in MSCs cultured in IMDM, whereas in MSCs cultured in OM, the OD value increased significantly with increasing concentrations of E2 (*p < 0.05). C. Concentration of osteocalcin in culture supernatants when MSCs were incubated in IMDM or OM at different concentrations of E2. With or without E2, osteocalcin was expressed at a low level in MSCs cultured in IMDM. In MSCs cultured in OM, the level of osteocalcin increased significantly with increasing concentrations of E2 (*p < 0.05).
To determine the role of E2 on HSCs, colony forming assays were used to determine the proliferation potential of HSCs, which can differentiate into colony-forming units-granulocyte/macrophage (CFUs-GM) progenitor in semisolid media with various cytokines, including GM-CSF, stem cell factor (SCF) and interleukin-3 (IL-3) (Fig. 3A).
As shown in Fig. 3B, E2 induced the proliferation of HSCs in a dose-dependent manner when incubated in all four types of culture media. Compared with HSCs incubated in IMDM, OM or IMDM co-cultured with MSCs with or without E2, the number of CFUs-GM increased significantly (p < 0.05) when HSCs were co-cultured with MSCs in OM. As the E2 concentration was increased to 10−6 mM, the number of CFUs-GM reached 1,230.0 ± 30.98/1.5 × 104 cells, whereas it was 828.0 ± 23.35/1.5 × 104 cells, 749.0 ± 72.45/1.5 × 104 cells and 884.0 ± 90.04/1.5 × 104 cells in the other three types of culture media, respectively.

E2 induces the proliferation of HSCs when co-cultured with MSCs in OM.
A. The morphology of colony-forming unit-granulocyte/macrophage (CFU-GM) derived from HSCs. B. Number of CFUs-GM derived from HSCs when HSCs were cultured in IMDM, OM, IMDM with MSCs or OM with MSCs at different concentrations of E2. Without E2 treatment, the number of CFUs-GM from HSCs cultured in OM with MSCs was significantly higher than from HSCs cultured in IMDM, OM or IMDM with MSCs (*p < 0.05). As the concentration of E2 increased, the difference between these culture systems became more and more significant (p < 0.05).
It is well known that osteoblasts regulate the biological activities of HSCs by secreting many kinds of cytokines. ELISAs were used to detect the concentrations of hematopoietic growth factors (GM-CSF and IL-6) in culture supernatants, which could further confirm whether hematopoietic growth factors participated in this process. As shown in Fig. 4A, compared with HSCs incubated in IMDM, OM or IMDM co-cultured with MSCs with or without E2, GM-CSF levels were significantly higher when HSCs were co-cultured with MSCs in OM (p < 0.05). Further, E2 promoted the secretion of GM-CSF in a dose-dependent manner when HSCs were co-cultured with MSCs in OM. When the E2 level was increased to 10−6 mM, the concentration of GM-CSF reached 2,856.47 ± 346.81 pg/mL, higher than the levels of 1,338.56 ± 223.45 pg/mL, 1,268.48 ± 378.19 pg/mL and 1,409.74 ± 567.88 pg/mL in culture supernatants from IMDM, OM and MSCs in IMDM, respectively. The changes in the concentrations of IL-6 were similar to GM-CSF, as shown in Fig. 4B. When E2 was increased to 10−6 mM, the concentration of IL-6 reached 215.85 ± 53.14 pg/mL, higher than that of 112.44 ± 26.79 pg/mL, 103.83 ± 32.16 pg/mL and 118.93 ± 37.33 pg/mL in culture supernatants from IMDM, OM, and IMDM with MSCs, respectively (p < 0.05).
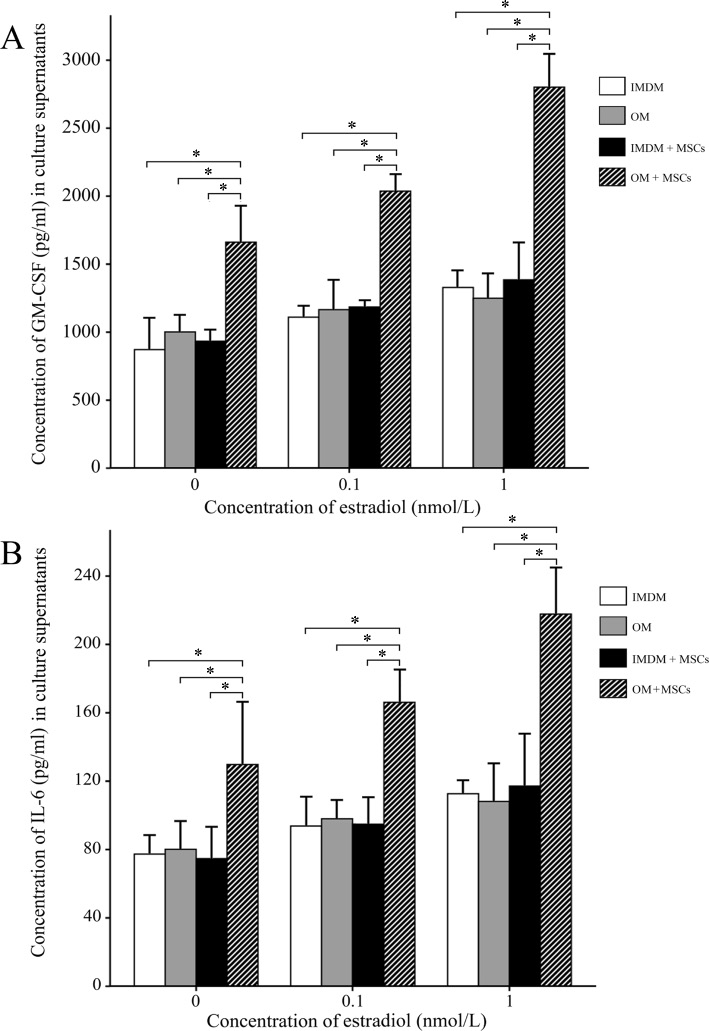
Regulation of hematopoietic growth factors.
A. Concentration of GM-CSF in culture supernatants when HSCs were cultured in IMDM, OM, IMDM with MSCs or OM with MSCs at different concentrations of E2. Without E2 treatment, the concentration of GM-CSF in culture supernatants from HSCs cultured in OM with MSCs was significantly higher than from HSC cultured in IMDM, OM or IMDM with MSCs (*p < 0.05). As the concentration of E2 increased, the difference between these culture systems became more and more significant (p < 0.05). B. Concentration of IL-6 in culture supernatants when HSCs were cultured in IMDM, OM, IMDM with MSCs or OM with MSCs at different concentrations of E2. Without E2 treatment, the concentration of IL-6 in culture supernatants from HSCs cultured in OM with MSCs was significantly higher than from HSC cultured in IMDM, OM or IMDM with MSCs (*p < 0.05). As the concentration of E2 increased, the difference between these culture systems became more and more significant (p < 0.05).
More and more studies have shown that estrogen plays a vital role in hematopoiesis, especially in regulation of HSCs. Nakada et al. (2014) recently confirmed that estrogen increases the self-renewal of HSCs in females during pregnancy. In our previous study in rats, when estrogen-deficiency was established by ovariectomy, hematopoiesis dysfunction occurred due to the decreased number of HSCs in the bone marrow (Qiu et al. 2012). Further, a reduction of osteoblasts also occured, which positively correlated with the volume of hematopoietic tissue (R = 0.506, p < 0.01). In this study, we aimed to investigate whether estradiol induces the proliferation of HSCs by promoting the osteogenic differentiation of MSCs in vitro and to explore its possible mechanism.
MSCs are one of the most important non-hematopoietic stem cells which have the ability of self-renewal and multiple differentiation potential. With different inductions, they can differentiate into osteoblasts, chondrocytes, adipocytes and so on (Jo et al. 2013; Liu and Sun 2014). In this study, we isolated and collected clusters of MSCs derived from rat bone marrow by density gradient centrifugation, adherent culture and serial passage. We found that these cultured cells were positive for CD44, CD90 and CD105 and negative for CD34 and CD45, which is in accordance with the generally accepted definition of rat MSCs (Chamberlain et al. 2007; Zhang and Chan 2010; Oh et al. 2014; Tsai et al. 2014).
Under appropriate induction in vitro, MSCs can undergo osteogenic differentiation as noted in 1999 (Pittenger et al. 1999). In this study, we found that E2 can promote the osteogenic differentiation potential of MSCs in a dose-dependent manner, which was confirmed by the OD value for calcified nodules dyed in red (R = 0.765, p < 0.05) and osteocalcin assays (R = 0.664, p < 0.05). Our results are in line with previous studies reported (Benayahu et al. 2000; Lei et al. 2009). It is considered that the role of E2 in the osteogenic differentiation of MSCs is mainly related to estrogen receptors (ERs) on MSCs (Bord et al. 2000; Wang et al. 2006). ERα is defined as an activator for the osteogenic differentiation of MSCs. Estrogen promotes the differentiation of MSCs into osteoblasts by up-regulating the expression of ERα, as well as other osteogenic differentiation genes such as ALP, Bone Morphogenetic Protein-2 (BMP-2) and transforming growth factor-β1 (TGF-β1) (Zhou et al. 2001; Leskela et al. 2006).
In this study, we also found that E2 increased the number of CFUs-GM differentiated from HSCs in a dose-dependent manner (R = 0.574, p < 0.01). This result is in accordance with our previous study in vivo (Qiu et al. 2012). The exact mechanism of E2 on HSCs is still obscure. In recent years, it was verified that there are several types of ERs exist on the surface of HSCs, such as ERα (Ray et al. 2008) and G-protein-coupled estrogen receptor 1 (GPER) (Di Vito et al. 2010). Presumably, E2 promotes the proliferation of HSCs through the interaction with ERs on HSCs. Moreover, when HSCs were incubated in OM with MSCs instead of the original culture medium, E2 increased the number of CFUs-GM from HSCs much more significantly than ever before (p < 0.05). There was also a positive correlation between osteoblasts and CFUs-GM (R = 0.797, p < 0.01). Taichman et al. (1997) found that HSCs co-cultured with osteoblasts could maintain their primitiveness much more fully, which is likely due to the secretion of IL-6 by osteoblasts. Osteoblast-deficiency causes a decrease in the number of HSCs and hematopoietic progenitors in a transgenic mouse model, and the reappearance of osteoblasts in vivo can recover medullary hematopoiesis (Visnjic et al. 2004). These facts, to some extent, support our results that the proliferation of HSCs is induced by the osteogenic differentiation of MSCs.
It is well known that osteoblasts belong to BMSCs, which provide the structure scaffolding that supports hematopoiesis (Gong 1978; Nilsson et al. 1996). The establishment of spatial relationship between osteoblasts and HSCs is based on the fact that primitive HSCs are closely approximated with endosteal surfaces (Lord 1990; Nilsson et al. 2001). Osteoblasts can synthesize a spectrum of hematopoietic growth-promoting cytokines, such as IL-1, IL-6, granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), GM-CSF, leukemia inhibitory factor (LIF), tumor necrosis factor (TNF-α) and TGF-β, which possibly accounts for the hematopoietic promoting activities of osteoblasts (Calvi et al. 2003; Zhang et al. 2003). In our study, we found that levels of GM-CSF and IL-6 in the culture supernatants of MSCs in OM increased significantly with an increase in E2, which is in accordance with the increase of CFUs-GM derived from HSCs in our study. GM-CSF and IL-6 in IMDM, OM and IMDM with MSCs were at a low level. These results suggest that GM-CSF and IL-6 may play a vital role in the interaction between osteoblasts and HSCs, which is in accordance with previous studies (Nelissen et al. 2000; Dormady et al. 2001). However, whether any other cytokines participate in this process and mutual effects of these cytokines are undefined and need to be further explored.
In conclusion, our study indicates that E2 facilitates the osteogenic differentiation of MSCs. E2 increases the number of CFUs-GM derived from HSCs especially when HSCs are co-cultured with MSCs in osteoblastic induction. GM-CSF and IL-6 may be involved in the growth-promoting process. However, whether any other cytokines are also involved in this process and their mutual effects are undefined and requires further investigation.
This work was supported by a grant of Natural Science Foundation of China (NSFC) (30950004). We appreciate the assistance of Zhejiang Chinese Medical University and thank Xiaoli Jin and Zhengkuan Xu for their professional technical support.
The authors declare no conflict of interest.