2015 年 235 巻 1 号 p. 39-46
2015 年 235 巻 1 号 p. 39-46
Chewing xylitol gum provides oral health benefits including inhibiting Streptococcus mutans plaque. It is thought to be especially effective in conditions where it is difficult to perform daily oral cleaning. Our study aim was to determine the effects of chewing xylitol gum on self-rated and objective oral health status under a condition interfering with oral hygiene maintenance. A randomized controlled intervention trial was conducted on 55 healthy ≥ 20-year-old men recruited from the Japan Ground Self Defense Force who were undergoing field training. Participants were randomly assigned to a test group (chewing gum; n = 27) or a control group (no gum; n = 28) and the researchers were blinded to the group assignments. The Visual Analog Scale (VAS) scores of oral conditions subjectively evaluated oral health, and the stimulated salivary bacteria quantity objectively evaluated oral health 1 day before field training (baseline) and 4 days after the beginning of field training (follow-up). VAS scores of all three oral conditions significantly increased in the control group (malodor: p < 0.001; discomfort: p < 0.001; dryness: p < 0.001), but only two VAS scores increased in the test group (malodor: p = 0.021; discomfort: p = 0.002). The number of salivary total bacteria significantly increased in the control group (p < 0.01), while no significant change was observed in the test group (p = 0.668). Chewing xylitol gum positively affects self-rated and objective oral health status by controlling oral hygiene under conditions that interfere with oral hygiene maintenance.
The use of sugar-free chewing gum may be especially effective in conditions where it is difficult to carry out daily oral cleaning and conventional oral maintenance. People in areas affected by earthquakes and tsunami were barely able to maintain oral health owing to a lack of water and oral hygiene materials, such as toothbrushes and toothpaste (Hosokawa et al. 2012). Other research on the oral health of earthquake and tsunami survivors also reported that periodontal conditions were worse and the number of remaining teeth was lower than the national average in Japan (Kishi 2012; Ministry of Health, Labor and Welfare 2012). Because chewing gum does not depend on oral hygiene materials and can be used even if water does not exist, the use of sugar-free gum may help maintain oral health and act as a buffer against stress even in natural disasters and similar situations, such as simulated training for natural disaster preparation in the Self-Defense Forces, police, and the like.
Xylitol chewing gum, a type of sugar-free chewing gum, is a well-known and effective strategy for preventing caries (Isokangas et al. 1988; Machiulskiene et al. 2001; Burt 2006), and acts primarily by inhibiting Streptococcus mutans plaque (Ly et al. 2006; Nakai et al. 2010; Seki et al. 2011; Shinga-Ishihara et al. 2012). Moreover, the use of xylitol chewing gum as an adjunct to tooth brushing is beneficial for oral hygiene including plaque control, based on a systematic review of the literature (Keukenmeester et al. 2013). Previous studies have also reported that chewing gum may relieve stress and depression (Scholey et al. 2009; Smith 2010; Smith et al. 2012; Yu et al. 2013).
There are a small number of studies evaluating the effects of chewing xylitol gum on oral health status using both self-rated and objective parameters. We hypothesized that chewing xylitol gum could affect oral conditions and the number of salivary total bacteria even in an environment where it is difficult to carry out daily oral hygiene and conventional oral health maintenance. Therefore, our aim was to confirm the effects of chewing xylitol gum on oral health in people under conditions that prevent oral hygiene maintenance by comparing the change in Visual Analog Scale (VAS) scores of oral conditions and the salivary bacterial population during Japan Ground Self Defense Force (JGSDF) field training using an exploratory randomized controlled trial design.
The present study was conducted in October 2011. Participants were recruited from JGSDF members undergoing field training at the Fukuoka base. Field training created an environment wherein the participant felt as if he was experiencing a disaster event in preparation for actual disaster and emergency events. During field training, it was very difficult for participants to maintain daily oral hygiene. We included healthy men aged over 20 years. The following individuals who were not able to provide sufficient data for the proper assessment of self-rated and objective parameters of oral health status were excluded: two individuals who used dentures or received a dopamine receptor antagonist. Thus, the remaining 55 randomly selected participants were deemed eligible and enrolled by the commanding officer of the JGSDF. All participants provided informed written consent after receiving sufficient explanation of the study. This study was approved by the ethics committee of the faculty of Kyushu University (23036) and registered with the UMIN Clinical Trials Registry (UMIN Clinical Trial Registration No. 000007962).
Study designThe trial design is summarized in Fig. 1. This study employed a randomized controlled trial methodology in accordance with the CONSORT Statement (Schulz et al. 2010); the commanding officer assigned the participants to the test group (n = 27) or control group (n = 28) using a sealed opaque envelope to conceal the random allocation sequence until interventions were assigned. The examiner and researchers were blinded to both the group sampling and assignments. The test group was instructed to chew two pieces of gum for 5 min 7 times daily during 4 days of field training based on self-reported information; the time taken to chew the gum was left to individual discretion. The control group did not receive gum during training. In both groups, VAS scores and stimulated saliva were collected to evaluate oral health 1 day before training (baseline survey) and on the final day of training (follow-up survey). Two weeks before training began, information regarding oral condition, hygiene habits, and subject demographics were obtained through an oral examination by a dentist and self-administered questionnaires. At the study conclusion, 5 subjects who declined the follow-up survey were excluded, and the remaining 50 participants (test group: n = 24; mean age, 30.8 ± 8.6 years; control group: n = 26; mean age, 28.4 ± 8.0 years) formed the final population for analysis. The main reason for dropout was that 5 subjects went on separate ways from the groups in the middle of the field training.

Schematic of trial design indicating the number of participants at each stage.
The sample size was calculated by statistical software (nQuery Advisor, Statistical Solutions, Saugus, MA, USA), based on changes in salivary bacterial cells and VAS scores in the prior literature (Ucak et al. 2011; Cochrane et al. 2012). A sample size of 20 per group was required for detection of a significant difference (80% power, two-sided 5% significance level).
Chewing gumThis study used a commercially available lime-mint flavored xylitol gum (Lotte Co. Ltd., Tokyo, Japan). The gum (1.5 g) contained 1.16-g carbohydrates, 0.5-g xylitol, 0.43-g maltitol, 0.3-mg phosphate calcium hydrogen, and 1.5-mg lait funoran extract. During the survey period, the test group chewed the gum at a xylitol dose of 7 g/day according to the following method recommended by Lotte Co. Ltd.: “Chew two pieces of gum for 5 min 7 times a day for promoting recalcification of tooth.” A previous review of the literature also suggested that a dose of 5-10 g/day xylitol divided into 3 or more frequencies of consumption are needed for therapeutic effects of chewing gum (Ly et al. 2008). We assessed the frequency that each subject in the test group chewed the gum by the following question just after training, “How many average times a day did you chew two pieces of xylitol gum during the training period?” The answer must be an integer between 0 and 7.
Self-rated oral health status using VASWe used the VAS to subjectively evaluate self-rated oral health status. The VAS has widely been used to measure clinical symptoms, such as oral malodor, discomfort, and dryness (Warde et al. 2000; Ohrn et al. 2001; Pai et al. 2001; Gerdin et al. 2005; Zaitsu et al. 2011). Assessed oral conditions included malodor, discomfort, and dryness. Each question consists of a 100-mm line with 0 representing a state of no awareness (minimum) and 100 representing a very strong (maximum) state of awareness. Participants were asked to mark the line to indicate the intensity of their oral condition.
Saliva collectionSaliva collections at baseline and follow-up were carried out in an optional place used for training at 1:30 pm and 9:00 am, respectively. During sampling, participants were instructed to sit in a chair and chew the same gum used in the intervention trial for 3 min, and the resulting saliva was collected into a sterile tube, weighed, and the weight was converted to volume based on 1 ml/g of specific gravity. The saliva was transported on ice in 2 hours and stored at −80°C until analysis.
Bacterial quantification using real-time polymerase chain reaction (PCR)Salivary total bacteria were quantified to objectively assess oral health status using a QuantiFast SYBR Green PCR kit (Qiagen, Hilden, Germany) in a StepOne Real-Time PCR System (Applied Biosystems) according to the manufacturer’s instructions. Real-time PCR is an advanced form of PCR that is an experimental method for amplifying specific regions of the DNA molecule by using DNA polymerase. The cycling conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. The universal bacterial primers 806F (5′-TTA GAT ACC CYG GTA GTC C-3′) and 926R (5′-CCG TCA ATT YCT TTG AGT TT-3′) were used. DNA melting curves for the 16S rRNA amplicons were assessed for any putative PCR artifacts or nonspecific PCR products. To reflect the oral hygiene of the individuals, we calculated the total bacterial quantities using the comparative Ct method with extracted Streptococcus mutans Xc DNA as the real-time PCR standard (Shibata et al. 2003; Takeshita et al. 2010). One colony-forming unit of Streptococcus mutans was defined to be one cell of the organism.
Oral examination and questionnairesThe oral examination was carried out by a single dentist (T.H.). Oral examination data included the decayed, missing, and filled tooth (DMFT) index, number of teeth present, number of teeth with a pocket depth (PD) ≥ 4 mm, bleeding on probing (BOP), and plaque index. The DMFT index is an accepted indicator for the prevalence of dental caries (Aida et al. 2008; Sheiham and Sabbah 2010). The six-point method of probing PD was performed on the remaining teeth, excluding the wisdom teeth, and the PD was recorded at the deepest part of each tooth (mm) as a periodontal parameter. BOP was used as an index of gingival inflammation (Lang et al. 1986). The plaque index was used as a measurement of dental plaque (Loe 1967). Examiner reliability for the oral examination was verified by an intra-examiner calibration of volunteers who had similar characteristics to the study participants; Cohen’s κ value was > 0.8, which indicated good intra-examiner reliability agreement. Each subject completed a self-administered questionnaire that assessed tooth brushing, smoking frequencies, and gum chewing frequencies. Tooth brushing frequency was classified as twice or more daily, once daily, or less than once a day (Wakaguri et al. 2011). Smoking frequency was classified as current, ever, or non-smoker (Takeuchi et al. 2014).
Statistical analysisDemographic differences between the control and test groups were ascertained using the chi-squared test for categorical variables and the Mann-Whitney U test for continuous variables. Changes within each group during the survey period were measured using the Wilcoxon signed-rank test. In addition, to assess the differences in the amount of changes in salivary bacteria during the survey period between the groups adjusting for baseline salivary bacteria quantity, a generalized linear model (GLM) with an identity link and normal distribution was conducted. In our outcome measurements, only a distribution of the amount of changes in salivary bacteria during the survey period approximately had a normal distribution. A value of p < 0.05 indicated statistical significance. All statistical analyses were performed using SPSS version 20 (IBM SPSS Japan, Tokyo, Japan).
Table 1 summarizes the participant demographics. The median age in the control group and the test group was 26.5 y (range, 23.0 to 32.3) and 28.0 y (range, 24.3 to 36.8), respectively. All the participants were men and had at least 27 natural teeth. Age, number of teeth present, DMFT, smoking frequency, gum chewing frequency, number of teeth with a PD ≥ 4 mm, BOP, and plaque index were not significantly different between the test and control groups at baseline. During field training, the test group participants chewed gum 4.77 ± 1.41 times (mean ± s.d.) per day and chewing frequency ranged from 1 to 7.
Table 2 summarizes tooth brushing frequency at baseline and during the training period. The prevalence of tooth brushing twice or more daily was 92.0% (test group: n = 22, 91.7%; control group: n = 24, 92.3%) before training and 18.0% (test group: n = 2, 8.3%; control group: n = 7, 26.9%) during training. Although tooth brushing frequency was not significantly different between the test and control groups at baseline (p = 0.933), there was a significant difference in tooth brushing frequency between the groups during training (p = 0.031). In addition, during the training period, subjects in both groups demonstrated significantly reduced tooth brushing frequency (test group: p < 0.001; control group: p < 0.001) compared to baseline using the Wilcoxon signed-rank test (data not shown).
Fig. 2 illustrates the oral condition VAS scores over the survey period. At baseline, there was no significant difference in the VAS scores between the control and test groups. However, at follow-up, the VAS scores in the control group were significantly higher than scores in the test group. In addition, all VAS scores in the control group significantly increased after training, while in the test group, only malodor and discomfort VAS scores increased, but dryness scores did not.
Fig. 3 illustrates the stimulated salivary volume and salivary total bacteria quantity. There was no significant difference in the stimulated salivary volume between the control and test groups at either baseline or follow-up; both groups showed a significantly increased amount of stimulated saliva after training. The salivary bacteria quantity was significantly higher in the test group than in the control group at baseline. However, there was no significant difference in salivary bacteria quantity between the groups at follow-up. In the control group, there was a significantly increased salivary bacteria quantity after training, which was not observed in the test group.
Table 3 summarizes the effect of gum use on the salivary bacteria quantity assessed using a GLM. There was a significantly greater increase in salivary bacteria quantity in the control group than in the test group after consideration of baseline salivary bacteria quantity (p = 0.035).
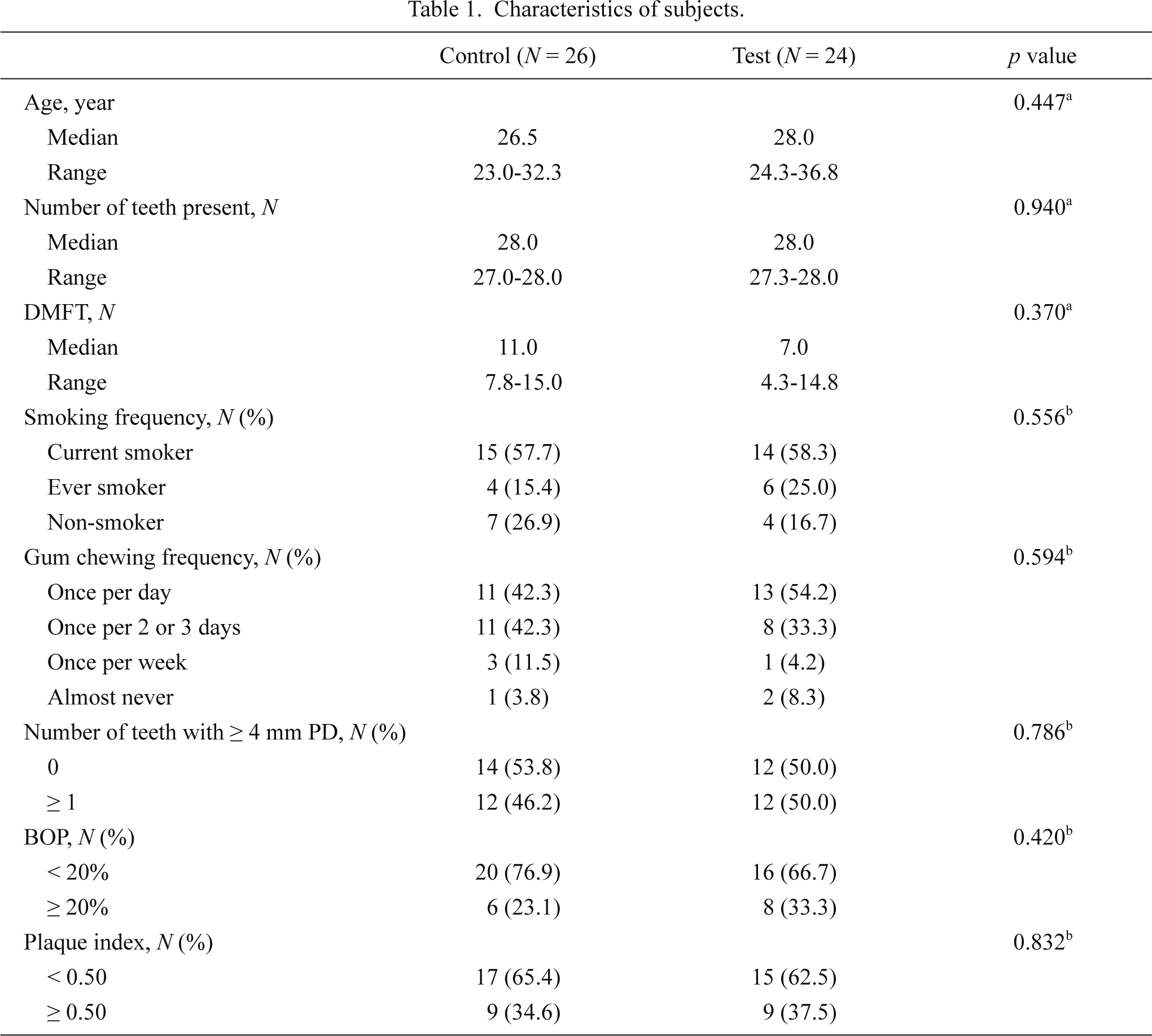
Characteristics of subjects.
DMFT, decayed, missing or filled tooth; PD, probing pocket depth; BOP, bleeding on probing.
aMann-Whitney U test.
bPearson’s Chi-square test.
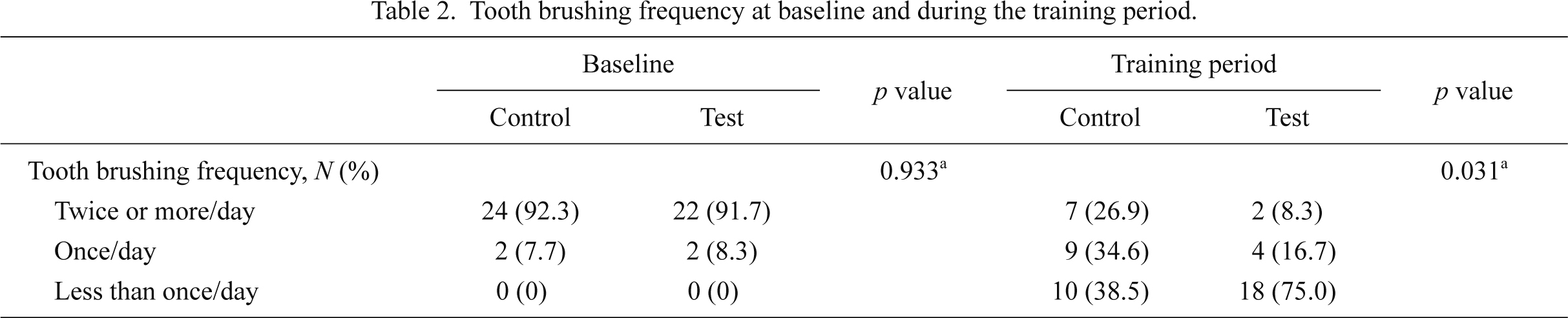
Tooth brushing frequency at baseline and during the training period.
aPearson’s Chi-square test.
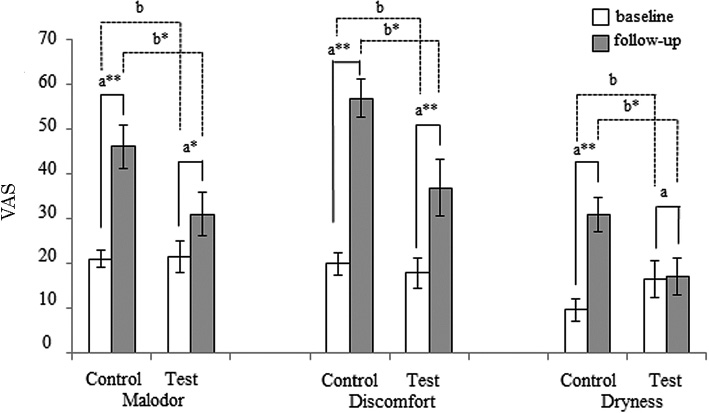
Reported changes in oral conditions (malodor, discomfort, and dryness).
VAS, Visual Analog Scale.
aWilcoxon signed-rank test.
bMann-Whitney U test.
**p < 0.01; *p < 0.05.

Quantified changes in the stimulated salivary volume and salivary bacteria quantity.
aWilcoxon signed-rank test.
bMann-Whitney U test.
**p < 0.01; *p < 0.05.

Effect of gum use on salivary bacteria quantity by GLM.
95% CI, 95% confidence intervals.
aGLM includes baseline salivary bacteria quantity (106 cells/ml) as covariate.
This randomized controlled intervention trial study has two major findings. First, this study indicates that chewing xylitol gum may improve self-rated oral health status in a situation that interrupts routine oral hygiene. Second, this study reveals that chewing xylitol gum may dampen the increase in salivary total bacteria quantity during this particular scenario, which serves as a marker of objective oral health status. Previous studies have investigated the relationship between the combined use of xylitol chewing gum and daily tooth brushing and oral health characteristics such as plaque, gingival index scores, stimulated salivary flow rate, and Streptococcus mutans quantity (Steinberg et al. 1992; Fure et al. 1998; Ly et al. 2006; Milgrom et al. 2006; Seki et al. 2011; Al-Haboubi et al. 2012). To our knowledge, the present study is the first to simultaneously examine the effects of chewing xylitol gum on both self-rated and objective indicators of oral health status in a simulating situation of natural disasters that interrupt daily tooth brushing.
Our findings are consistent overall with those of previous studies reporting that chewing xylitol gum is beneficial to oral health. A randomized controlled intervention trial (Campus et al. 2011) revealed that healthy adult individuals who chewed xylitol gum had a significantly lower concentration of salivary Streptococcus mutans compared to baseline. A systematic review of the literature suggested that the use of xylitol chewing gum as an adjunct to tooth brushing provided a beneficial reduction in plaque scores in a systematic review (Keukenmeester et al. 2013). Previous studies have also reported a positive correlation between the number of salivary total bacteria and the amount of tooth surface plaque (Schaeken et al. 1987; Price et al. 2007). Therefore, our results concerning the number of salivary total bacteria partially reflect the oral hygiene of the individuals. Our findings also suggest the plausible mechanism linking xylitol gum chewing and oral bacteria quantity. The xylitol contained in xylitol chewing gum has been known to inhibit the growth, metabolism, and polysaccharide production of Streptococcus mutans (Söderling et al. 1987, 2008; Söderling 2009). The subsequent decreased ability of biofilm formation may lead to a decrease in the amount of some kind of bacteria involved in the biofilms. Thus, chewing xylitol gum might suppress an increase in the number of salivary total bacteria.
All oral VAS scores were significantly increased in both the test and control groups when oral hygiene could not be maintained, except for the dryness VAS score in the test group. However, while there were significant differences in the three VAS scores after training, there was not any significant difference before training, as shown in Fig. 2. In parallel with the dampened increase in salivary bacteria quantity, incremental VAS scores were suppressed after chewing xylitol gum (Figs. 2 and 3). Presumably, an increased salivary bacteria quantity may contribute to increased VAS scores of oral conditions. This is consistent with previous studies reporting that malodor is correlated with oral hygiene status, including plaque control records and tongue-coating scores (Tanaka et al. 2003; Pham et al. 2011), and that the use of chewing gum helps improve oral discomfort (Warde et al. 2000). In contrast to a previous finding that decreased saliva was correlated with oral dryness (Guggenheimer and Moore 2003), we found a significant increase in the dryness VAS score in the control group, while saliva increased after training. In addition, we observed a significant increase in the amount of stimulated saliva in both groups after training, as shown in Fig. 2. We surmise that baseline salivation may be suppressed by stress before and during training (Queiroz et al. 2002).
Our study assumed that participants were unable to perform daily oral hygiene during difficult field training conditions. As expected, tooth brushing frequency decreased during training in both groups, though the control group had a higher daily tooth brushing frequency compared to the test group during training. This likely indicates that the test group subjects may have felt less need for daily oral hygiene because they were chewing xylitol gum. However, after training, the increase in salivary bacteria was significantly lower in the test group than the control group. Therefore, the use of xylitol chewing gum can suppress an increase in the salivary bacterial population after accommodating for potential confounding factors, including tooth brushing frequency.
Our study has several limitations and strengths. Despite selecting a randomized controlled intervention trial, which enabled us to control for potential confounding variables that could have altered the results, the study design did prove problematic. We could not standardize the tooth brushing frequency and allotment of xylitol chewing gum during the JGSDF field training. In addition, our intervention study did not include a crossover method. Therefore, we could not eliminate possible deviations from the study design, and the clinical effect of the intervention may be open to interpretation. However, the study benefits from the consistent lifestyle of the participants, such as daily meals during field training. Second, given the limited period of data collection, we prioritized salivation quantification and could not assess the plaque index at follow-up. Thus, it was impossible to control for oral hygiene changes over the course of training. Third, participants chewed the same gum used in the intervention trial during saliva collection at both baseline and follow-up; therefore, saliva collected from the control group might be contaminated by xylitol chewing gum. In addition, saliva collections at baseline and follow-up were performed at different times of the day because of the field training schedule. However, sampling was carried out by two groups at the same time. Thus, there is a possibility that the effect of gum use on oral health was underestimated. On the other hand, we believe that it is not necessary to address the antimicrobial effects of xylitol, as we froze the sampled saliva until analysis and performed a real-time PCR method to assess salivary bacteria quantity, including dead or damaged bacteria. Fourth, our research data about the gum chewing frequency were derived from self-reported questionnaires, raising issues of information bias regarding the accurate average chewing time. Fifth, our intervention study did not include a group where participants chewed a chemical-free gum as a control group. Therefore, our study design could not determine whether mechanical salivary stimulation or a chemical effect of xylitol contributed more to the improvement of oral conditions. Sixth, although the reliability and validity of VAS for measuring oral dryness already have been confirmed (Pai et al. 2001; Gerdin et al. 2005), the reliability and validity of VAS for measuring oral malodor and discomfort were still have not been validated. Therefore, our measurement might have not accurately reflected self-rated oral health status. Seventh, this study only evaluated men; therefore, we could not examine potential gender differences in the effects of xylitol chewing gum on oral health. However, there are no known previous studies reporting any significant association between gender and chewing gum use in adults. Finally, the 4-day intervention period was quite short. Our study schedule greatly depended on the field training schedule. Therefore, adjusting and extending our study intervention period was difficult. A long-term intervention is expected to reveal a more distinct effect of chewing xylitol gum on oral health.
The present results show that the use of xylitol chewing gum positively affects self-rated and objective oral health status by improving oral conditions and inhibiting the increase of salivary bacteria. These findings provide evidence for the use of xylitol chewing gum as a beneficial oral cleaning tool in conditions that prevent daily oral hygiene and conventional oral health maintenance.
We thank the Japan Ground Self Defense Force for their help in the study. We especially thank the individuals who participated in this study. This study was supported by a Grant-in-Aid of Scientific Research (24659935) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This study was also supported by Lotte Co. Ltd. through the provision of xylitol chewing gum. The authors report no conflicts of interest related to this study.
The authors declare no conflict of interest.
T.H., K.T., Y.S., and Y.Y. conceptualized and designed the study. T.H. and T.T. collected the data. K.T., Y.S., and T.T. interpreted and analyzed the data. T.H. and K.T. drafted the manuscript. Y.Y. revised the manuscript critically for important intellectual content. All authors read and approved the final version of this manuscript.