2015 Volume 235 Issue 1 Pages 29-37
2015 Volume 235 Issue 1 Pages 29-37
Both osteoporosis and tooth loss are health concerns that affect many older people. Osteoporosis is a common skeletal disease of the elderly, characterized by low bone mass and microstructural deterioration of bone tissue. Chronic mild stress is a risk factor for osteoporosis. Many studies showed that tooth loss induced neurological alterations through activation of a stress hormone, corticosterone, in mice. In this study, we tested the hypothesis that tooth loss early in life may accelerate age-related bone deterioration using a mouse model. Male senescence-accelerated mouse strain P8 (SAMP8) mice were randomly divided into control and toothless groups. Removal of the upper molar teeth was performed at one month of age. Bone response was evaluated at 2, 5 and 9 months of age. Tooth loss early in life caused a significant increase in circulating corticosterone level with age. Osteoblast bone formation was suppressed and osteoclast bone resorption was activated in the toothless mice. Trabecular bone volume fraction of the vertebra and femur was decreased in the toothless mice with age. The bone quality was reduced in the toothless mice at 5 and 9 months of age, compared with the age-matched control mice. These findings indicate that tooth loss early in life impairs the dynamic homeostasis of the bone formation and bone resorption, leading to reduced bone strength with age. Long-term tooth loss may have a cumulative detrimental effect on bone health. It is important to take appropriate measures to treat tooth loss in older people for preventing and/or treating senile osteoporosis.
Osteoporosis is a common skeletal disease characterized by decreased bone mass and deteriorated bone microstructure, leading to increased fracture risk (National Institutes of Health 2000). With the aging of the population, the burden of osteoporosis and its related fracture is rapidly expanding. Osteoporosis had become an increasingly important health and socioeconomic issues. Osteoporosis has a complex and multifactorial etiology. Several risk factors, including menopause, physical inactivity, smoking, alcohol use, corticosteroid use and inadequate calcium and vitamin D intake have been implicated in the development of osteoporosis (National Institutes of Health 2000; Pothiwala et al. 2006; Weinstein 2011). Recently, repeated or continuous stress is also discussed in this context. The relationship between stress and health has been the research focus over the years. Various chronic psychological or physical stress is linked to the development of many diseases, including anxiety, Alzheimer’s disease, inflammatory disease, cancer, cardiovascular disorder, obesity and diabetes (Marin et al. 2011; Proietti et al. 2011; Reber 2012; Greenberg et al. 2014). There is a lot of evidence indicating the association between chronic stress and bone loss (Patterson-Buckendahl et al. 1995; Yirmiya et al. 2006; Cizza et al. 2009; Furuzawa et al. 2014).
Masticatory activity plays an important role in the whole body homeostasis. Teeth are essential for masticatory process. Teeth also play a critical role in overall nutrition and general health. Teeth provide a uniquely discriminating sense of touch and directional specificity for occlusal awareness, intra-oral contact for management of a food bolus, discrimination of food texture and hardness, and control of jaw muscles for mastication and swallowing (Klineberg et al. 2012). These specific features of teeth are closely linked with the periodontal sensitivity, suggesting an integrated role of the pulp-dentine-enamel complex (Farahani et al. 2011). Many studies have shown that poor mastication due to tooth loss, acting as stress, causes various reactions of the autonomic nervous system and endocrine system, activates the hypothalamic-pituitary-adrenal (HPA) axis and transmits to the hypothalamus to increase the release of corticotrophin-releasing hormone (CRH), inducing the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary, which stimulates the adrenal cortex to produce glucocorticoids, such as cortisol in humans and corticosterone in mice (Levine 2005). Evidence from clinical studies indicated that chronically stressed patients exhibited alterations in HPA axis activity (Aguilera 2011). Animal experiments showed that the plasma corticosterone level of the toothless mice was significantly higher than those of the control mice. Pretreatment with metyrapone, which suppresses the stress-induced rise in plasma corticosterone levels, prevented the toothless condition-induced increase in plasma corticosterone levels (Onozuka et al. 2002; Ichihashi et al. 2007; Kubo et al. 2007). However, the association between tooth loss and bone status remains unclear. In the present study, we examined the bone quantity and quality to determine whether tooth loss early in life accelerates age-related bone loss in the senescence-accelerated mouse strain P8 (SAMP8).
The senescence-accelerated mouse (SAM) strains are a group of related inbred strains that have been developed by selective inbreeding for accelerated senescence (SAMP strains) and for a normal senescence profile (SAMR strains) from the progeny between AKR/J and unspecified strain(s) of mice. Molecular genetic analyses of SAM strains have revealed two noteworthy features. One is that the nuclear genome of SAM strains is a complex mosaic of that of parental AKR/J and unspecified strain(s), with each strain having a distinct mosaic pattern. The other feature is that SAM strains harbor rare or even unique nucleotide polymorphisms in the nuclear and mitochondrial genomes. Because of the wide variation in the experimental approaches, ages, organs and brain areas, clear genetic variation among SAMP8 and its original strain ARK/J was not determined until now (Mori and Higuchi 2013). SAMP8 mice retain the albino phenotype (white coat with red eye). SAMP8 mice show an accelerated senescence and age-associated pathologies such as deficits in learning and memory, brain atrophy (Takeda et al. 1994). SAMP8 mice were also used to mimic senile osteoporosis (Derave et al. 2005; Lo et al. 2009; Wauquier et al. 2012; Furuzawa et al. 2014). In this light, SAMP8 mice correctly mimic aging features and pathophysiologic environment regarding senile osteoporotic establishment in humans.
SAMP8 mice were obtained from our breeding colony, maintained as an inbred stain from breeders originally provided by the Council for SAM research and housed in the Division of Animal Experiment, Asahi University School of Dentistry.
All animals were maintained on a standard pellet chow for rodents (CE-2, CLEA Japan, Inc., Tokyo, Japan) available ad libitum. In the present study, only male mice were used for experiments. The mice were housed in groups of 5 in plastic cages under temperature- and humidity-controlled conditions (23 ± 1°C, 55 ± 25%) and a 12:12 h light/dark cycle (light period, 6:00-18:00; dark period, 18:00-6:00). The experiment was undertaken in accordance with the guidelines for care and use of laboratory animals, Asahi University.
Mice were randomly divided into the control and toothless groups. Both groups were subdivided into 3 age groups of 10 each, totaling 60 animals. Removal of the upper molar teeth was performed as described previously (Kubo et al. 2005). Briefly, at one month of age, mice were anesthetized with sodium pentobarbital and all upper (maxillary) molar teeth were extracted using dental tweezers. Control animals underwent the same manipulation except that the molar teeth were not extracted. After the procedure, all mice were housed separately with respect to the control or toothless mice in a group of 5 per cage during the post-operative period.
Blood sampling and the plasma corticosterone measurementAt the time of euthanasia, all mice were administered by an overdose of sodium pentobarbital. As the peak blood corticosterone level occurred at 20:00 (Onozuka et al. 2002), blood was sampled at 20:00. The plasma corticosterone level was measured by radioimmunoassay as reported previously (Furuzawa et al. 2014; Onishi et al. 2014).
Biomechanical testingBiomechanical testing of the femur was performed at room temperature using a mechanical strength analyzer (Shimadzu, EZ Graph, Japan), as reported previously (Watanabe et al. 2009). The left femur was positioned horizontally on a special holding device with supports located at a distance of 5 mm apart. For stabilization, the mid-diaphysis of the femur was placed on a special holding device with supports of the test apparatus that were 5 mm apart. The load of a three-point bending test was applied in the anteroposterior direction midway between the two supports. A bending force was applied with the cross head at a speed of 0.1 mm/min, until the bone collapsed. The load-displacement curve was recorded. The maximum load (N) and stiffness (N/mm) were calculated according to previously published equations (Turner and Burr 1993).
Micro-CT scanning and 3D microstructural analysisAfter the experiment, the femurs and the 4th lumbar vertebrae (L4) were dissected and cleaned of adherent soft tissues. The bone samples were analyzed using cone-beam X-ray micro-CT system (MCT-CB100MF, Hitachi Medical Corporation, Kashiwa, Japan) as described previously (Chen et al. 2008, 2009; Chen and Kubo 2012). The metaphyses of the distal femur and L4 vertebral body were scanned at the resolution of 10 µm, with a tube voltage of 50 kV, tube current of 0.1 mA.
After micro-CT scanning, the image data was transferred to a workstation. The vertebral trabecular bone region was outlined for each micro-CT slice, excluding both the cranial and caudal endplate regions. Within these regions, trabecular bone was separated from cortical bone with boundaries defined by the endocortical bone surfaces. As for the distal femur, the region of interest was defined using 100 slices at approximately 0.5-2.0 mm away from the growth plate. The structural indices were calculated using a 3D trabecular bone analysis software TRI/3D-BON (Ratoc System Engineering Co. Ltd., Tokyo, Japan). TRI/3D-BON builds 3D models from serial tomographic datasets for visualization and morphometric analysis as described (Chen et al. 2011; Chen and Kubo 2012).
Histomorphometric measurementsBone samples were harvested for histomorphometric analysis. Each mouse received an intraperitoneal injection of 30 mg/kg of calcein (Sigma-Aldrich, St. Louis, MO, USA) eight days before euthanasia and a second injection two days before euthanasia for measurement of bone formation by double labeling. For plastic sectioning, vertebrae L3 were fixed in 70% ethanol and embedded in undecalcified in methylmethacrylate. Sections were cut at 150 µm thickness using a low speed diamond saw (Isomet, Buehler, Lake Bluff, IL, USA), and then hand ground to a thickness of 20 µm for histomorphometric analyses.
Specimens for paraffin sectioning were fixed in freshly prepared 4% paraformaldehyde, decalcified in 10% EDTA and embedded in paraffin by standard histologic procedures, as described previously (Fen et al. 2002). Then 5-µm-thick sections were prepared for tartrate-resistant acid phosphatase (TRAP) staining to evaluate osteoclast number per bone perimeter (N.Oc/B.Pm). All bone histomorphometric measurements were performed using Adobe Photoshop, as recently described (Egan et al. 2012).
Statistical analysisResults were repressed as means ± s.d. Statistical analysis was performed by repeated measures analysis of variance or factorial analysis of variance followed by Scheffe’s post hoc multiple comparison tests. Probability values < 0.05 were considered statistically significant.
Mean body weight was comparable between the control and toothless mice on the day of surgery. The body weight of the toothless mice showed significantly lower compared with the age-matched molar-intact control mice (Fig. 1A). The plasma corticosterone levels for both control and toothless mice are shown in Fig. 1B. The plasma corticosterone levels of both control and toothless mice increased with age. The plasma corticosterone level of the toothless mice at 2 months of age had a tendency to be higher. At 5 and 9 months, the plasma corticosterone level of the toothless mice was significantly higher compared with the age-matched control mice (Fig. 1B). As the chronic stress causes a significant increase in the circulating corticosterone levels, we consider that tooth loss early in life acts as chronic stress.

The mean body weight and the plasma corticosterone level in the control and toothless mice.
The body weight of the toothless mice showed significantly lower compared with the age-matched molar-intact control mice (A). The plasma corticosterone levels of both control and toothless mice increased with age. Compared with the control mice, the plasma corticosterone level of the toothless mice at 5 and 9 months of age was significantly higher (B). All data represent as mean ± s.d. *P < 0.05.
Fig. 2 shows the mean values of the femoral mechanical parameters. As is evident, among the three age groups, the values of maximum load and stiffness were highest at 5 months of age in both control and toothless groups. Compared with the age-matched control mice, femoral maximum load and stiffness had a tendency to be lower in the toothless mice at 2 months of age. There was a significant decrease of maximum load and stiffness in the toothless mice at 5 and 9 months.
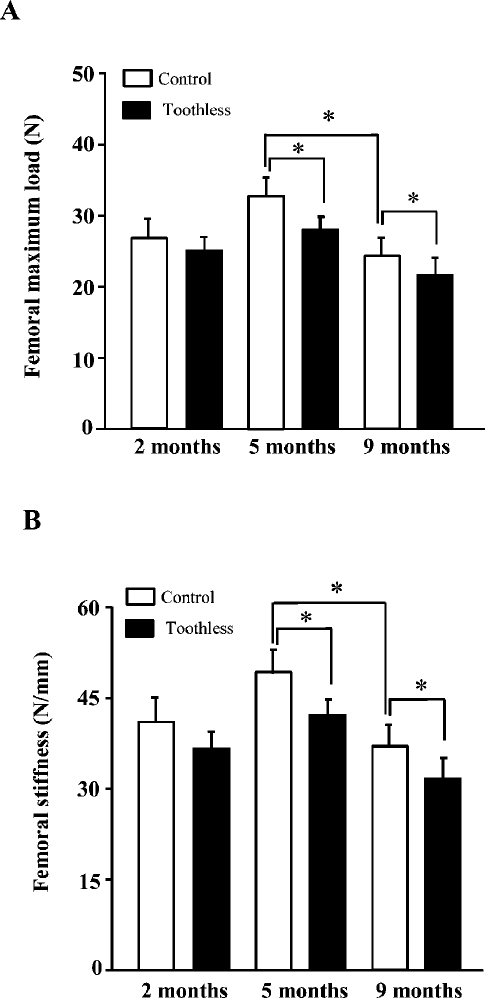
The femoral maximum load and stiffness in the control and toothless mice.
The femoral maximum load (A) and stiffness (B) were significantly lower in the toothless mice at 5 and 9 months of age, compared with the age-matched control mice. All data represent as mean ± s.d. *P < 0.05.
The microstructural parameters of the vertebral trabecular bone are shown in Fig. 3. Among the three age groups of the control mice, the 5-month-group had the highest value of trabecular bone volume fraction (BV/TV). BV/TV decreased between control and toothless mice by around 38%, 55%, and 59% for 2-, 5- and 9-month groups respectively. The change pattern of trabecular number (Tb.N) and trabecular thickness (Tb.Th) was quite similar with that of BV/TV. As compared with the control mice, trabecular number (Tb.N) of the toothless mice was significantly lower at 5 and 9 months of age. Trabecular thickness (Tb.Th) was decreased in the toothless mice of 2- and 5-month-groups. Consistently, trabecular separation (Tb.Sp) was significantly higher in the toothless group at 2, 5 and 9 months of age (Fig. 3).
Fig. 4 shows the typical three-dimensional reconstructed micro-CT images of the L4 vertebral body. Images of the sample with BV/TV that was closest to the mean BV/TV were reconstructed in each group. The trabecular bone was highest at 5 months of age among the three age groups. There was a significant trabecular bone loss in the toothless group compared with the age-matched control group (Fig. 4).

Three-dimensional microstructural properties of the vertebral trabecular bone in the control and toothless mice.
Compared with the control mice, bone volume fraction (BV/TV) was significantly decreased (A) in the toothless mice at 2, 5 and 9 months. Trabecular thickness (Tb.Th) was declined at 2 and 5 months (B), and trabecular number (Tb.N) was reduced at 5 and 9 months (C). Trabecular separation (Tb.Sp) was significantly increased in the toothless mice at 2, 5 and 9 months (D). All data represent as mean ± s.d. *P < 0.05.

Three-dimensional micro-CT images representative the fourth lumbar vertebral body in the control and toothless mice.
The trabecular bone was highest at 5 months of age among the three age groups. There was a significant trabecular bone loss in the toothless mice, compared with the age-matched control mice at 2, 5 and 9 months of age. Bar = 0.3 mm.
a: 2-month-control; b: 2-month-toothless; c: 5-month-control; d: 5-month-toothless: e: 9-month-control; f: 9-month-toothless.
The microstructural parameters of the distal femoral trabecular bone are shown in Fig. 5. The change pattern of the trabecular bone microstructural properties in the distal femur resembled that of the vertebra. BV/TV of the distal femur was much lower than that of the vertebra. BV/TV of the toothless group was significantly lower compared with the control group among the three age groups. Tb.N was significantly lower at 5 months, and Tb.Th was decreased at 2 and 5 months of age. Tb.Sp was significantly higher in the toothless mice at 5 and 9 months of age (Fig. 5).

Three-dimensional microstructural properties of the distal femoral metaphyseal trabecular bone in the control and toothless mice.
Compared with the control mice, BV/TV was significantly decreased in the toothless mice at 2, 5 and 9 months (A). Tb.Th was declined at 2 and 5 months (B), and Tb.N was reduced at 5 months (C). Tb.Sp was significantly increased in the toothless mice at 5 and 9 months (D). All data represent as mean ± s.d. *P < 0.05.
The bone formation rate/bone surface (BFR/BS) of both control and toothless mice tended to decrease with age. Compared with the control mice, BFR/BS was significantly lower in the toothless mice at 5 and 9 months of age (Fig. 6). The number of TRAP-positive osteoclasts per bone perimeter was shown in Fig. 7. TRAP-positive osteoclast number (N.Oc/B.Pm) was significantly increased in the toothless group compared with the age-matched control group.

The bone formation rate per bone surface (BFR/BS) in the control and toothless mice.
Compared with the control mice, BFR/BS was significantly lower in the toothless mice at 5 and 9 months of age. All data represent as mean ± s.d. *P < 0.05.

TRAP-positive osteoclast number per bone perimeter (N.Oc/B.Pm) in the control and toothless mice.
N.Oc/B.Pm was significantly increased in the toothless mice at 2, 5 and 9 months, compared with the age-matched control mice. All data represent as mean ± s.d. *P < 0.05.
The key finding of this study is that tooth loss since an early age accelerated the bone deterioration morphologically and functionally with age. Both human and animal studies showed that various chronic stress inhibits osteoblast bone formation and stimulates osteoclast bone resorption, leading to bone loss (Yirmiya et al. 2006; Cizza et al. 2009; Furuzawa et al. 2014). The results of the present study are in line with these findings. We found that tooth loss early in life caused a decrease of osteoblast bone formation and an increase of osteoclast bone resorption in SAMP8 mice at 2, 5 and 9 months of age. We did the same experiments using C57BL/6 and ddY mice. Bone loss was also accelerated in toothless mice with age (unpublished data). Therefore, we consider that tooth loss early in life accelerated age-dependent osteoporosis is the phenomenon common in general. Tooth loss induced significant reduction of trabecular bone volume fraction both in vertebra and femur. Age-related reduction of BV/TV in toothless mice is due to decreases in Tb.Th and Tb.N, and increases in Tb.Sp, that has formed the basis of the plausible hypothesis for trabecular bone loss in osteoporotic patients (Riggs and Parfitt 2005; Chen et al. 2008, 2010). In this study, we found that the mean body weight of the toothless mice was lower than that of the control mice by 6-8%. However, we did not find any marked differences of the daily food intake and the general nutritional state between the control and toothless mice. It was reported that weight gain or weight loss within 10 percent had little effect on bone mass (Meyer et al. 2008). It is, therefore, reasonable to assume that the low body weight is not the main factor that affects the bone condition of the toothless mice.
The adult skeleton undergoes continuous bone remodeling, in which a homeostatic balance between osteoblast and osteoclast activity plays a pivotal role. The bone remodeling is strictly regulated by many neurotransmitters, hormones and various cytokines. Exposure to chronic stressors, such as restraint, immobilization, forced swimming and social or mental stressors activates the HPA axis, leading to sustained increase of the circulating corticosterone. Previous studies indicated that chronic stress induced enlargement of adrenal gland and elevated circulating corticosterone level (Konkle et al. 2003; Yirmiya et al. 2006; Furuzawa et al. 2014). Adrenalectomy abolished the stress-induced bone loss, implicating glucocorticoids in this effect (Yirmiya et al. 2006). Sustained loss of masticatory stimulation induced by toothless condition since an early age not only accelerated the aging process of hippocampus-dependent cognitive function, but also developed abnormal behavior of locomotor hyperactivation at their advanced age (Iinuma et al. 2014; Kawahata et al. 2014). SAMP8 mice undergo normal maturation up to the age of 6 months, and then exhibit accelerated aging (median life span 12 months compared with 2 or 3 years for other strains). SAMP8 mice are a proposed experimental murine model for senile osteoporosis (Furuzawa et al. 2014). It was reported that the plasma corticosterone level was significantly higher in the mature and old toothless SAMP8 mice (Iinuma et al. 2014). As chronic stress causes a significant increase in the plasma corticosterone levels (Onozuka et al. 2002; Furuzawa et al. 2014; Iinuma et al. 2014), we consider that tooth loss early in life may act as chronic stress in adult and aged SAMP8 mice.
Previous studies demonstrated that exposure to various stressors, such as restraint, immobilization, forced swimming, psychological and social stressor led to sustained increase in the circulating corticosterone level. Osteoporosis induced by glucocorticoid (cortisol in humans and corticosterone in mice and rats) is the most common form of secondary osteoporosis (Van Staa et al. 2000). Glucocorticoid is associated with detrimental effects on bone caused by suppression of bone formation and stimulation of bone resorption via glucocorticoid receptor in bone cells. Glucocorticoid excess induces a decrease in osteoblastogenesis and impairs osteoblastic differentiation and maturation, leading to decreases in the number and function of osteoblasts (Weinstein et al. 1998). Glucocorticoid increases the expression of the macrophage colony stimulating factor (M-CSF) and the receptor activator of NF-κB ligand (RANKL), and decreases the expression of its decoy receptor, osteoprotegerin (Canalis et al. 2007; Compston 2010). As a result, there is an early increase in osteoclastogenesis and a prolongation of the osteoclast lifespan (Compston 2010). Recent clinical investigations demonstrated that there was a significant relationship between molar tooth number and osteoporotic status (Darcey et al. 2013; Numoto et al. 2013). Teeth are fundamental to quality of life throughout human life. Early tooth loss not only causes masticatory disorders, but also acts as chronic stress, inducing constant increase of the circulating corticosterone level. The elevated corticosterone level has negative effects on genes and molecular signaling pathways in bone cells, impairing the osteoblast-osteoclast dynamic homeostasis, leading to bone loss with age.
In conclusion, this study showed that tooth loss early in life acted as chronic stress, induced constant increase in the circulating corticosterone level. The elevated level of corticosterone impaired the dynamic homeostasis of bone formation and bone resorption, accelerated age-related bone deterioration in SAMP8 mice. Long-term tooth loss may be a risk factor for age-dependent osteoporosis. Adequate dental treatments for tooth loss of the older people are important for preventing and/or treating osteoporosis.
The authors declare no conflict of interest.