2015 年 236 巻 3 号 p. 199-207
2015 年 236 巻 3 号 p. 199-207
Haloperidol is a neuroleptic drug used for a medication of various psychoses and deliria. Its administration is frequently accompanied by cardiovascular side effects, expressed as QT interval prolongation and occurrence of even lethal arrhythmias. Despite these side effects, haloperidol is still prescribed in Europe in clinical practice. Haloperidol binds to sigma receptors that are coupled with inositol 1,4,5-trisphosphate (IP3) receptors. Sigma receptors are expressed in various tissues, including heart muscle, and they modulate potassium channels. Together with IP3 receptors, sigma receptors are also involved in calcium handling in various tissues. Therefore, the present work aimed to study the effects of long-term haloperidol administration on the cardiac function. Haloperidol (2 mg/kg once a day) or vehiculum was administered by intraperitoneal injection to guinea pigs for 21 consecutive days. We measured the responsiveness of the hearts isolated from the haloperidol-treated animals to additional application of haloperidol. Expression of the sigma 1 receptor and IP3 receptors was studied by real time-PCR and immunohistochemical analyses. Haloperidol treatment caused the significant decrease in the relative heart rate and the prolongation of QT interval of the isolated hearts from the haloperidol-treated animals, compared to the hearts isolated from control animals. The expression of sigma 1 and IP3 type 1 and type 2 receptors was increased in both atria of the haloperidol-treated animals but not in ventricles. The modulation of sigma 1 and IP3 receptors may lead to altered calcium handling in cardiomyocytes and thus contribute to changed sensitivity of cardiac cells to arrhythmias.
Haloperidol is an antipsychotic drug used for a medication of both acute and chronic psychosis, mainly schizophrenia, mania and psychomotor agitation. Oral or parenteral administration according to acute or chronic protocols is possible, with respect to patient’s condition and diagnosis. Rather frequent side effects are observed, neurological, mental, and last but not least, cardiovascular. The last mentioned comprise mostly QT interval prolongation; occasionally, it is accompanied by arrhythmias, such as Torsade de Pointes, eventually leading to ventricular fibrillation and even sudden cardiac death (Remijnse et al. 2002).
Haloperidol is a non-specific drug with affinity to numerous receptors, including dopamine D2 receptors and serotonin 5HT2 receptors. It is also known as a prototypic ligand of sigma receptors. These receptors were first discovered in the central nervous system (Martin et al. 1976). Later, their presence was proven in various tissues (Su and Junien 1994), including the central nervous system (Alonso et al. 2000) and numerous peripheral tissues, such as immune system (Wolfe and De Souza 1993), digestive tract, liver and kidney (Hellewell et al. 1994), endocrine and reproductive systems (Wolfe and De Souza 1993; Kushner and Zukin 1994), and also the heart muscle (Dumont and Lemaire 1991; Novakova et al. 1995). Three subtypes of sigma receptor are distinguished. In the heart muscle, sigma 1 and sigma 2 receptors are expressed (Ela et al. 1994; Novakova et al. 1995). Their stimulation leads to a release of Ca2+ from the intracellular store into the cytosol (Novakova et al. 1995, 1998; Maurice and Su 2009). In 2001, Hayashi and Su (2001) reported the existence of the trimeric structure consisting of sigma 1 receptor, inositol 1,4,5-trisphosphate (IP3) receptor and ankyrin isomer 220 on the membrane of endoplasmic reticulum in cultured rodent cells. Dissociation of this triplet and translocation of either a dimer sigma 1 receptor/ankyrin (in case of sigma receptor agonist binding) or sigma 1 receptor alone (after antagonist binding) has been described in the same model (Hayashi and Su 2003). Due to the effect on calcium current in cardiomyocytes (Tarabova et al. 2009) and an impact on the regulation of intracellular calcium store, sigma receptors are suggested to play a role in modulation of cardiac functions. Moreover, activation of sigma 2 receptors results in inhibition of inward rectified potassium channels (Monassier et al. 2007). Haloperidol acts as a potent sigma 1 antagonist and a sigma 2 agonist (Colabufo et al. 2004; Cobos et al. 2007).
Haloperidol is not the only sigma ligand used as an efficient drug in clinical practice. Many other neuropsychiatric drugs such as fluvoxamine and donepezil are sigma 1 agonists, and opipramol and sertraline are sigma 1 antagonists. Bhuiyan and co-workers (2010) suggested that selective serotonin reuptake inhibitors, such as fluvoxamine, have a cardioprotective effect in case of pressure-overload-induced dysfunction of the rat heart by upregulating sigma 1 receptor expression and stimulating sigma 1 receptor-mediated Akt-eNOS signalling. Recently, sigma 1 receptors were reported as a target for cardioprotection (Bhuiyan and Fukunaga 2011). On the other hand, administration of sigma 1 antagonist results in negative cardiovascular effects. A recent study described coronary artery atherosclerosis after sertraline treatment in female premenopausal primates (Shively et al. 2015).
We reported that repeated exposure to haloperidol, a sigma receptor ligand, increased expression of sigma 1 receptor and IP3 receptor type 1 and type 2 in rat atria (Novakova et al. 2010). In ventricles, only expression of the sigma 1 receptor was increased. In another study, silencing of both, the type 1 and type 2 of IP3 receptors resulted in a decrease of sigma 1 receptor’s expression (Novakova et al. 2007). This fact suggests the mutual direct or indirect interaction(s) of abovementioned receptors.
Despite possible side effects, haloperidol is still prescribed in Europe in clinical practice. Therefore, the present work aimed to study the effects of long-term haloperidol treatment on guinea pig hearts and the responsiveness of these hearts to the additional doses of haloperidol. Expression of the sigma 1 receptor and IP3 receptors type 1 and type 2 in guinea pigs repeatedly exposed to haloperidol was also studied.
The study was performed on 30 guinea pigs (unspecified breed, male only, 3 months old). Animals were housed in Laboratory Animal Breeding and Experimental Facility, Faculty of Medicine, Masaryk University, Brno, Czech Republic. All animal experiments were carried out according to the recommendations of the European Community Guide for the Care and Use of Laboratory Animals and according to the experimental protocol approved by the Committee on the Protection of Animals, Faculty of Medicine, Masaryk University and by the Committee of Ministry of Agriculture of the Czech Republic.
Animals were divided into two groups: haloperidol-exposed (group H; 20 animals; average body mass 320.9 ± 48.8 g) and control (group C; 10 animals; average body mass 322.4 ± 54.6 g). Haloperidol (Sigma Aldrich, USA; 2 mg/kg once a day by intraperitoneal injection) or vehiculum (13.3 ml of 2% alcohol solution per 1 kg of actual body mass) was administered for 21 consecutive days. The dose of haloperidol was chosen according to the previous studies (Inoue et al. 2000; Fialova et al. 2009). The proper dose of haloperidol or vehiculum was calculated daily for each animal according to its actual body mass. Weighing and application was done always at the same daytime (around noon). No anaesthetic or analgesic agents were used in this part of experiment. Gentle handling and quiet approach was applied to reduce discomfort of animals during the manipulation.
Isolated heart experimentsTwenty-four hours after the last haloperidol dose, guinea pigs were deeply anaesthetised by isoflurane (2%). The heart was rapidly removed from the thorax, placed in a cold (4°C) Krebs-Henseleit solution and prepared for cannulation. The aorta was cannulated and the heart was perfused according to Langendorff at constant perfusion pressure (80 mmHg) with Krebs-Henseleit solution (NaCl, 118 mM; NaHCO3, 24 mM; KCl, 4,2 mM; KH2PO4, 1.2 mM; MgCl2, 1.2 mM; CaCl2, 1.25 mM; glucose, 5.5 mM) aerated with 95% O2 and 5% CO2. The temperature was maintained constant (37°C) throughout the experiment. Experimental protocol consisted of four 20-min lasting consecutive phases: stabilization, the first haloperidol exposure (H1), washout with Krebs-Henseleit solution, and the second haloperidol exposure (H2). Haloperidol was administered diluted in Krebs-Henseleit solution at the concentration of 10 nmol/l. This concentration was chosen in order to approximate clinical situation; free plasma levels of haloperidol in patients are usually between 10-200 nmol/l (Flanagan 1998). Throughout the entire experiment, electrogram as three orthogonal leads was continually recorded by touch-free method (Fialova et al. 2009).
The recorded signals were subsequently analysed: ten successive RR intervals were averaged at the end of the 5th, 10th, 15th, 20th, 25th, and 30th minute of each phase and the heart rate was calculated. In the same way, QT interval was measured. QT interval correction according to Bazzet formula (QTc) was performed. Manual detection of arrhythmias was done: each arrhythmia was classified and the time of its appearance was noted.
Expression of sigma 1 and IP3 receptorsTwenty-four hours after the last haloperidol dose, guinea pigs were deeply anaesthetised by isoflurane (2%). The heart was rapidly removed from the thorax, placed in a cold (4°C) Krebs-Henseleit solution and washed from the blood. Small samples of myocardium from free wall of right and left atria and both ventricles were obtained and placed into RNA later (Roche, Switzerland) or formaldehyde (Sigma, USA) for stabilization until further processing. The expression of IP3 and sigma 1 receptors in heart tissues were proved using RT-PCR and immunohistochemistry.
RNA isolation, cDNA preparationFor RNA isolation High pure RNA Tissue isolation kit (Roche, Switzerland) was used. Tissue samples or cell samples were homogenized in 200 µL of lysis buffer. Subsequently, lysates were transferred into the column and RNA isolation was carried out according to manufacturer’s instructions. Isolated RNA was used for cDNA synthesis. Total RNA (600 ng) was transcribed using Transcriptor first strand cDNA synthesis kit (Roche, Switzerland) according to manufacturer’s instructions. Prepared cDNA (20 µL) was diluted with RNase-free water to 100 µL and directly analyzed by real-time polymerase chain reaction.
Real-time reverse-transcription polymerase chain reactionReal-time reverse-transcription polymerase chain reaction (PCR) was performed in triplicates using the TaqMan gene expression assay system with the 7500 real-time PCR system (Applied Biosystems, CA, USA). The amplified DNA was analyzed by the comparative Ct method using β-actin as an endogenous control. The primers and probe set for β-actin (Assay ID: Cp03755211_g1; Applied Biosystems), sigma 1 receptor (Assay ID: Cp03755850_m1, Applied Biosystems), IP3R1 (CpLOC100713023, Applied Biosystems), and IP3R2 (CpLOC100717278, Applied Biosystems) were used. Real-time PCR was performed under the following amplification conditions: total volume of 20 µL, initial denaturation 95°C/10 min, then 45 cycles 95°C/15 sec, 60°C/1 min.
Immunohistochemical stainingImmunostaining of sigma 1 and type 1 IP3 receptors was performed on 3-4 µm-thick formaldehyde fixed, paraffin-embedded tissue sections, which were deparrafinized using xylen, rehydrated by decreasing ethanol concentration washes, and then processed for antigen retrieval. Antigen retrieval was performed by heating the slides in 10 mM citrate buffer (pH 6) at 95-99°C for 40 min. Samples were then pre-incubated in PBS (137 mmol/l NaCl, 2.7 mmol/l KCl, 1 mmol/l KH2PO4, and 6.5 mmol/l Na2HPO4; pH 7.4) with 0.1% Triton X100 (Sigma, USA) and 10% normal goat serum (Sigma, USA) for 1 h at room temperature. After washing in PBS the samples were incubated with primary antibodies at 4°C overnight. Anti-Inositol 1,4,5-Triphosphate Receptor (Type I) (Sigma, USA) and Anti-OPRS1 ab 53852 (sigma-receptor; Abcam) both produced in rabbit and diluted at 1% bovine serum albumin (BSA) at PBS were used as primary antibodies. After washing in PBS, secondary antibody (goat anti rabbit IgG conjugated to Alexa Fluor 488; Invitrogen) was added and samples were incubated for 1 hour at room temperature in the dark. Slides were then washed as described above and stained in DAPI (1 μg/ml) for 15 min, rinsed again in PBS, then mounted with antifade Vectashield (Vector Laboratories, USA). Negative controls were obtained by overnight incubation in 1% BSA instead of primary antibody. Fluorescent images were taken using a confocal microscope Zeiss LSM 700 (Zeiss, Germany) using 488 nm laser.
Statistical analysisMean relative expressions of the genes in group C were set as a reference values. Values of expression in the group H were expressed in relation to the reference values. Twofold or higher/lower value in group H than in group C was accepted as statistically significant. Results of the heart rate and QTc were expressed as means ± SEM. The heart rate in H1, washout and H2 phases were expressed in percent of the heart rate value in the 30th minute of the stabilisation period of the respective experiment. Standard parametric and nonparametric descriptive statistics (mean, median, range) were done for each phase. The differences between consecutive phases of the experiment were analysed by Student’s paired t-test. Differences between individual phases of each experiment and its stabilisation phase were analysed by one-sample t-test. Differences between two experimental groups were analysed by Student’s unpaired t-test. P values were calculated, two-side p < 0.05 was considered significant. Analyses were performed using GraphPad Prism® 5 (version 5.01, GraphPad Software, Inc., San Diego, CA).
Animals were randomly divided into two groups: haloperidol-exposed (group H; 20 animals; average body mass 320.9 ± 48.8 g) and controls (group C; 10 animals; average body mass 322.4 ± 54.6 g). At the end of stabilization, no significant difference in the heart rate of isolated hearts was observed (group H 197.70 ± 13.19 beats/min, and group C 195.00 ± 29.00 beats/min, respectively).
Significant decrease in relative heart rate was observed in group H from the beginning of phase H1 to the end of experiment; heart rate in group C was basically stable (p < 0.05; Fig. 1). In group H, the mean values of heart rate showed decreasing tendency during the H1 phase and this decrease was statistically significant (p < 0.05, compared to the end of stabilization) from the 5th minute of H1 phase. At the end of H1, the mean heart rate in group H was 91.8% ± 1.1% and its values did not further change. Also, mean value of QTc in group H was significantly higher than in group C (362 ± 4 ms in group H vs. 329 ± 15 ms in group C, p < 0.05). This difference between the groups was preserved and mostly significant (p < 0.05) during the experiment (Fig. 2).
Mean QTc showed increasing tendency during the H1 phase, being statistically significant in group H (p < 0.05) from the 15th minute (compared to the end of stabilization). At the end of H1 phase, mean QTc in group H was 384 ± 6 ms. During washout, insignificant decrease was observed. In H2 phase, mean QTc values increased again, but this change was already insignificant. In group C, the same, but insignificant trends were observed.
Incidence of arrhythmias was low in both groups. Sporadic supraventricular premature beats were the only detected type of arrhythmia. No ventricular electrical disturbance was observed.
Expression of sigma 1 receptor in the heart atria was significantly increased in group H (in left atrium 5.67-times and in right atrium 2.84-times, respectively; Fig. 3a, b). Expression of the IP3 receptors type 1 and 2 was also elevated (in left atrium type 1 IP3 receptor 3.01-times and type 2 IP3 receptor 6.81-times, and in right atrium IP3 receptor 1 3.23-times and IP3 receptor 2 4.08-times, respectively; Fig. 3a, b). In ventricles, no significant changes were detected (Fig. 3c, d).
Immunohistochemistry demonstrated both sigma 1 and type 1 IP3 receptors in the cytoplasm of cells of atria and ventricles in both control and haloperidol-exposed groups. Compared to the control, slightly increased signal for both receptors was observed in the cytoplasm of these cells in the haloperidol-exposed animals (Fig. 4c, d).

Changes of relative heart rate in isolated hearts.
Changes of the relative heart rate in isolated hearts of haloperidol-exposed guinea pigs (squares; n = 10) and controls (circles; n = 3). Haloperidol (10 nM) was administered in two phases of experiment (H1, H2), first exposure was followed by washout period. Significant decrease in relative heart rate in group H caused by the first haloperidol administration as compared to controls was observed; this effect was not washable. Results are expressed as mean ± SEM. Statistical significance: *p < 0.05 compared to the 30th minute of stabilisation (one-sample t-test); op < 0.05 compared to group C (Student’s unpaired t-test).

Changes of QTc in isolated hearts.
Changes of QTc in isolated hearts of haloperidol-exposed guinea pigs (squares; n = 10) and controls (circles; n = 3). Haloperidol (10 nM) was administered in two phases of experiment (H1, H2), first exposure was followed by washout period. QTc was significantly prolonged in group H as compared to controls. Results are expressed as mean ± SEM. Statistical significance: *p < 0.05 compared to the 30th minute of stabilisation (one-sample t-test); op < 0.05 compared to group C (Student’s unpaired t-test).

Changes in relative gene expression.
Changes in relative gene expressions of the sigma 1 receptor, IP3 receptor type 1 and type 2 in guinea pig hearts after repeated haloperidol exposure. The column graphs present the mean relative mRNA levels of sigma 1 receptor (SigmaR), IP3 receptor type 1 (InsP3R1) and type 2 (InsP3R2) in left atrium (LA; a), right atrium (RA; b), left ventricle (LV; c), and right ventricle (RV; d) in guinea pig hearts. Haloperidol significantly increased the gene expression of sigma 1 receptor, IP3R1 and IP3R2 in both atria (a, b), but not in ventricles (c, d). Each column is displayed as mean ± SEM and represents an average of 3-10 heart samples. Mean relative mRNA levels in the controls (group C; hatched columns) were set as a reference values. Mean relative mRNA levels in haloperidol-exposed guinea pigs (group H; black columns) were expressed in relation to the reference values. Statistical significance: op < 0.05 in group H as compared to group C.
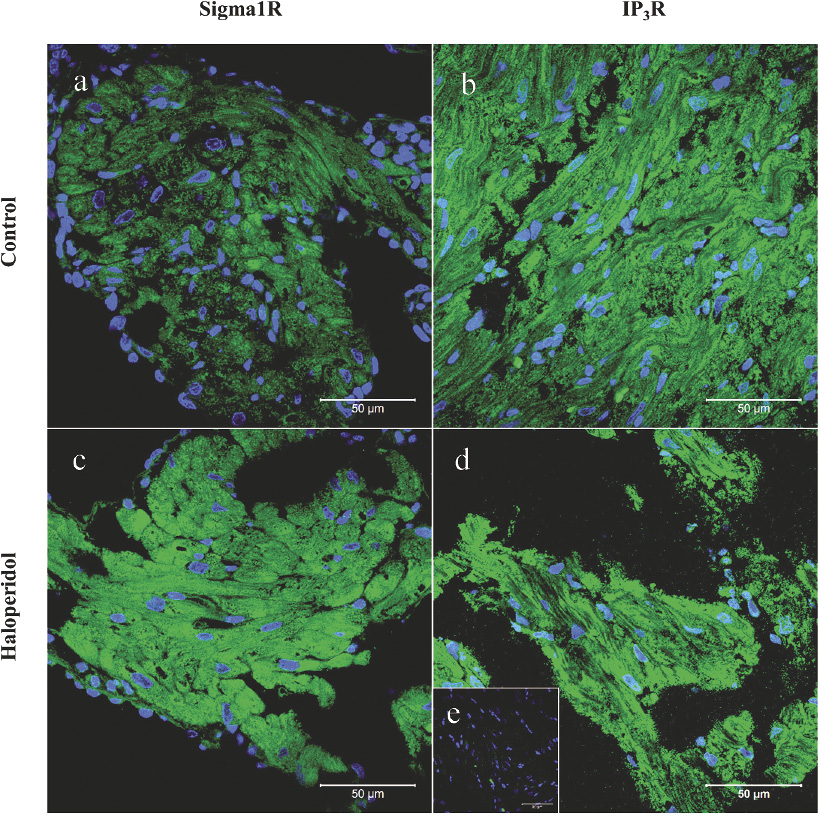
Immunohistochemical staining of the sigma 1 receptors and the IP3 receptors type 1.
Immunohistochemical staining of the sigma 1 receptors and the IP3 receptors type 1 in the left cardiac atrium in controls and haloperidol-exposed guinea pigs. (a) Sigma 1 receptors stained in controls, (b) IP3R1 stained in controls, (c) sigma 1 receptors stained in haloperidol-exposed animals, (d) IP3R1 stained in haloperidol-exposed animals. In haloperidol-exposed guinea pigs, slightly increased signal for both receptors (c, d) was visible. Nuclei were stained by DAPI. Inset (e) shows the negative control, where primary antibody was omitted. Scale bar represents 50 μm.
Although haloperidol administration often resulted in various cardiovascular side effects, in many countries it is still prescribed as an efficient antipsychotic agent. Clinical aspects of acute treatment with haloperidol and its impact on cardiovascular system, primarily QT interval prolongation and associated arrhythmias, were studied repeatedly (Metzger and Friedman 1993; Hennessy et al. 2002; Beach et al. 2013). Effects of the acute and/or chronic exposure to haloperidol have been also studied on various biomodels and species, including rat (Fialova et al. 2009), guinea pig (Testai et al. 2007; Fialova et al. 2009), and rabbit (Dhein et al. 2008).
In this study, effects of chronic exposure to haloperidol were studied in guinea pig hearts. Guinea pig represents a suitable model for studying drug effects on electrocardiogram, since guinea pig cardiac cells exhibit specific ion channels quite comparable to those of humans (Busch et al. 1994). Furthermore, outcomes of our previous studies indicate that long-term exposure to haloperidol affects electrogram parameters of isolated heart in rat and guinea pig and also expression of sigma receptors in the rat heart (Fialova et al. 2009; Novakova et al. 2010).
In this work it was clearly shown that three weeks exposure of guinea pigs to haloperidol resulted in significant decrease in the relative mean heart rate after the additional haloperidol administration to isolated heart, although basal HR of these hearts did not differ. However, further washout of this bolus and another bolus did not cause any consecutive change in this parameter. This pattern resembles changes in the relative mean heart rate of isolated rat hearts of animals exposed to haloperidol for three weeks (Fialova et al. 2009; Novakova et al. 2010).
QT interval reflects the duration of the ventricular action potential and depends on ventricular repolarization. Ventricular repolarization is ensured mainly by various outward potassium currents. Two main delayed rectifying currents operate to achieve repolarization, a rapid [IKr] and a slow [IKs] current. When these currents are reduced, repolarization is prolonged, the ventricular action potentials broaden and the duration of the QT interval increases. Interference with the IKr current is the most common mechanism of QT prolongation (reviewed by Witchel and Hancox 2000). In humans, IKr is conducted by human ether-a-go-go related gene (hERG) potassium channels (Sanguinetti et al. 1995). Heterologous hERG was blocked by haloperidol when expressed in Xenopus oocytes (Suessbrich et al. 1997). Monassier et al. (2007) showed that haloperidol blocks human recombinant hERG potassium channels in COS-7 monkey kidney cells via stimulation of sigma 2 receptors. On the other hand, haloperidol has affinity for dopamine D2 receptors, which are located in guinea pig heart (Gomez et al. 2002). Dopamine D2 agonists may increase QT interval duration (Gomez et al. 2002). However, haloperidol acts as a dopamine D2 antagonist and the dopaminergic effect on the heart rate and QT interval duration is disputable. As far as we know, the direct effect of haloperidol on the heart activity mediated via dopaminergic receptors has not been studied yet. QT interval changes are related to the appearance of arrhythmias; therefore, monitoring of the QT interval is important in the identification of warning signs that precede serious rhythm disturbances, such as Torsade de Pointes and eventually sudden cardiac death.
In this work, acute exposure to haloperidol increased QTc duration in guinea pig isolated hearts from both groups, although in group C this increase was insignificant. Haloperidol concentration of 10 nmol/l used in our experiments on isolated hearts is close to IC50 for hERG blocking (Redfern et al. 2003; Katchman et al. 2006). Therefore, one of the mechanisms of haloperidol QT prolongation in guinea pigs might be connected with decreasing activity of IKr.
Our previous work (Novakova et al. 2010) showed that the rate-corrected QT interval in isolated hearts from haloperidol-exposed rats was shorter than in control animals. Fialova et al. (2009) described a wide range of arrhythmias in isolated hearts of control rats, including Torsade de Pointes and sustained ventricular fibrillation. In isolated hearts of haloperidol-exposed rats, an absence of haloperidol-induced arrhythmias after additional bolus of haloperidol was described (Fialova et al. 2009). Such inter-species differences may be attributed to different equipment of ventricular cardiac cells with specific ion channels. Guinea pig ventricular cardiomyocytes do not develop transient outward potassium current, whereas rat ventricular myocytes do. Furthermore, delayed rectifier potassium current of relatively high amplitude found in guinea pig cardiac cells, but is negligible or even absent in rat (Varró et al. 1993).
Except for direct effects on potassium channels, there is another explanation how haloperidol might affect the action potential duration and consequently QT interval duration in ventricular cardiac cells. Haloperidol is a prototypic ligand of sigma receptors. Concentration of haloperidol used in isolated heart experiments is close to Kd value of the haloperidol binding on cardiac sigma receptors (Novakova et al. 1995). In the heart muscle, both sigma 1 and sigma 2 receptors are expressed (Ela et al. 1994; Novakova et al. 1995). Modulation of sigma 1 receptors by haloperidol might at least partially explain changes in electrophysiological parameters that were detected in isolated guinea pig hearts in this study since it has been reported in various models that sigma receptors modulate potassium channels behaviour (e.g. McKay and Kaczmarek 2002). In order to elucidate the putative role of sigma receptors in our experimental setup, expression of the sigma 1 receptors was determined. Moreover, expression of IP3 receptors type 1 and 2, which are coupled with sigma 1 receptors (Novakova et al. 1998), was studied. The study did not focus on IP3 receptors type 3 since there is no evidence of the presence of IP3 receptor type 3 in guinea pig heart. According to available data IP3 receptor type 3 is expressed in ferret, rat, human and mouse cardiac cells, including conductive system (reviewed in Kockskamper et al. 2008). Moreover, type 3 IP3 receptor has a lesser impact on regulation of heart function than the other two subtypes in all mentioned species (Kockskamper et al. 2008). An increase of sigma 1 receptor’s expression in the heart atria of haloperidol-exposed animals, compared to control ones was observed. On contrary to the rat hearts (Novakova et al. 2010), in guinea pig ventricles no changes of sigma 1 receptor expression were observed. This difference might account for different species. Moreover, daily manipulation of animals during chronic treatment represents a moderate level of stress for animals. Mild stress modulates expression of cardiac sigma receptors (Novakova et al. 2007) and this reaction may differ in rat and guinea pig. Hand by hand with sigma 1 receptor overexpression, expression of the IP3 receptors type 1 and 2 were increased in atria only. The same effect of long-lasting haloperidol administration on IP3 receptor’s expressions was reported previously in rat hearts (Novakova et al. 2010).
Immunohistochemical staining detected both sigma 1 and IP3 type 1 receptors in the same compartment of cardiomyocytes, namely in the cytoplasm. Hayashi and Su (2001, 2003) described functional coupling of sigma 1 receptor with IP3 receptor and ankyrin 220 in a rodent cell line, NG-108. However, in our study, co-localization of sigma 1 and IP3 type 1 receptors was not studied. Previous studies revealed that stimulation of sigma receptors lead to a release of Ca2+ from the intracellular store into the cytosol (Novakova et al. 1995, 1998; Maurice and Su 2009). The direct effect of haloperidol on calcium current in isolated rat cardiomyocytes was also described (Tarabova et al. 2009). Therefore we have proposed that haloperidol treatment affects cytosolic calcium availability in the cardiac cells and as a consequence changes arrhythmogenic threshold.
Question arises about the functional consequences of altered expression of sigma 1 and IP3 receptors in the heart. Numerous local actions, where the sigma 1 receptor plays a key role, were reported, but its general function remains unclear. IP3 receptors type 1 and 2 are known as the intracellular calcium channels participating in calcium release from the endoplasmic reticulum (Lencesova and Krizanova 2012). Novakova and co-workers (2010) found that prolonged exposure to haloperidol significantly increased mRNA levels of sigma 1 receptors in both rat atria and ventricles. Sigma 1 receptor mRNA was increased also in isolated cardiomyocytes. Haloperidol affected the expression of IP3 receptors type 1 and 2 in cardiac atria, but not in cardiac ventricles. In the heart, higher levels of IP3 receptors type 1 in cardiac ganglia and IP3 receptors type 2 in cardiomyocytes were described (Krizanova et al. 2008). Haloperidol causes changes in both electrical activity and mechanical performance of the heart. Decrease in heart rate, increase in atrioventricular effective refractory period, the atrioventricular conduction time, and increase in left ventricular developed pressure in isolated rat hearts were reported (Medlin et al. 1996). Approving results were shown in canine models (Sugiyama et al. 2001; Rasty et al. 2004). Similarly, significantly decreased heart rate and diastolic blood pressure were reported in schizophrenics treated with haloperidol (Agelink et al. 1998).
In summary, in guinea pig heart haloperidol has a significant effect on heart rate and QTc interval duration. This effect might be realized partly through the modulation of potassium currents, partly through the increase in the gene expression of sigma 1 receptors and IP3 receptors, which was proven by RT-PCR and endorsed by immunohistochemical staining. The changes in the expression of these receptors may lead to altered calcium handling in cardiomyocytes and thus contribute to changed sensitivity of cardiac cell to arrhythmias.
However, further experiments are required to elucidate the effect of haloperidol—both in acute and chronic administration—on IKr current, on co-localization of sigma 1 and IP3 receptors, and last but not least on cytosolic calcium availability in the cardiac cells.
The study was performed at Masaryk University as part of the project “Cardiovascular system from the cell to patient’s bed” MUNI/A/1326/2014 with the support of the Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2015. The financial support from the European Regional Development Fund Project FNUSA-ICRC No. CZ.1.05/1.1.00/02.0123 is also highly acknowledged. The authors wish to thank prof. Pavel Braveny for critical reading of the manuscript, prof. Petr Dobsak for fruitful discussion above the manuscript, and Mrs. Branislava Vyoralova for excellent technical assistance during the experiments.
The authors declare no conflict of interest.