2015 Volume 237 Issue 2 Pages 77-82
2015 Volume 237 Issue 2 Pages 77-82
Interleukin 35 (IL-35) is a newly discovered anti-inflammatory cytokine. Recent studies have indicated that it plays a crucial role in the pathogenesis of autoimmune diseases. In humans, IL-35 is predominantly secreted from regulatory T cells. This study aimed to measure serum IL-35 levels in patients with rheumatoid arthritis (RA) and in control individuals, and analyze its association with disease indicators of RA. One hundred patients with RA were recruited, and 50 volunteers were enrolled as healthy controls. Serum IL-35 levels were measured using an enzyme-linked immunosorbent assay kit. RA patients showed significantly lower serum levels of IL-35 compared with healthy controls (p < 0.001). RA patients suffering from erosive arthritis (n = 31) had lower IL-35 levels than those with non-erosive arthritis (n = 69, p = 0.022). In addition, serum IL-35 level was significantly lower in 22 patients with elevated percentage (> 75%) of neutrophils (p < 0.001). Correlation analysis indicated a significantly negative association between IL-35 and age, rheumatoid factor (RF), or percentage of neutrophils. In contrast, the serum IL-35 levels were not significantly different between patients with anti-cyclic citrullinated peptide (CCP) antibodies (n = 78) and those without anti-CCP antibodies (n = 22). However, among patients without anti-CCP antibodies, the serum IL-35 levels were lower in patients with erosive arthritis (n = 8) than those patients without erosion (n = 14) (p < 0.001), although no significant difference was detected in patients with anti-CCP antibodies. In conclusion, IL-35 plays a protective role in the pathogenesis of RA.
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by inflammatory cell infiltration of the synovium, synovial hyperplasia, angiogenesis, and cartilage and bone erosion (Turk et al. 2014). RA represents a typical T-cell-mediated disease. Cytokines released by T-cells or other joint-infiltrating cells may play an important role in the pathogenesis of RA. The cytokine environment in the peripheral lymphoid tissues and the target organ (the joint) has a strong influence on the outcome of the initial events that trigger autoimmune inflammation (Astry et al. 2011; Burska et al. 2014; Striz et al. 2014). Cytokines are typically categorized as predominantly pro-inflammatory or anti-inflammatory. These two types of cytokines together promote a cytokine environment that may alter immune cell function and other effector responses in arthritis, acting in either a pathogenic, pro-inflammatory or a protective, anti-inflammatory fashion. With respect to RA, studies on cytokines have generally focused on clarifying pathogenesis or developing effective therapeutic targets. The roles of cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-17, and interferon-γ (IFN-γ), in disease progression in RA have been analyzed extensively; in fact, cytokine inhibitors against TNF-α, IL-1, and IL-6 have been developed successfully (Bessis and Boissier 2001; Smolen and Maini 2006). Treatment with these inhibitors may provide a breakthrough in the management of a portion of patients with RA (van den Berg and McInnes 2013; Alghasham and Rasheed 2014).
IL-35 is a newly discovered cytokine belonging to the IL-12 family. IL-35 is a heterodimeric protein consisting of two subunits named IL-12p35 and Epstein-Barr virus-induced gene 3 (EBI3). Among the various types of CD4+ T cells, p35 and EBI3 are expressed predominantly in forkhead box protein 3 (Foxp3)+ regulatory T cells (Treg), and not in the effector CD4+ T cells, while only Treg cells constitutively secrete IL-35 protein as an p35/EBI3 dimer (Devergne et al. 1997; Collison et al. 2007). Because Treg cells play a major role in autoimmune control and protect from inflammation, IL-35 may participate in the inflammatory process of RA development (Gee et al. 2009; Gabay and McInnes 2009; Clavel et al. 2013). Both EBI3 and p35 knockout mice show overt autoimmunity and inflammatory disease, suggesting that the EBI3-p35 heterodimer may be an important immunomodulator (Collison et al. 2007). The role of IL-35 was further validated in two studies that demonstrated that IL-35 treatment could ameliorate the severity of collagen-induced arthritis in mice (Niedbala et al. 2007; Kochetkova et al. 2010). However, in a recently published study, IL-35 was demonstrated to promote chronic inflammation in mice with collagen-induced arthritis, when performing a non-viral gene transfer strategy (Thiolat et al. 2014). Because IL-35-research is a novel field, the presence and functional existence of IL-35 in humans have not been proven and remain controversial. Therefore, the significance of IL-35 in human autoimmunity awaits further detailed characterization.
In this study, we aimed to determine the concentration of IL-35 in the sera of patients with RA, and to analyze possible associations between IL-35 and indicators of RA.
Serum samples were collected from 100 patients with RA (62 women and 38 men; mean age: 55.7 ± 13.9 years) and 50 healthy controls (34 women and 16 men; mean age: 52.5 ± 12.0 years). The blood samples were collected when these patients and control subjects first visited the hospital. RA patients with current infections were excluded, and none of the included patients had any other autoimmune diseases. All the RA patients fulfilled the American College of Rheumatology (ACR) criteria for RA (Arnett et al. 1988). Patient charts were reviewed for demographic information, clinical diagnosis, radiographic information, and other clinical or laboratory data. The characteristics of the RA patients have been described previously (Wang et al. 2014). RA that had developed within less than 2 years was defined as recent onset disease. CRP levels > 20 mg/L were considered as elevated levels. For erythrocyte sedimentation rate (ESR), levels > 20 mm/h for women and > 15 mm/h for men were considered as elevated levels. Highly active disease was defined as a disease activity score (DAS28) > 5.1 (van Gestel et al. 1998). Neutrophil count > 75% was considered as elevated. The demographic data for 100 RA patients are listed in Table 1.
Written informed consent was not obtained because of the nature of the study design, which utilized serum samples taken after routine tests. All subjects recruited in this study were informed of the nature of the project, and verbal informed consent was obtained from each patient. This was recorded by the physician who explained the study procedure. The study protocol and verbal consent document were approved by the Ethics Committee of Xiangya Hospital, Central South University, where the study was performed.

Demographic data and disease indicators of 100 patients with rheumatoid arthritis.
Note that the demographic data and most of disease indicators were published in our report (Wang et al. 2014), because we analyzed the same group of RA patients in the present study.
aCategorical variables are given as the %; normally distributed data are shown as the mean ± SD; other continuous variables are shown as the median (range).
RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide antibodies; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DAS28, disease active score in 28 swollen and 28 tender joints.
Serum IL-35 levels in patients and control individuals were measured using a commercial enzyme-linked immunosorbent assay (ELISA; LEGEND MAX™, BioLegend, San Diego, CA, USA). The concentration of IL-35 was calculated using a standard curve.
Anti-cyclic citrullinated peptide (CCP) antibodies detectionAnti-CCP antibodies were detected by a commercial ELISA kit (Immunoscan CCPlus, Euro-Diagnostica, Malmo, Sweden) according to the manufacturers’ instructions.The cut-off value for a positive reaction was set at 25 U/mL, as suggested by the manufacturer.
Statistical analysesStatistical analysis was performed using SPSS 16.0 for Windows. As the serum IL-35 levels in 100 RA patients lacked normal distribution, differences between groups were assessed by the Mann-Whitney U test, and correlations were determined by computing Spearman rank correlation coefficients. The results were expressed as the median (range) value. P values of < 0.05 were considered statistically significant.
The distribution of IL-35 concentrations in RA patients and healthy controls is shown in Fig. 1. The mean level of serum IL-35 in RA patients was 2.19 ng/mL (range: 0.94-23.67 ng/mL), while that in healthy controls was 6.86 ng/mL (range: 3.55-43.92 ng/mL). The serum IL-35 concentration was significantly lower in patients with RA, compared with healthy controls (p < 0.001).

Serum levels of IL-35 in rheumatoid arthritis patients and healthy controls.
Serum IL-35 levels were estimated in 100 patients with rheumatoid arthritis (RA), and 50 healthy controls (HC) using a commercial enzyme-linked immunosorbent assay kit. The solid bars represent the median IL-35 levels.
IL-35 levels were compared in 100 RA patients on the basis of gender and various disease indicators. RA patients who suffered erosive arthritis (n = 31) had lower IL-35 levels than those with non-erosive arthritis (n = 69, p = 0.022; Fig. 2A). In addition, serum IL-35 level was significantly lower in patients with elevated percentage of neutrophils (n = 22, p < 0.001; Fig. 2B). Correlation analysis indicated that serum IL-35 concentration was negatively associated with age (r = −0.296, p = 0.003), rheumatoid factor (RF; r = −0.265, p = 0.008), and percentage of neutrophils (r = −0.241, p = 0.016; Fig. 3). A significant association was observed between IL-35 level and C-reactive protein (CRP; r = −0.197, p = 0.050).
The serum IL-35 levels were not significantly different between patients with anti-CCP antibodies (n = 78) and those without anti-CCP antibodies (n = 22) (p = 0.265). We then analyzed the association between IL-35 and disease indicators in these two patient groups. In the anti-CCP antibodies-positive patients, there was no significant difference in IL-35 levels between patients with erosive arthritis (n = 23) and non-erosive arthritis (n = 55) (p = 0.056). However, the IL-35 levels were lower in patients with elevated percentage of neutrophils (n = 20) than those with non-elevated percentage of neutrophils (n = 58) (p < 0.001). Correlation analysis revealed that serum IL-35 level was negatively associated with age (r = −0.240, p = 0.034), RF (r = −0.251, p = 0.027), and percentage of neutrophils (r = −0.224, p = 0.048).
Among patients who showed anti-CCP antibodies negative (n = 22), the serum IL-35 levels were significantly lower in patients suffering erosive arthritis (n = 8) than those patients without erosion (n = 14) (p < 0.001). Correlation analysis further indicated that serum IL-35 level was negatively associated with age (r = −0.534, p = 0.010) and ESR (r = −0.574, p = 0.005), but positively associated with immunoglobulin (Ig) M level (r = 0.442, p = 0.039).
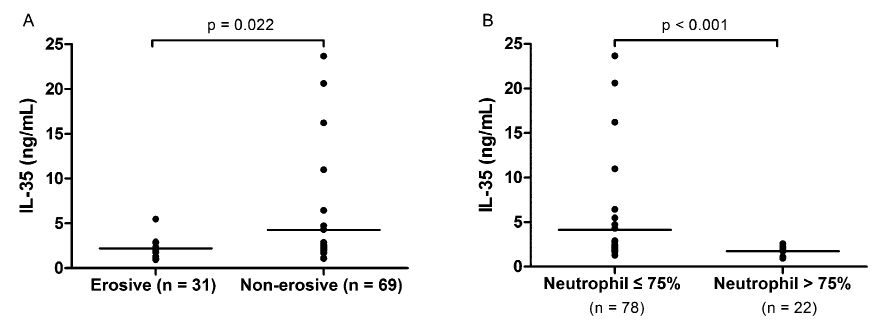
Comparisons of IL-35 levels between patient groups based on bone erosion status and percentage of neutrophils in 100 RA patients.
The difference was assessed by the Mann-Whitney U test. RA patients who suffered erosive arthritis (n = 31) had lower IL-35 levels than those with non-erosive arthritis (A) (n = 69, p = 0.022). In addition, serum IL-35 level was significantly decreased in patients with elevated percentage of neutrophils (B) (n = 22, p < 0.001). The solid bars represent the mean levels of IL-35.

Correlations between IL-35 levels and age, rheumatoid factor (RF), and percentage of neutrophils.
Spearman’s analysis was used for the correlation analysis. In 100 RA patients, weak but significant negative correlations were observed between serum IL-35 concentration and age (A) (r = −0.240, p = 0.034), RF (B) (r = −0.251, p = 0.027), and the percentage of neutrophils (C) (r = −0.224, p = 0.048).
IL-35 has been identified as a novel immunosuppressive/anti-inflammatory cytokine that may be involved in various autoimmune diseases. In this study, to investigate whether the expression of IL-35 is altered in patients with RA, we determined the serum levels of IL-35 in patients with RA, and further analyzed the possible associations between IL-35 and disease indicators for RA. Our results suggested that IL-35 was expressed at a significantly lower level in the sera of RA patients than in the sera of healthy controls. In addition, IL-35 was negatively associated with certain disease indicators such as age, bone erosion, percentages of neutrophils, and RF, indicating its participation in the inflammatory processes associated with RA development.
The IL-12 family members include IL-12, IL-23, IL-27, and IL-35. These cytokines are unique and pleiotropic, and play an important role in T-cell fate. IL-12, IL23, and IL-27 are primarily expressed in antigen-presenting cells (APCs), such as macrophages, monocytes, and dendritic cells, whereas bioactive IL-35 is secreted mainly from CD4+ Foxp3+ Treg cells (Devergne et al. 1997; Collison et al. 2007). Preliminary data have revealed that IL-35 is concerned primarily with Treg effector function, and as such, this may be of considerable interest in the autoimmune disease field (Pope and Shahrara 2013). To date, the majority of studies on the effects of IL-35 have been carried out in mice or other experimental models of arthritis. Collison et al. (2007) reported that treatment of naive effector T cells with either ectopically expressed or recombinant IL-35 suppressed T cell proliferation in a murine model. The role of IL-35 was further validated in two studies that demonstrated that IL-35 treatment ameliorated the severity of collagen-induced arthritis (Niedbala et al. 2007; Kochetkova et al. 2010). With respect to human autoimmune diseases, decreased IL-35 concentration was detected in systemic lupus erythematosus (SLE), and a negative association with disease severity was observed (Ouyang et al. 2014). However, in another study, increased levels of plasma IL-12 family cytokines, including IL-35, were observed in 30 patients newly diagnosed with severe SLE patients, but these levels decreased significantly after glucocorticoid therapy, indicating that glucocorticoid treatment may have a suppressive effect on IL-35 expression (Qiu et al. 2013). In the present study, a significant decline in serum IL-35 levels was demonstrated in the RA cohort compared with the healthy controls. Whether the decreased expression of IL-35 in the RA cohort was affected by medication remains to be validated.
Further analysis indicated that IL-35 levels were negatively correlated with disease-related indicators such as RF, percentage of neutrophils, and articular erosion. RF is a disease marker for RA that is widely used in diagnosis, monitoring, and prognosis. RF-positive RA is consistently reported to be more severe, functionally and radiographically, than RF-negative RA. Higher RF concentration is believed to be associated with increased disease activity, and RF level falls markedly after therapy (van Boekel et al. 2002; Bobbio-Pallavicini et al. 2004). On the other hand, IL-35 attenuates collagen-induced arthritis in mice via its ability to inhibit Th17 cell development and induce Treg cell differentiation (Niedbala et al. 2007). Because Th17-response is pro-inflammatory, it may facilitate the production of autoantibodies. Thus, the weak but significant correlation between IL-35 and RF may be explained by the fact that IL-35 has an inhibitory effect on Th17 cell development and subsequent downstream reactions. Nevertheless, we failed to find any association between serum IL-35 level and anti-CCP antibody concentration or anti-CCP antibodies positivity. However, a distinct pattern of IL-35 concentration was observed between anti-CCP antibodies-positive and anti-CCP antibodies-negative patients. These data indicate that the biological function of IL-35 is influenced by certain factors that mediate disease status, such as autoantibody profiles in RA. Consistent with the anti-inflammatory activity of IL-35, we observed a weak but significant negative correlation between IL-35 and the percentage of neutrophils in the peripheral blood of RA patients. In RA, synovial fluid often contains a large number of neutrophils that could lead to joint inflammation and/or destruction. In most cases, the percentage of neutrophils in the circulation and their count also increase with inflammation of joints (Wright et al. 2014). Neutrophils are regulated by a set of bioactive molecules, including cytokines. Thus, IL-35 may have a regulatory effect on neutrophils; however, the exact mechanism underlying this phenomenon remains to be elucidated.
Although we failed to detect an association between IL-35 and DAS28 of RA patients, a significantly different distribution of IL-35 levels between patients with and those without erosive arthritis was observed. RA patients with erosive arthritis (n = 31) had significantly lower IL-35 levels than those with non-erosive arthritis (n = 69, p = 0.022). Bone erosion is a critical feature of RA, and it influences disease severity and functional outcome. Initial stages of bone erosion involve synovitis, which suggests the involvement of immune cells, synovial fibroblasts, and bioactive molecules, including cytokines (Schett and Gravallese 2012). IL-6 and TNF are two important pro-inflammatory cytokines, and treatment with direct inhibitors of these cytokines is one of the most effective approaches to slow down or arrest bone erosion in RA (Schett et al. 2008). In addition, the roles of IL-17 and IL-15 in RA pathogenesis have been highlighted previously. Because IL-17 and IL-15 possess both proinflammatory and pro-osteoclastogenic properties, they are considered candidate therapeutic targets (Ogata et al. 1999; Miossec et al. 2009). For a long time, the majority of research on RA pathogenesis was concentrated on the roles of pro-inflammatory cytokines in bone erosion in RA. However, with the identification of novel anti-inflammatory cytokines, research attention is now being focused on their role in autoimmunity. A recently published study has indicated that IL-35-producing B cells play an important role in the regulation of immunity, and a potential therapeutic value for IL-35 production by B cells in autoimmune and infectious diseases has been proposed (Shen et al. 2014). In another study, Nakano et al. (2015) focused on the regulatory role of IL-35 in T cells of patients with RA. They observed that IL-35 exhibited an immunosuppressive role in T cell activation during the immune response elicited in RA. However, these authors found a significantly negative correlation between IL-35 level and DAS28, while no significant associations were observed in our study. The discrepancy might be attributed to the disease status of patients at the time of blood sampling. Another reason might be the difference of the sample size: 100 patients in our study compared to 28 in the previous study. Nevertheless, collectively, these findings indicate toward the suppressive effect of IL-35 on inflammation in RA patients. Referring to our observation, which indicated different expression levels of IL-35 in patients with erosive arthritis and those without, we speculated that pro-inflammatory and anti-inflammatory cytokines promote the distinctive cytokine microenvironment at the site of synovitis. Synovitis inflammation further triggers the process of bone erosion; the microenvironment may be altered accordingly. Thus, to some extent, the different expression levels of IL-35 between patients with erosive and non-erosive arthritis may be attributed to differences in inflammatory microenvironment between the two patient groups. Furthermore, additional factors such as therapeutic strategy or genetic background might be responsible for the differential expression of IL-35. However, the exact regulatory mechanism underlying IL-35 expression in patients with RA needs to be elucidated in the future with systemic studies.
In summary, we observed the lower level of circulating IL-35 in patients with RA, when compared with healthy controls, indicating that the expression of IL-35 in RA patients might be down-regulated or suppressed. Further analysis revealed that IL-35 concentration is negatively associated with RF, percentage of neutrophils, and bone erosion in patients with RA. These results suggested a possible protective role for IL-35 in the pathogenesis of RA, especially in the inflammation process. In the future, studies will focus on the mechanism underlying the protective effect of IL-35 in RA development or investigating the therapeutic value of IL-35.
The study was supported by “The Fundamental Research Funds for the Central Universities,” Central South University (Grant Number: 2012QNZT118).
The authors declare no conflict of interest.