2015 Volume 235 Issue 4 Pages 295-304
2015 Volume 235 Issue 4 Pages 295-304
Visit-to-visit systolic blood pressure variability (VTV-SBPV) is correlated with cardiovascular complications. However, it still remains unclear whether VTV-SBPV is related to cardiovascular outcomes in patients with peritoneal dialysis (PD), who often manifest hypertension. We, therefore, evaluated the association of VTV-SBPV with all-cause mortality, cardiovascular complications, or the loss of residual renal function (RRF) that is a powerful predictor of mortality and morbidity in PD patients. We retrospectively reviewed the medical records for patients undergoing maintenance PD for at least 12 months at Seoul National University Hospital. The patients were divided into quartiles of VTV-SBPV based on the standard deviation of systolic blood pressure (SBP). We checked the SBP of the patients for up to 2 years after the initiation of PD. Among 216 PD patients, 16 primary outcome events (cardiovascular complications and all-cause mortality) occurred. VTV-SBPV was not associated with primary outcomes. During the follow-up, RRF loss occurred in 46 patients. The hazard ratios (HRs) for the loss of RRF in the 4 quartiles of VTV-SBPV, based on the highest to the lowest variability, were as follows: 6.201 (95% CI: 1.982-19.401, p = 0.002), 2.542 (95% CI: 0.859-7.523, p = 0.092), and 2.133 (95% CI: 0.635-7.165, p = 0.246), respectively. The loss of RRF was more frequently detected in patients with higher VTV-SBPV. VTV-SBPV was indicated as an independent risk factor for the loss of RRF. Therefore, the degree of variations in SBP should be carefully monitored to preserve the RRF of these patients.
Hypertension is prevalent in peritoneal dialysis (PD) patients, and it is a well-known and important risk factor for cardiovascular events (Cocchi et al. 1999). Many clinical guidelines and therapeutic interventions focus on mean blood pressure (BP) in PD patients to help prevent cardiovascular-related complications (Chobanian et al. 2003, James et al. 2014). Recent studies have revealed that visit-to-visit blood pressure variability (VTV-BPV) is strongly correlated with cardiovascular complications (Rothwell 2010, Rothwell et al. 2010). Many studies have examined BP and short- and long-term variations in BP in the general population (Muntner et al. 2011), as well as in patients with hypertension (Kawai et al. 2012b; Kostis et al. 2014), diabetes mellitus (DM) (Kilpatrick et al. 2010; Jennersjo et al. 2011), chronic kidney disease (Mallamaci et al. 2013), and end-stage renal disease (ESRD) requiring hemodialysis (HD) (Rossignol et al. 2012; Chang et al. 2014). By contrast, only a few studies have focused on VTV-BPV and outcomes in PD patients (Tonbul et al. 2002; Shigenaga et al. 2009). Therefore, we have evaluated the association of VTV-BPV with cardiovascular outcomes and all-cause mortality in PD patients.
The BP of PD patients improves initially but then steadily worsens over the years (Menon et al. 2001). Residual renal function (RRF) is a powerful predictor of mortality and morbidity in PD patients (Bargman et al. 2001), we also investigated the relationship between VTV-BTV and the loss of RRF. We used systolic blood pressure (SBP) as a major indicator for tracking BPV, because the variability in diastolic blood pressure (DBP) was found less informative in evaluating cardiovascular outcomes in epidemiological populations (Rothwell et al. 2010; Muntner et al. 2011).
We retrospectively reviewed the medical records of 249 adult patients who initiated PD between May 2005 and August 2012 at Seoul National University Hospital (SNUH). We excluded 6 patients who died, 9 patients whose dialysis modality changed to HD, 4 patients who were on maintenance HD for at least 3 months because of peritonitis or mechanical problems, and 10 patients who received kidney transplantation within 12 months of initiating PD. We also excluded 2 patients who were lost to follow-up and 2 patients who had no BP data in their medical records. The final study included 216 patients who were on maintenance PD for at least 12 months. This study was approved by the institutional review boards of the SNUH and conducted according to the principles of Declaration of Helsinki.
Baseline characteristicsDemographic and health-related data were collected from the patients’ medical records. The characteristics investigated include age, sex, body mass index, smoking status, underlying DM, hypertension, malignancy, congestive heart failure, a history of coronary artery disease or stroke, and the number and class of antihypertensive medications. Hypertension was defined according to the JNC 7 report (Chobanian et al. 2003) (i.e., SBP > 140 mmHg, DBP > 90 mmHg, or taking antihypertensive medications). Sitting BP was measured at every visit during 2 years using device that automatically measures BP.
Cardiovascular comorbidities were defined as a history of myocardial infarction, angina, or congestive heart failure. Romhilt-Estes “diagnostic” criteria (Romhilt and Estes 1968) were used to calculate the baseline peak wave velocity (PWV) and left ventricular hypertrophy (LVH) of the patients on electrocardiogram (ECG) within 1 month of initiating PD. The initial left ventricular mass index (LVMI) of the patients was also assessed on echocardiogram within 1 month of initiating PD. The following PD-related parameters were examined within 1 month from the initiation of PD: initial RRF, which was defined as creatinine clearance + urea clearance)/2 (mL/min/1.73 m2), Kt/V (K, clearance; T, treatment time; V, volume of urea distribution), peritoneal equilibration test (PET), and normalized protein nitrogen appearance (nPNA). Sitting BP was measured after a 5-minute rest. BP measured at every visit using an automatic BP measuring device (JAWON, EASY X 800).
Primary and secondary outcomesThe primary outcomes were the composites of cardiovascular death, nonfatal myocardial infarction, unstable angina, stroke, revascularization, hospitalization for heart failure, resuscitation cardiac arrest, and all-cause mortality. The primary outcomes were assessed from 1 year after the initiation of PD.
The secondary outcome was loss of RRF. Patients with an estimated urine output less than 200 mL/24 h were considered to have lost their RRF, and no further timed urine samples were collected. The 24 h RRF was measured at baseline (i.e., within 1 month of beginning PD) and thereafter at 6-month intervals.
Calculation of systolic blood pressure variability and definition of visit-to-visit systolic blood pressure variabilityThe visit-to-visit systolic blood pressure variability (VTV-SBPV) was defined as the variability between consecutive SBP measurements taken at different times. Although several parameters can be used to measure the variation in SBP over time, we determined the standard deviation (SD) as the variation of the VTV-SBP. The SD of the VTV-SBP is the most commonly used index of variability in a set of BP measurements. We retrospectively collected SBP data from baseline to 2 years after the initiation of PD. As the patient’s volume status may change after initiating PD, we excluded SBP data collected up to 3 months after the initiation of PD when calculating the SD of the SBP. All the included patients were divided into quartiles based on VTV-SBPV (i.e., highest to lowest variability).
Statistical analysisThe participants were grouped into quartiles based on SD of their SBP in each outpatient clinic visit. All the parameters are presented as the mean ± s.d. An ANOVA was used to compare the mean of the baseline characteristics. Linear regression was employed to assess the association between the participants’ characteristics and the SD in their SBP. Logistic regression models and Kaplan-Meier survival analysis were used to explore the association of the primary outcomes with the VTV-SBPV, when compared with other well-known risk factors. Multiple logistic regression analysis with the backward elimination technique was employed to identify predictors of the primary outcomes. The association of VTV-SBPV with the loss of RRF was evaluated by Cox proportional hazard models. Statistical analysis was performed with IBM SPSS Statistics 20. A value of p < 0.05 was considered statistically significant.
This study included 216 patients on maintenance PD for at least 12 months at SNUH. The demographic and clinical characteristics of the 216 patients at the initiation of PD are shown in Table 1. The mean age of the patients was 46.9 ± 14.0 years, with a range of 17 to 88 years, and the proportion of men was 59.3%. Most patients (n = 197, 91.2%) were hypertensive at the start of PD. The average number of antihypertensive medications was 2.6 ± 1.9, with a range of 0 to 10. Seventeen (7.9%) patients had a history of coronary artery disease, and 16 (7.4%) had a history of stroke. The mean SBP and DBP, from 4 months to 24 months after the initiation of PD, were 130.3 ± 11.4 mmHg and 78.9 ± 8.2 mmHg, respectively. The average SD of the VTV-SBPV was 12.90 ± 5.37 mmHg. When the participants were grouped by quartile of intra-individual SD of SBP, the SD values of the first (Q1), second (Q2), third (Q3), and fourth (Q4) quartiles were 0.85-9.57, 9.58-12.27, 12.28-15.80, and 15.81-46.06 mmHg, respectively. The median duration of PD was 442 months. The number of BP measurements ranged from 4 to 21, and the mean value did not differ in each quartile groups: (Q1: 13.8 ± 4.2 [range: 4-21], Q2: 15.0 ± 3.9 [range: 7-21], Q3: 14.9 ± 4.2 [range: 4-21], and Q4: 14.9 ± 3.9 [range: 4-21], p = 0.388).

Baseline characteristics of all the participants (total number of patients = 216).
CAD, coronary artery disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; RRF, residual renal function; Kt/V, urea clearance; PET, peritoneal equilibration test; CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cyclic peritoneal dialysis; NIPD, nocturnal intermittent peritoneal dialysis; nPNA, normalized protein nitrogen appearance; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Ca, calcium; P, phosphorous; PWV, peak wave velocity; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; ECG, electrocardiogram.
Patients with higher VTV-SBPV smoked more frequently. They took a higher number of antihypertensive medications, including beta-blockers and calcium channel blockers. Moreover, we found that these patients had higher C-reactive protein (CRP) levels (Table 2).
The baseline urine volume, RRF, Kt/V, PET type, use of icodextrin and nPNA, did not differ between the quartiles based on the SD of SBP. The biochemical results, excluding CRP, did not differ between the groups. The factors associated with the SD of SBP across visits after adjusting for age, sex, and multiple variables are shown in Table 3. A multivariable model showed that higher mean DBP, pulse pressure, CRP, and number of antihypertensive medications were associated with higher SD of SBP across visits.

Clinical characteristics by quartile of participants in groupings based on systolic blood pressure variability: standard deviation (mmHg), (total number of patients = 216).
CAD, coronary artery disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; RRF, residual renal function; Kt/V, Urea clearance; PET, peritoneal equilibration test; CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cyclic peritoneal dialysis; NIPD, nocturnal intermittent peritoneal dialysis; nPNA, normalized protein nitrogen appearance; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Ca, calcium; P, phosphorous; PWV, peak wave velocity; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; ECG, electrocardiogram. ∫p < 0.05, ∫∫p < 0.01, ∫∫∫p < 0.001.

Mean differences in the standard deviation of systolic blood pressure associated with participant characteristics.
CVD, cardiovascular diseases; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LVH, left ventricular hypertrophy; ECG, electrocardiogram. ∫∫p < 0.01, ∫∫∫p < 0.001.
*Model 1: Age and sex adjusted.
**Model 2: All the variables associated with the SD of SBP (p < 0.05) in Model 1. (The mean SBP variable was excluded due to the existence of multicollinearity.)
The numbers in the table are the differences in SBP (SD).
Over a median of 44 months of follow-up (range 12-102 months), 16 primary outcome events occurred. In the Q1 group, 2 primary outcome events occurred, while 3 occurred in the Q2, 5 occurred in the Q3, and 6 occurred in the Q4 groups, with no statistically significant differences between the quartile groups. Fig. 1 shows the Kaplan-Meier curves related to the primary outcomes according to the quartile of the SD of VTV-SBP.
The odds ratios (ORs) for cardiovascular events and all-cause mortality associated with quartiles of the SD of SBP, with the lowest quartile serving as the referent, were initially unadjusted and then adjusted for demographic characteristics, such as age and sex. A subsequent model included demographic characteristics and variables which were significantly correlated with the primary outcomes in the univariate analysis. Age, number of antihypertensive medications, cardiovascular comorbidities, and initial LVH on ECG that were associated with the primary outcomes (p < 0.05) in the univariate analysis are listed in Table 4. In the multiple logistic regression analysis, older age (OR 1.106, 95% CI: 1.042-1.174, p = 0.001) and LVH on ECG (OR 5.806, 95% CI: 1.569-21.483, p = 0.008) were associated with more cardiovascular events and all-cause mortality. Higher VTV-SBPV was not related to cardiovascular events and all-cause mortality in our study.

Primary outcomes based on the quartiles in the SD of VTV-SBPV.
Primary outcomes: cardiovascular death, nonfatal myocardial infarction, unstable angina, stroke, revascularization, hospitalization for heart failure, resuscitation cardiac arrest, and all-cause mortality.
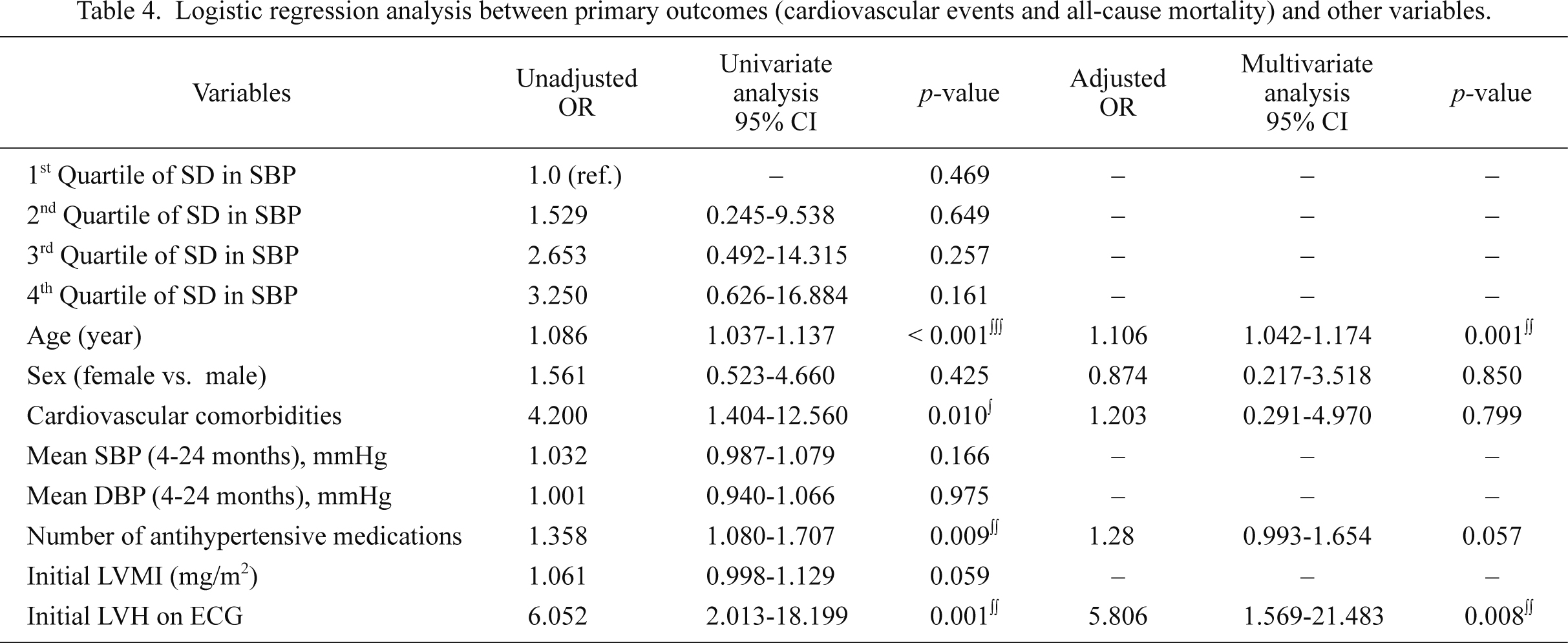
Logistic regression analysis between primary outcomes (cardiovascular events and all-cause mortality) and other variables.
SBP, systolic blood pressure; DBP, diastolic blood pressure; LVMI, left ventricular mass index; LVH, left ventricular hypertrophy; ECG, electrocardiogram.
∫p < 0.05, ∫∫p < 0.01, ∫∫∫p < 0.001.
During the follow-up, loss of RRF occurred in 46 of the 216 patients with PD. The loss of RRF occurred in 5 patients in the Q1, 9 in the Q2, 15 in the Q3, and 17 in the Q4 groups. The mean time to the loss of RRF was 28.9 ± 17.1 months. The Kaplan-Meier curves related to the secondary outcomes according to the quartile of the SD of VTV-SBP are presented in Fig. 2.
The hazard ratios (HRs) for the loss of RRF associated with quartiles of the SD of SBP, with the lowest quartile serving as the referent, were initially calculated unadjusted and then adjusted for age, sex, mean SBP (4-24 months), mean DBP (4-24 months), initial creatinine, Kt/V, urine volume, initial RRF, peritonitis rates (episodes/year), and use of icodextrin. The univariate analysis revealed that the factors associated with the loss of RRF were quartile group by the SD of SBP, mean SBP (4-24months), mean DBP (4-24months), initial creatinine, Kt/V, urine volume, RRF measured within 1 month from PD initiation, and rate of peritonitis. After adjustment for variables associated with the loss of RRF in univariate analysis, and age and sex, the risk of the loss of RRF was greater in the higher VTV-SBPV group (Table 5). The HRs for the loss of RRF in the 4 groups from the highest to the lowest quartiles of SD of SBP were Q4: 6.201 (95% CI: 1.982-19.401, p = 0.002), Q3: 2.542 (95% CI: 0.859-7.523, p = 0.092), and Q2: 2.113 (95% CI: 0.635-7.165, p = 0.220), respectively (Table 5).

Secondary outcomes based on the quartiles in the SD of VTV-SBPV.
Secondary outcomes: Loss of residual renal function.

Cox proportional hazard regression model results for selected risk factors secondary outcome: loss of residual renal function (anuria event).
SBP, systolic blood pressure; DBP, diastolic blood pressure; Kt/V, urea clearance; RRF, residual renal function.
∫p < 0.05, ∫∫p < 0.01, ∫∫∫p < 0.001.
This retrospective single tertiary hospital-based study looked for an association between long-term VTV-SBPV and outcomes cardiovascular outcomes, all-cause mortality, or the loss of RRF that is important in PD patients. Recent studies investigated the potential correlation between VTV-SBPV and renal function outcomes, such as the development of nephropathy in DM patients (Okada et al. 2012), and the prevalence rate of clinical albuminuria and microalbuminuria with VTV-SBPV (Kawai et al. 2012a). However, the correlation between VTV-SBPV and outcomes of PD patients remains unclear.
In our study, the patients with higher VTV-SBPV had more cardiovascular risk factors, including history of smoking, higher CRP, and greater use of beta-blockers. Smoking is an important risk factor for atherosclerosis and peripheral artery disease (Fowkes et al. 1992). Experimental study has shown an association between heavy smoking and variations in BP increases (Groppelli et al. 1992). It is possible that patients in the higher VTV-SBPV group have greater risk for chronic inflammatory response in the walls of arteries because of smoking. CRP is also a predictive marker for cardiovascular outcomes (Blake et al. 2003), exerting its effect on vascular smooth muscle cells by augmenting the production of endothelin-1 and upregulating the angiotensin 1 receptor (Verma et al. 2002; Wang et al. 2003). In our study, the VTV-SBPV group had higher CRP levels, which may be related to CRP’s effect of vasoconstriction.
A previous general population-based study found no significant association between VTV-BPV and DBP (Muntner et al. 2011). However, the mean DBP was associated with higher VTV-SBPV in our study, because the mean SBP variable in this study was excluded from analysis due to the existence of multicollinearity. A recent meta-analysis of data from randomized trials comparing antihypertensive medications showed that the use of calcium channel blockers and diuretics reported an association with lower variability in BP, whereas the use of angiotensin-converting enzyme (ACE) inhibitors and beta-blockers was associated with higher variability (Webb et al. 2010). However, the patients in our study who had higher VTV-SBPV used more calcium channel blockers and diuretics, which did not agree with the findings of the previous study.
Previous studies also reported a significant relationship between VTV-SBPV and cardiovascular outcomes and all-cause mortality (Rothwell et al. 2010; Muntner et al. 2011). However, we did not find a similar relationship in the PD patients in the present study, with few primary outcome events recorded in our institute. Our study reported a smaller number of primary outcome events in the higher VTV-SBPV group than those reported in previous studies; this contradicted the results of previous studies. Only age and higher LVH on ECG were independent risk factors for cardiovascular events and all-cause mortality as determined by the multiple logistic regression analysis. We also found no significant difference in the outcomes of the PD patients based on coefficient of variation of SBP or average real variability index of SBP.
Many studies have investigated the underlying mechanism of VTV-SBPV. One study found an apparent association with inconsistent adherence to antihypertensive medications (Kostis et al. 2014). The authors of the Systolic Hypertension in the Elderly Program (SHEP) study examined cardiovascular mortality in a follow-up of up to 17 years. The VTV-SBPV in the SHEP study was significantly higher in the placebo group than in the active treatment group. In our study, the patients with higher VTV-SBPV took more antihypertensive medications, but this finding was not statistically significant during the follow-up period. Another study reported a relationship between VTV-BPV and the arterial baroreflex function, which is important in buffering changes in BP and may be influenced by carotid endarterectomy (Timmers et al. 2004). An animal study with rats revealed that sinoaortic denervation had no effect on the mean BP, but the BPV increased (Eto et al. 2003). The underlying mechanism of this phenomenon was impaired endothelial function. A previous study found that genes of the renin-angiotensin-system (RAS) play a role in BPV, with the researchers reporting a correlation between VTV-BPV and RAS gene polymorphisms, especially ACE insertion/deletion (I/D) polymorphisms, in hypertensive patients (Kawai et al. 2012b).
Maiorca et al. (1995) first reported the important association between RRF and survival in PD patients, with the preservation of RRF conferring a survival benefit on those patients. Our study showed that higher VTV-SBPV was an independent risk factor for the loss of RRF, defined as anuria. A recent study revealed that RRF and BP were significantly related to LVMI (Ataş et al. 2014). An earlier study found a significant correlation between the decline in residual glomerular filtration rate and greater LVMI, and those patients had increased cardiovascular risk factors, such as increased arterial pulse pressure, anemia, and hypoalbuminemia (Wang et al. 2002).
It has been reported that BPV is a more critical determinant of glomerular damage than the BP level (Mancia and Parati 2003). A recent cross-sectional study of the relationship between VTV-SBPV and diabetic nephropathy in patients with type 2 DM demonstrated that patients with higher VTV-SBPV had a greater risk of developing albuminuria (Okada et al. 2013). Kawai et al. (2012a) used renal Doppler ultrasonography to analyze the relationship between VTV-BPV and the resistive index, which is a measure of renal vascular resistance, and found that subjects with higher SBPV had a significantly higher resistive index and that the VTV-SBPV was correlated with renal arteriosclerotic changes. Pulse pressure, which is an index of arterial stiffness, was associated with higher VTV-SBPV in our study, as reported in a previous study (Muntner et al. 2011). These findings suggest that end-stage organ damage is an important mechanism underlying the loss of RRF induced by VTV-SBPV. Although further animal studies and prospective studies are needed to determine the precise mechanism of the adverse effects of VTV-BPV on RRF, our study suggests that VTV-SBPV is an independent risk factor for the loss of RRF. Earlier studies revealed that PD patients administered icodextrin PD fluid had lower BP and better maintenance of urine volume (Davies et al. 2003; Selby et al. 2005). However, our study revealed no relationship between use of icodextrin and each of VTV-SBPV and the loss of RRF.
Our study has several limitations. First, it was a single center study with a small number of primary outcome events, especially cardiovascular outcomes and all-cause mortality; consequently, the results have limited statistical significance. Second, it was a retrospective study, so no control BP data were analyzed. Third, we did not identify a mechanism linking VTV-SBPV with the loss of RRF.
In conclusion, PD patients with higher VTV-SBPV had more cardiovascular risk factors including higher CRP levels and greater number of antihypertensive medications. No relationship was evident between VTV-SBPV and cardiovascular and/or all-cause mortality in our study population. However, in this study, we determined that higher VTV-SBPV was related to the loss of RRF. These results suggest that we should pay attention to the BP level as well as to its variation in PD patients.
The authors declare no conflict of interest.