2015 Volume 236 Issue 4 Pages 297-304
2015 Volume 236 Issue 4 Pages 297-304
Small cell lung cancer (SCLC) is an aggressive disease characterized by rapid growth and metastases. It has been recognized that the inflammation of the microenvironment plays a critical role in the development of malignancies. However, little is known about the role of multiple inflammatory and hematological markers in the prognosis of SCLC. The aim of this study was to determine the clinical significance of pre-treatment inflammation-based scores and characteristics as prognostic indicators for the survival of SCLC patients. A retrospective analysis of 919 SCLC cases was performed. Patients’ characteristics and hematologic tests data at initial diagnosis were collected. The univariate analysis of all SCLC patients indicated that favorable prognostic factors were age ˂ 70 years, non-smokers, good performance status, limited disease and response to treatment. Moreover, univariate analysis of inflammation-based scores and other blood parameters showed that neutrophil-lymphocyte ratio ≥ 5, platelet-lymphocyte ratio ≥ 250, systemic immune-inflammation index (SII) ≥ 1,600 × 109/L, prognostic nutritional index (albumin + 5 × lymphocyte) < 45, and elevated serum lactate dehydrogenase (LDH) predicted poor prognosis in SCLC patients. SII represents the score that is calculated as follows: platelet count × neutrophil count/lymphocyte count. In the multivariate analysis, SII, together with serum LDH, stage and response to therapy, were associated with overall survival (OS). This study demonstrated that the combination of platelet count and neutrophil-lymphocyte ratio could help to predict poor prognosis in SCLC. Our findings will facilitate the understanding of survival differences in SCLC patients in clinical practice.
Small cell lung cancer (SCLC) is a malignant neuroendocrine tumor consisting of small cells that originate in the lung (Zakowski 2003). SCLC accounts for approximately 15-20% of all lung cancers according to histological types and is the most aggressive form of lung cancer subtypes, characterised by rapid growth and early development of widespread metastases (Brigham et al. 1978). Although the incidence of SCLC has decreased markedly in the U.S. due to the advocation of smoking cessation, unfortunately for the 80% with limited-stage cancer and for all patients with extensive-stage cancer, the outcomes remain poor despite temporary responses to initial chemotherapy and radiotherapy (Dowell 2010). According to National Cancer Institute (NCI) data, the median survival time for patients with limited-stage SCLC is less than 24 months, and most of the patients with extensive-stage SCLC, even with the treatments currently available, survive no more than 12 months (National Cancer Institute at National Institutes of Health 2015).
Outcomes for patients with cancer are determined by tumor characteristics, patient-related factors and laboratory parameters. Poor performance status (PS), extensive-stage disease, male gender and old age are poor prognostic indicators for patients with SCLC (Albain et al. 1990, 1991). Also, several pre-treatment blood parameters are also prognostic factors for SCLC. Of these, elevated lactate dehydrogenase (LDH) was confirmed to be a very poor prognostic factor for SCLC (Albain et al. 1990, 1991; Bremnes et al. 2003). Most current data have been from studies in non-Asian SCLC, and little is known about the prognostic factors in Chinese patients with SCLC.
It has increasingly been recognized that the inflammation of the microenvironment plays a critical role in the development and progression of malignancies by influencing the proliferation and survival of tumor cells, promoting angiogenesis and metastasis, and reducing responses to anti-cancer agents (Mantovani et al. 2008). Recently, several biomarkers and hematological indices representative of systemic inflammatory responses, including the neutrophil-lymphocyte ratio (NLR), the platelet-lymphocyte ratio (PLR), a combination of platelet count and neutrophil-lymphocyte ratio as systemic immune-inflammation index (SII), the C-reactive protein (CRP) and a combination of albumin and lymphocyte count as the prognostic nutritional index (PNI) that have been associated with poor prognosis in various cancers (Proctor et al. 2011; Kinoshita et al. 2012; Lee et al. 2013; Hu et al. 2014; Paramanathan et al. 2014). Other hematological indices, including platelet count, hemoglobin (HGB), and the mean corpuscular volume (MCV) have been reported as potential predictors of survival, and have responded to chemotherapeutic agents in a limited number of studies (Dellapasqua et al. 2012; Qu et al. 2014). However, little is known about the role of multiple inflammatory and hematological markers in the prognosis of SCLC.
The aim of this study was to determine the clinical significance of pre-treatment inflammation-based scores and characteristics as prognostic indicators for the survival of Chinese patients with SCLC.
The medical records of 919 patients who had been diagnosed with SCLC from January 2000 to December 2012 at Harbin Medical University Cancer Hospital were retrospectively reviewed. All patients diagnosed with SCLC were confirmed by histologic pathology. The study was approved by the medical ethics committee of Harbin Medical University Cancer Hospital. The need for patient informed consent was waived because of the retrospective nature of the study.
Data collected for the analysis included: gender (male vs. female), age (< 70 years vs. ≥ 70 years), smoking history (non-smokers vs. current smokers), PS (0-1 vs. ≥ 2), body mass index (BMI: ≥ 18.5 vs. < 18.5), disease stage (limited vs. extensive), date of diagnosis and death, treatment regimens (no systemic treatment, surgery, radiotherapy, chemotherapy) and response to initial treatment (complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD)). Current smokers included patients with a smoking index of ˃ 400 pack per year throughout their lives and having quit smoking for ˂ 5 years; the remaining patients were defined as non-smokers. Staging of the patients with SCLC was defined according to the Veterans Administration Lung Study Group (VALG) classification. PS was assessed using the Eastern Cooperative Oncology Group (ECOG) scores criteria. The response to therapy was assessed according to the RECIST 1.1 criteria. Overall survival (OS) was calculated from the date of diagnosis to the time of death (due to any cause), or until December 2014 for patients who remained alive.
Data were collected from hematologic tests carried out at initial diagnosis and before any anti-cancer treatments. These data included neutrophil-, lymphocyte- and platelet counts, HGB, MCV, mean platelet volume (MPV), serum LDH and blood albumin.
An elevated platelet count was taken to be higher than 300 × 109/L. Low HGB was defined as < normal levels (120 g/L in males and 110 g/L in females). MCV levels ˃ 85 fl were considered as high, and low MPV was defined as < 8.5 fl. Based on serum LDH, patients were divided into normal LDH (≤ upper limit of normal (ULN)), high LDH (> ULN and ≤ 2 × ULN) and higher LDH groups (> 2 × ULN) (the ULN was 190 U/L before January 2006 and the ULN was 250 U/L after January 2006).
The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count, and the PLR was defined as the platelet count multiplied by the absolute lymphocyte count. NLR ≥ 5 and PLR ≥ 250 were considered as elevated levels, as described previously (Proctor et al. 2011). The SII was defined as follows: SII = platelet count × neutrophil count/lymphocyte count (Hu et al. 2014), and an SII ≥ 1,600 × 109/L was considered as the cut-off value. The PNI was calculated as albumin + 5 × lymphocyte and PNI < 45 was considered as abnormal, as described previously (Kinoshita et al. 2012).
Statistical analysisAll statistical analyses were carried out using IBM SPSS statistical software (version 19.0). Numeric variables are presented as means ± SD. Univariate survival analysis was performed using the Kaplan-Meier method with the log-rank test. To determine the independent prognostic factors, multivariate survival analysis and a calculation of hazard ratios (HR) were performed using a Cox regression model. Factors that were significant on the univariate analysis were included. The categorical variables were compared by Spearman tests. All P-values were two-sided, and P < 0.05 was considered to indicate statistical significance.
The characteristics of the 919 patients analyzed are summarized in Table 1. The median survival time of all patients with SCLC was 10.4 months (range 0.2-68.0 months). The 1-, 2- and 3-year survival rates were 42.3, 12.3 and 2.9%, respectively. The median age of patients was 56 years (range 16-84 years) and 70% of the patients were male. A total of 82.7% of patients had undergone systemic treatments, and the responses were evaluated. Of these, 83.2% had shown a good response (CR+PR) to initial treatment, including chemotherapy and/or radiotherapy.

Patient distribution and characteristics associated with OS.
*There are significant differences statistically in comparison between any two groups and all of P values are < 0.001.
The univariate analysis of all the patients indicated that favorable prognostic factors were age ˂ 70 years, non-smokers, and good PS (Table 1). The median OS was similar between non-smokers and smokers. However, a difference was observed after 12 months, and 2-year survival rates were 15.9 and 9.3% in non-smokers and smokers, respectively (P = 0.004). Patients with limited-stage SCLC had longer OS than those with extensive-stage SCLC. Although female patients had longer survival time than male patients, the difference was not significant. The prognosis value of BMI was analyzed, and it showed no significant correlation with the prognosis of SCLC in this study (P = 0.221).
Of the total number of patients, 17.3% did not receive systemic anti-cancer treatments and their survival time was 4.9 months. Patients showing no response (SD+PD) to initial therapies had longer survival time compared to those not receiving any treatment; their OS was shorter than patients who did respond (CR+PR) to treatments (P < 0.001) (Table 1). In subset analysis according to stage, the therapeutic response remained a strong prognostic indicator for survival in limited-stage and extensive-stage SCLC. The limited-stage SCLC patients that responded to treatment showed a median OS of 14 months (range 12.8-15.2 months), whereas the extensive-stage patients not in receipt of systemic therapies had a median OS of only 4.3 months (range 3.7-4.9 months).
Impact of inflammation-based scores and other blood parameters on OSThe results of the univariate analyses of the pre-treatment blood variables are listed in Table 2. The median values for platelet count, HGB, MCV and MPV were 244 × 109/L (range 38-669), 136 g/L (range 52-209), 90.8 fl (range 32.3-111.0) and 7.2 fl (range 1.7-18.9), respectively. No significant correlations were observed between platelet count, HGB, MCV, MPV and the prognosis of SCLC in this study. Kaplan-Meier survival analysis of the stratified limited-stage and extensive-stage SCLC groups did not demonstrate any significant relationships with OS.
A total of 20.6% (189/919) of the patients had an NLR ≥ 5, which was associated with gender (male vs. female, 24.1 vs. 12.7%, P < 0.001), PS (0-1 vs. ≥ 2, 20.1 vs. 28.9%, P = 0.004) and baseline platelet count (normal vs. elevated, 18.1 vs. 28.5%, P = 0.001). Additionally, patients with a NLR < 5 had higher response rates (84.4%) compared to those with a NLR ≥ 5 (76.9%) (P = 0.046). PLR ≥ 250 was observed in 183 patients (19.9%) and was associated with a worse PS (30.2 vs. 17.8%, P < 0.001). SII ≥ 1,600 × 109/L was observed in 127 patients (13.8%) and correlated with gender (male vs. female, 14.6 vs. 12.0%, P = 0.023) and HGB levels (low vs. normal, 40.0 vs. 12.2%, P < 0.001). A total of 162 patients (17.6%) had PNI < 45, and this was more frequent in patients with extensive-stage SCLC (21.3 vs. 15.2%, P = 0.019). Univariate analyses showed that NLR ≥ 5, PLR ≥ 250, SII ≥ 1,600 × 109/L and PNI < 45 predicted poor prognosis in SCLC patients (Fig. 1). Stratified with the limited-stage SCLC and extensive-stage SCLC groups, a PLR ≥ 250 and SII ≥ 1,600 × 109/L predicted worse OS for limited-stage SCLC (P = 0.014 and P = 0.001, respectively) and PNI < 45 was a poor prognostic factor for extensive-stage SCLC (P = 0.009).
Serum LDH ranged from 51.5-1,921.7 U/L with a median value of 181.0 U/L. Elevated serum LDH levels were observed in 343 patients (37.3%), of which 55 (6.0%) had higher LDH values (> 2 × ULN). Analysis of the relationship between LDH and baseline characteristics revealed more patients with elevated LDH in extensive-stage SCLC compared with limited-stage SCLC (76.2 vs. 11.6%, P < 0.001). Elevated LDH was associated with worse PS (54.1 vs. 33.8%, P < 0.001). The correlation between LDH and responses to treatments in 760 patients receiving systemic therapies was analyzed further. The result revealed more therapy-resistant patients in the elevated LDH group compared with the normal LDH group (21.1 vs. 14.6%, P = 0.016). Kaplan-Meier survival analysis showed that elevated LDH was a strong prognostic factor for OS in SCLC (P < 0.001) (Fig. 2). Stratified analysis by disease stage showed that elevated LDH also predicted a worse prognosis both in limited-stage SCLC (P < 0.001) and extensive-stage SCLC (P < 0.001).

Association between hematologic tests data and OS.
*There are significant differences statistically in comparison between any two groups and all of P values are < 0.001.

Kaplan Meier survival curves for OS according to inflammation-based scores in 919 SCLC patients.
A. 189 patients with NLR ≥ 5 had shorter median OS than 730 patients with NLR < 5 (8.8 vs. 10.9 months, P = 0.007). B. 183 patients with PLR ≥ 250 had shorter median OS than 736 patients with PLR < 250 (8.9 vs. 10.9 months, P = 0.004). C. 127 patients with SII ≥ 1,600 × 109/L had worse prognosis than 792 patients with SII < 1,600 × 109/L (8.6 vs. 10.9 months, P < 0.001). D. 162 patients with PNI < 45 had worse prognosis than 757 patients with PNI ≥ 45 (8.7 vs. 11.0 months, P < 0.001).

Kaplan Meier survival curve for OS according to serum LDH levels in 919 SCLC patients.
The patients with elevated serum LDH (55 patients with LDH values of > 2 × ULN and 288 patients with one of > ULN and ≤ 2 × ULN) had shorter median survival time than 576 patients with normal LDH (6.2 vs. 9.1 vs. 11.8 months, P < 0.001).
To assess the independent prognostic factors, multivariate Cox proportional hazard analysis was performed on the data of all 919 patients. In the multivariate analysis, serum LDH and SII, together with stage and response to therapy, were identified to be associated with OS, after adjustment for other characteristics (Table 3).
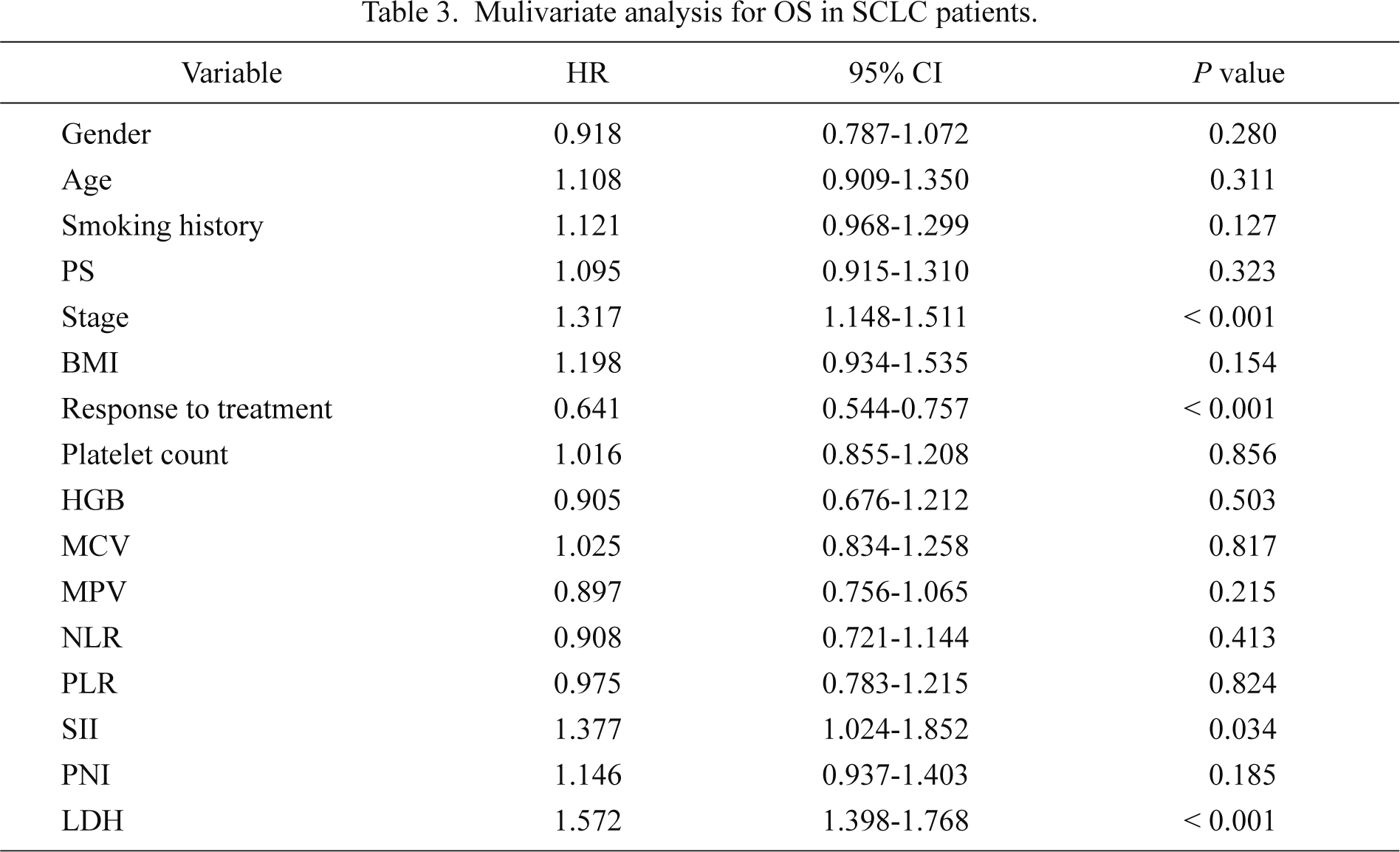
Mulivariate analysis for OS in SCLC patients.
Over recent decades, numerous biological targets such as targeted agents and new cytotoxic drugs for NSCLC have been identified for anticancer therapies and significant progress has been made in the treatment of malignancies (Sandler et al. 2006; Socinski et al. 2006; Mok et al. 2009). However, little progress has been made in the treatment of SCLC and no targeted agents have prolonged the OS for SCLC patients. SCLC represents a significant healthcare problem worldwide because of its highly aggressive nature and propensity for relapse. Good PS, young age, female sex, and single metastatic lesion are favorable prognostic features for patients with SCLC. Previous studies showed that white ethnicity was an independent favorable prognostic factor for SCLC, indicating possible differences between Asian and non-Asian patients (Albain et al. 1990, 1991). The present study also identified good PS, young age, and the limited stage as favorable prognostic factors, consistent with the results of previous studies (Albain et al. 1990, 1991; Bremnes et al. 2003; Foster et al. 2009). Smoking status before diagnosis in the prognosis of SCLC was also evaluated and the results confirmed that non-smokers with SCLC had better prognosis than smokers, and this was consistent with results from Korea (Sun et al. 2015). Smoking status was especially useful as a predictor of long-term survival in this study. The combination of cisplatin plus etoposide (EP) remains the most widely used regimen for therapy-naïve SCLC. Moreover, EP plus thoracic radiotherapy for patients with limited-stage SCLC and a combination chemotherapy for extensive-stage SCLC can produce a response rate of ˃ 70% and prolong the median survival time (Roth et al. 1992; Takada et al. 2002). In our study, 760 patients received systemic therapies, including combination chemotherapy and/or thoracic radiotherapy. The results showed an 83.2% response to initial treatment, and patients with CR+PR had significantly longer survival time than those who were resistant to treatment. Multivariate analysis showed that therapy response was an independent variable, similar to previous reports (Evans et al. 1985; Noda et al. 2002). Therefore, the consistency of roles of patients and therapy characteristics for prognosis with previous studies illustrated the reliability of case data in this study. The impact of BMI on prognosis in SCLC, considering its effects on the prognosis of several cancers (Pai et al. 2012; Han et al. 2013) was evaluated. The results showed a lack of any association between BMI and clinical outcomes in patients with SCLC.
In addition to patient characteristics and treatments, biomarkers represent another useful prognostic factor for cancer. LDH can be released during tissue damage and is involved in tumor initiation and metabolism. Numerous studies have shown that LDH activity is an indicator of metastatic risk and could be an important prognostic factor in a variety of cancers (Weide et al. 2013; Cetin et al. 2014; Philipp et al. 2014; Ulas et al. 2014; Liu et al. 2015). The use of LDH as a prognostic factor in SCLC has also been demonstrated over recent decades (Tas et al. 2001; Bremnes et al. 2003; Hermes et al. 2010). The present study showed that LDH was a strong prognostic factor for survival in limited-stage and extensive-stage SCLC patients, confirming its importance in determining the prognosis of SCLC.
The inflammatory responses of cancer reflect the non-specific responses to tumor hypoxia, tissue injury, and necrosis, indicating a correlation between cancer cells, the immune system, and the inflammatory response. Inflammation promotes tumor proliferation, survival, angiogenesis and metastasis through the production of inflammatory mediators, including cytokines and chemokines, secretion of CRP and induction of neutrophilia (Mantovani et al. 2008). The inflammatory microenvironment of tumors is characterized by the presence of host leucocytes, and tumor-infiltrating lymphocytes may contribute to cancer growth and spread, and immunosuppression in malignancies (Balkwill and Mantovani 2001). In particular, the systemic inflammatory response can be assessed in cancer patients using several scores, including a combination of serum CRP and albumin as the modified Glasgow Prognostic Score (mGPS), NLR, PLR, the combination of CRP and white cell counts in Prognostic Index (PI) and PNI (McMillan 2009; Proctor et al. 2011; Paramanathan et al. 2014). A recent study in China demonstrated that the assessment of mGPS could assist in the identification of patients with poor prognosis for SCLC (Zhou et al. 2015). Regrettably, the detection of CRP is not routinely carried out at the Harbin Medical University Cancer Hospital, and, for this reason, mGPS and PI were not included in this study. The NLR and PLR represent the most common indices, as they are easy to obtain and measure. Previous studies have shown that elevated NLR and PLR at the baseline were associated with worse OS and disease-free interval in resected and advanced cancers (Proctor et al. 2011; Lee et al. 2013; Paramanathan et al. 2014; Pinato et al. 2014). PNI is not commonly used as a score for the measurement of systemic inflammatory responses, and a study of hepatocellular carcinoma (HCC) found that PNI was an independent and externally validated predictor of poor OS in patients with HCC (Pinato et al. 2012). Only two studies have focused on the value of NLR for prognosis in SCLC, with the results indicating that elevated NLR could predict worse OS and PFS (Kang et al. 2014; Wang et al. 2014). There is, therefore, a lack of data from studies with adequate sample sizes and multiple inflammation-based scores. The aim of our study was to increase the value of multiple inflammation-based scores for prognosis in 919 cases of SCLC. Univariate analysis demonstrated that NLR, PLR, and PNI were significantly associated with OS in patients with SCLC. However, multivariate analysis showed they were not independent prognostic factors in SCLC.
The impact of SII on prognosis in SCLC was also assessed. SII represents the score based on the combination of platelet count and neutrophil-lymphocyte ratio, showing systemic inflammatory responses. SII was initially used to indicate the host inflammatory and immune status in resected HCC patients (Hu et al. 2014); the results confirmed that SII was superior to NLR and PLR as a prognostic factor for poor outcomes and that high SII scores were possibly related to higher circulating tumor cell levels. The present study confirmed that SII was superior to NLR, PLR, and PNI as an independent factor for poor prognosis in patients with SCLC. This result could be because the SII score was based on lymphocyte, neutrophil and platelet counts and was more comprehensive in reflecting the balance of host inflammatory and immune status than the other scores. The difference between this study and the HCC study (Hu et al. 2014) was that the cut-off value of the SII used; namely, in this study, it was 1,600 compared to 330 in the HCC study. The reason for the difference could be because SCLC is an aggressive disease, and the majority of patients in this study were at an advanced cancer stage.
In this study, the significance of other hematological indices such as platelet count, HGB, MCV and MVP were assessed, as they had been reported as potential predictors of survival in a limited number of studies (Dellapasqua et al. 2012; Qu et al. 2014). These indices did not appear to be significant prognostic indicators for OS in this study.
However, as this is a retrospective study, there are some limitations. First, the assessment of inflammation-based scores did not include mGPS and PI scores due to lack of CRP values. Second, there was no analysis of PFS due to the data collection methods used. Nonetheless, to our knowledge, this represents the first study to evaluate the impact of relatively comprehensive inflammation-based scores on the prognosis of SCLC in a large population cohort.
In conclusion, the present study demonstrates that SII, a score of the combination of platelet count and neutrophil-to-lymphocyte ratio, could help to predict a poor prognosis in Chinese patients with SCLC. The identification of these factors will facilitate the comparisons of different populations of patients and also contribute to interpreting the relationship between the treatments administered and the differences in survival in various groups in clinical practice. Further prospective studies are needed to evaluate the cut-off values of the SII and to confirm these results.
The authors declare no conflict of interest.