2015 Volume 237 Issue 3 Pages 219-226
2015 Volume 237 Issue 3 Pages 219-226
One of the sexual hormones, estrogen, increases elasticity of human connective tissue such as the anterior cruciate ligament during the menstrual cycle in women. In the present investigation, the plantar fascia was investigated to see if there is a difference in elasticity with the menstrual cycle. Fifteen young healthy females in the age range of 18-35 years old with a regular menstrual cycle were tested twice throughout one full menstrual cycle; once during the early follicular phases and once at ovulation. Foot length, while standing on both feet and one foot were used to assess plantar fascia elasticity, ultrasound measured plantar fascia thickness while lying and standing, and posture sway and tremor using a balance platform during 8 different balance tests were assessed to see the impact of elasticity changes. Foot length increased significantly at ovulation compared to menstruation when standing on two feet (p = 0.03) and standing on one foot (p < 0.001). There was also a significant increase in plantar fascia in thinning per kilogram weight applied to the foot at ovulation compared to menstruation (p = 0.014). Associated with this increase in elasticity at ovulation, there was a reduction in balance in the most difficult balance tasks and an increase in tremor during ovulation (p < 0.05). Plantar fascia elasticity change during the menstrual cycle might have effects on posture sway and tremor, which could have a potential risk of falling. Therefore, healthy professionals working with young female adults should recognize these physiological effects.
Walking is one of the most effective forms of exercise to reduce the incidence of diseases such as cardiovascular disease and diabetes (Tully et al. 2005). It offers low impact and reduced risk of injury compared to other forms of exercise (Reiner et al. 2013). Other more intense forms of exercise such as running even offer more benefits in terms of reduction in long term health care costs and back and hip pain as individuals age (Andersen et al. 2010; Resaland et al. 2011). An average adult takes between 5,000 and 10,000 steps each day (men: an average of 7,192 steps per day; women: an average of 5,210 steps per day) (Tudor-Locke and Bassett 2004). One common problem that arises in physical activity is inflammation of the plantar fascia (Waclawski et al. 2015). The plantar fascia is one of the most important parts of the foot supporting the arch of the foot to protect the foot during walking, jumping and running. Plantar fasciitis is a common condition and can occur as a single occurrence or repeatedly throughout life (Digiovanni et al. 2006; Hyland et al. 2006). It is most common in people who exercise frequently or especially in people with a high body mass index (BMI) (Digiovanni et al. 2006; Weiner 2006; Moraes do Carmo et al. 2008).
The plantar fascia is a thick fibrous connective tissue which originates at the medial tuberosity of the calcaneus and inserts into the proximal phalanges (Kumar et al. 2013). The central portion is the thickest, attaches at the posterior aspect of the medial tuberosity of the calcaneus posterior to the origin of the flexor digitorum brevis tendon, and is 1.5 to 2.0 cm in width, distally, at the level of the metatarso-phalangeal joints. The central portion of the plantar aponeurosis divides into five fascicles, one for each of the toes (Goff and Crawford 2011). The lateral portion of the plantar aponeurosis arises from the lateral aspect of the medial tuberosity of the calcaneus and its distal medial and lateral bands attach to the plantar plate of the fourth toe and to the base of the fifth metatarsal respectively and is 1.0 to 1.5 cm in width.
Edema in the plantar fascia therefore affects movement in the foot by inhibiting normal tendon movement to flex and extend the foot, especially when body weight is applied (Goff and Crawford 2011; Kumar et al. 2013). The elastic fascia stretches as the ball of the foot touches the ground, lengthening the arch of the foot. The arch is supported with the strong tissue of the plantar fascia until the heel contacts the ground; so the plantar fascia offers stretch and support, as well as bearing some of body weight. Also, it plays an important role in balance during the various phases of gait (Holme et al. 1999). While the thickness of the plantar fascia in normal and injured patients does not vary between men and women (Sconfienza et al. 2013), little has been done to examine the effect of sex hormones in women on the plantar fascia.
When ankle instability was measured in 512 athletes, 23% had instability, with women having more instability than did men in both high school and college students (Tanen et al. 2014). Loading during jumping is also different during landing in women and men (Debiasio et al. 2013). Plantar loading is greater in the mid-foot in men than women during soccer (Sims et al. 2008). In general though, for all types of athletics, there is evidence of more ankle injuries in women than men (Willems et al. 2005a, b). Further, higher occurrence of knee injuries has been observed in women compared to men, women having 2-8 times the knee injury rate as men (Murphy et al. 2003; Vauhnik et al. 2008; Park et al. 2009a, b). This gender difference is at least partly due to the effect of estrogen on ligament laxity (Deie et al. 2002; Eiling et al. 2007; Park et al. 2009a, b; Petrofsky et al. 2013; Lee et al. 2014a). There are 17-β estradiol receptors in human connective tissues (Yu et al. 1999; Yudt et al. 1999) that cause relaxation of connective tissue such as the anterior cruciate ligament (ACL) at the time of ovulation when estradiol concentration peaks (Petrofsky et al. 2013; Lee et al. 2014a). Since the plantar fascia is an elastic band of tissue, it should be also affected by estradiol during the menstrual cycle as does the ACL.
Interestingly, however, there is a greater incidence of plantar fasciitis in males than females (Middleton and Kolodin 1992). Males are generally heavier, which, when combined with the greater speeds, increased ground reaction forces, and less ligament elasticity, may explain the greater injury tendency even though no evidence has been reported. For women, most injuries in sports occur to the lower limb with most of these to the foot and ankle. Yet, interest in sex differences in injuries is usually given to the knee injuries and not the foot (Sims et al. 2008) and differences in the plantar fascia laxity during the menstrual cycle have not been made.
Previous studies have reported that sex hormones might have an impact on postural control as well as muscular coordination. There are both alpha and beta receptors in tendons, ligaments and in skeletal muscle altering neuromuscular control and myofascial force transmission pathways during the menstrual cycle (Lemoine et al. 2003; Huijing and Jaspers 2005; Zazulak et al. 2006). The effect of estrogen on these receptors both directly and indirectly affects the female neuromuscular system (Rozzi et al. 1999). Sarwar and colleagues (1996) reported quadriceps strength increases and a significant slowing of muscle relaxation occurs during the ovulatory phase of the menstrual cycle. Thus estrogen relaxes collagen cross bridges in ligaments as well as altering actomyosin ATPase activity in skeletal muscle. Even if estrogen has no central neuromuscular effects, the relaxation in ligaments and slowing in muscle contraction speed is compensated for by more muscle activity to maintain motor control at joints such as the knee (Khowailed et al. 2015a, b).
A greater postural sway in the early follicular phase of the menstrual cycle has been reported (Darlington et al. 2001). It could be explained due to a complex interaction between knee and ankle laxity as cited above. In another study, Ericksen and Gribble found less ankle stability in women than men but no effects of the menstrual cycle. However, they examined their subjects 5 days before and 5 days after ovulation; estradiol peaks at ovulation and then falls (Ericksen and Gribble 2012).
Eight different balance tests were used in the previous research. These altered somatosensory input, visual input or the base of support to provide a series of increasing challenges to balance. The concept was that for easier balance challenges, the body could compensate using the vestibular system, for example, when vision is removed as in input (Tse et al. 2013). However, if there are slower muscle contractions and more tendon flexibility with estrogen, this may overload the system and, for the more difficult balance tests, cause a loss of balance. One way of detecting this is by tremor. Physiological tremor shows the compensation of the motor system to control sway. If tremor increases, the body is having a more difficult time maintaining balance (Petrofsky et al. 2012; Petrofsky and Khowailed 2014).
Therefore, the purpose of this study was to investigate the differences in plantar fascia elasticity during the two phases of the menstrual cycle where estradiol concentration is lowest and highest. This was accomplished by measuring the flexibility of the plantar fascia to increased load by measuring foot length under two different weights on the foot and by using high resolution ultrasound to assess planar fascia thickness when the foot was under two different loads of weight. If there is a change in flexibility of the plantar fascia, it may also cause a change in postural sway on a balance platform and alter neuromuscular tremor due to changes in plantar fascia elasticity. Therefore posturography was accomplished as well as measures of tremor during balance tasks.
The subjects in this study were 15 young healthy females between the ages of 18 and 30 years old, with a regular menstrual cycle. All subjects were screened for 3 months for a regular 28.2 ± 2.3 days of menstrual cycle and physically inactive with a BMI between 15 and 30. Subjects were excluded if they had any history of pregnancy, cardiovascular disease, hepatic disease, diabetes, and were taking any medication that would affect sex hormones. Also, they did not have any orthopedic abnormalities or injuries to the knee or foot. Subjects were screened to have normal arches and not have flat feet. Basic characteristics of the subjects are described in Table 1. All subjects signed a statement of informed consent as approved by the institutional review board of Loma Linda University.

Mean (SD) of general characteristic (N = 15).
SD, standard deviation.
Measurement of foot length: Foot length was measured with the subject standing on a polypropylene sheet covered in talcum powder. This allowed the foot to have a natural shape since the friction is low while measuring foot length. The length of the foot was measured with a digital caliper that could be extended to as long as 300 mm (Guanglu LTD, Shanghai, China). The length was measured under two conditions, with the person standing on one foot and on two feet.
Measurement of plantar fascia thickness: Musculoskeletal ultrasound is a useful imaging tool in confirming a diagnosis of plantar fasciitis by measuring plantar fascia thickness before and after a given treatment regimen to gauge the treatment’s efficacy. The standard “normal” or asymptomatic thickness value reported for the plantar fascia is 2.3 to 4.0 mm, averaging 3.4 mm (Abul et al. 2015). Each involved foot was evaluated sonographically with a L14-6 MHz linear array transducer (Sonosite Titan, Seattle, Washington) and acoustic coupling gel was applied to the plantar surface of the foot. The plantar fascia was examined with the patient in two positions; the prone position, with the affected foot hanging over the edge of the examination table with his or her ankle in neutral position and while standing on a platform. The ultrasound probe was applied vertically to the plantar aspect of the heel. The sagittal thickness of the proximal insertion of the plantar fascia was measured, at a standard reference point 5 mm from the proximal insertion at the anterior aspect of the inferior border of the calcaneus.
Balance tasks: Eight quiet standing balance tasks, each lasting for 6 seconds, were included in this study (Tse et al. 2013). Sensory variables such as the vision, base of support and surface compliance were altered individually or simultaneously in the balance tasks. To alter the visual input, two levels of vision (eyes open and eyes closed) were used in the balance tasks. To alter the somatosensory input, two different surface compliances (firm surface and foam surface) were used. The Aeromat balance block, a PVC/NBR foam with size 16 × 19 × 2.5 inches and density around 0.04-0.06 g/cm3 (AGM Group, Aeromat Fitness Product, Fremont, CA), was placed on top of the balance platform as the foam surface in this study. Participants were asked to stand in two different stance positions with feet apart (centers of the calcaneus in the same distance as the two anterior superior iliac spine) or in tandem (feet in a heel-toe position with non-dominant foot in front).
Measurement of postural sway: The displacement of the subject’s center of pressure was measured using a validated and reliable balance platform of 1 m × 1 m in size and 0.1 m in height (Petrofsky et al. 2009). Four stainless steel bars, each with four strain gauges, were mounted at the four corners under the platform (TML Strain Gauge FLA-6, 350-17, Tokyo, Japan). The output of the 4 Wheatstone strain gauge bridges was amplified with BioPac 100C low-level bio-potential amplifiers and recorded on a BioPac MP-150 system through a 24-bit A/D converter. The sampling rate is 1000 samples per second (Petrofsky et al. 2009).
To calculate the load and the center of the pressure of the force on the platform, the output of the four sensors was used to measure the X and Y coordinates of the center of gravity of the subject. This data was converted to a movement vector giving a magnitude and angular displacement. By averaging the vector magnitude over 6 seconds, mean and standard deviation (SD) were obtained for this measure. From this, the coefficient of variation (CV) was calculated (SD/mean × 100) as a measure of the postural sway (Petrofsky et al. 2009).
Procedures: All subjects were advised of the study goals, protocol, and inclusion and exclusion criteria. To measure plantar fascia elasticity, two different measures were made. First, the foot was loaded at two different weights (half body weight and full body weight) and foot length from the longest toe to the heel was measured on a polypropylene sheet that was powdered. The thickness of the plantar fascia was also measured with no load and full body weight to assess the elasticity of the plantar fascia as well. Finally, eight different balance tasks were assessed with a balance platform.
Sample size estimation: G-Power 3.1.9.2 software was used to calculate the sample size required so that a reasonable expectation would be likely to detect an expected effect size of 0.8 between the two dependent means (matched pairs). A sample size of 15 was required to show statistical significance when clinically significant differences with 82% of power.
Statistical analysis: Data was analyzed using SPSS for Windows version 22.0. Characteristics of the subjects were summarized using mean and SD. The distribution of the quantitative variables was examined using One-Sample Kolmogorov-Smirnov Test. A paired t-test was used to compare foot length, plantar fascial thickness, and each balance test between the menstruation and ovulation. The level of significance was set at alpha (α) is less than 0.05.
Foot length significantly increased both during menstruation and ovulation (p < 0.001) when weight was shifted onto the foot by bearing weight evenly on both feet (half body weight on each foot) and when standing on one leg (full body weight on one foot). The difference between foot length comparing ovulation to menstruation was significant for standing on two feet (p = 0.03) and standing on one foot (p < 0.001) (Fig. 1). The change in foot length averaged 5.0 ± 2.5 mm at ovulation and 3.5 ± 1.4 mm when shifting full body weight on one foot during menstruation. Using the change in body weight and the change in foot length, the elasticity of the plantar fascia and associated ligaments in the foot was calculated as 0.17 ± 0.08 mm/kg body weight at ovulation and 0.12 ± 0.04 mm/kg at menstruation, a significant difference (p = 0.01).
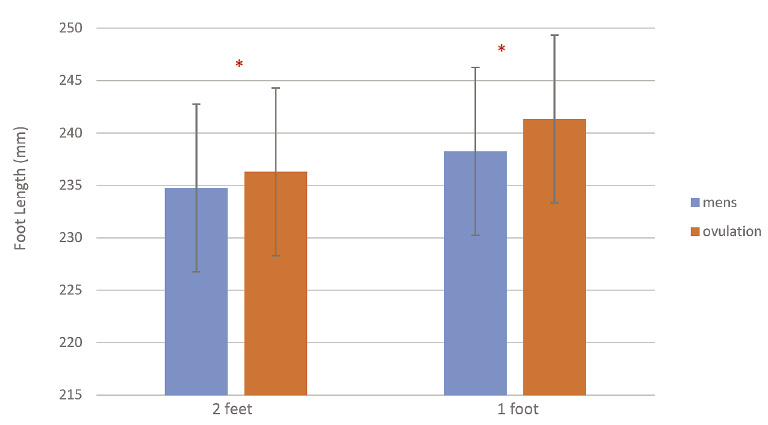
The measured foot length when standing on two feet and one foot at menstruation and ovulation.
*Significant difference in the foot length between ovulation and menstruation.
There was no significant difference in plantar fascia thickness both at menstruation and ovulation with the subject laying on the plinth (p > 0.05). However, the thickness decreased by 0.187 mm during menstruation and 0.164 mm at ovulation with weight applied to the foot. The reduction in thickness for both phases was significant (p < 0.001) as was the difference between the thickness in menstruation and ovulation (p = 0.012). When the change in thickness was divided by body weight, the change in thickness per kilogram body weight was 0.00051 mm/kg in the group at menstruation and 0.00087 mm/kg at ovulation, a significant increase in thinning per kilogram weight applied to the foot (p = 0.014, Fig. 2).
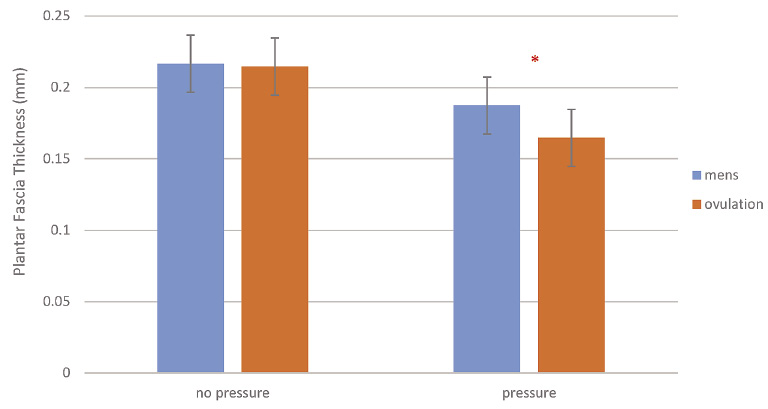
The measured thickness of the plantar fascia at menstruation and ovulation with no weight on the foot and full body weight.
*Significant difference in plantar fascia thickness between ovulation and menstruation.
The results of balance testing are shown in Figs. 3, 4 and 5. As shown in Fig. 3, there was no difference in sway during the menstrual cycle for the five least difficult balance tests (p > 0.05), these being, feet apart eyes open firm surface (FAEO Firm), feet apart eyes closed firm surface (FAEC Firm), feet apart eyes open on foam (FAEO Foam), feet apart eyes closed on foam (FAEC Foam) and tandem eyes open on firm (TEO Firm). For the three most difficult balance tasks, these being, tandem eyes open Foam (TEO Foam), tandem eyes closed firm surface (TEC Firm) and the most difficult task, tandem eyes closed foam (TEC Foam), there was a significantly greater sway at ovulation compared to menstruation (p = 0.007, p = 0.02, p = 0.002 respectively).
Sway on the balance platform was filtered at two specific frequencies, 8 Hz and 24 Hz. Eight Hz has been shown to represent the frequency of the stretch reflex (Sakamoto et al. 1998) whereas 24 Hz represents central motor loop tremor (McAuley et al. 1997). Here, for the five least difficult balance tasks, tremor at the 8 and 24 Hz bands averaged 1.1 ± 0.45 and 0.11 ± 0.004 respectively. For the three most difficult balance tasks, there was a significant higher tremor at ovulation compared to menstruation as shown in Figs. 4 and 5 with tremor increased with the complexity of the balance tasks (p = 0.002).
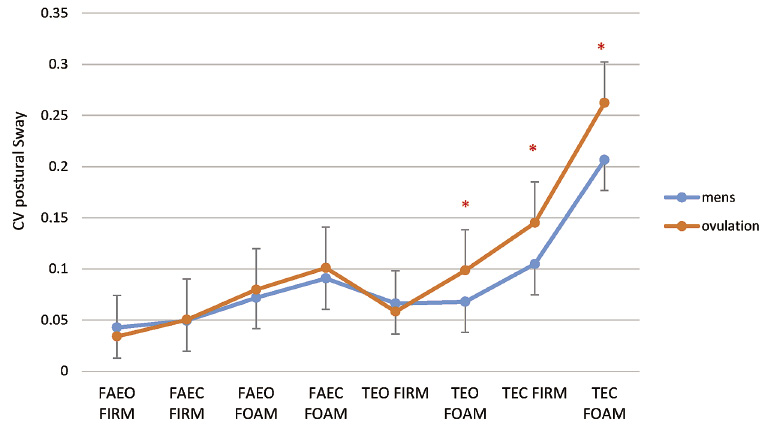
The postural sway (coefficient of variation) during 8 balance tasks at menstruation and ovulation.
*Significant difference in the posture sway between ovulation and menstruation.

The average tremor in the 8 Hz bandwidth for the 3 most difficult balance tasks at menstruation and ovulation.
*Significant difference in the 8 Hz bandwidth between ovulation and menstruation.

The average tremor in the 24 Hz bandwidth for the 3 most difficult balance tasks at menstruation and ovulation.
*Significant difference in the 24 Hz bandwidth between ovulation and menstruation.
17-β estradiol receptors are found in human connective tissues throughout the body (Sarwar et al. 1996; Rozzi et al. 1999; Woolley 1999a, b). They modulate the immune system and elicit changes in tissue blood flow and tissue growth (Petrofsky et al. 1975, 1976; Monica Brauer and Smith 2015). The normal menstrual cycle produces low serum levels of estrogen and progesterone in the early follicular phase (menstruation, day 1 to 6), and then estrogen is elevated in the late follicular phase: (day 7 to 14), and progesterone is elevated during the luteal phase: (day 15 to 28) while estrogen remains elevated and slowly returns to baseline (Beynnon and Fleming 1998; Constantini et al. 2005). Numerous studies have centered on the laxity of the ACL of the knee (Petrofsky et al. 2007; Adachi et al. 2008; Lee et al. 2013, 2014b). This is due to the fact that injuries of the knee are 2 to 8 times greater in women than men and occur most often at menstruation (Beynnon et al. 2005). Some studies that have examined knee ACL laxity found no difference during the menstrual cycle (Beynnon et al. 2005) but that women had more laxity than did men on average (Shultz et al. 2012). But these studies were flawed in that they did not control tissue temperature. Body temperature and skin and limb temperature varies during the menstrual cycle (Petrofsky et al. 1976, 2007; Lee et al. 2014a) and ligament elasticity varies with temperature as well (Ciccone et al. 2006; Petrofsky et al. 2013; Lee et al. 2014a). Therefore, without controlling tissue temperature the true effect of estrogen on ligaments cannot be determined. In fact, many studies that were conducted without controlling room temperature failed to show consistent changes in laxity probably due to lack of control of environmental temperature. When environmental temperature was controlled, in a previous study, the effect of estrogen could be seen (Lee et al. 2014a). Further by using a controlled temperature water bath on the knee, the menstrual cycle temperature effects could be seen separately from the estrogen effects (Lee et al. 2014a).
In the present investigation, room temperature was controlled and subjects rested for 20 minutes prior to measurements. Here, two indirect measures of plantar fascia elasticity were used. First, by measuring foot length on a powder board in response to two different loads, the elasticity of the plantar fascia could be seen. Observationally, with greater loads, the arch in the foot was compressed showed the loading and stretching of the plantar fascia. This, then, resulted in an increase in foot length. The greatest increase was at ovulation. When the change in length was divided by the change in weight applied to the foot, a measure of elasticity could be seen and at ovulation, the change in length per kilogram body weight. There was an almost 50% increase in length per kilogram body weight at ovulation compared to menstruation. When the thickness of the plantar fascia was measured at rest and when body weight was applied to the foot, the plantar fascia decreased in thickness also about 40% more at ovulation with the same applied load. These data then cross confirm each other supporting the idea that the plantar fascia is varying elasticity during the menstrual cycle. Other studies have confirmed that when weight bearing, women had higher arches than did men. This was abolished when the weight was removed from the foot showing the elasticity of the plantar fascia in both men and women (Fukano and Fukubayashi 2012).
We assume that if there were greater plantar fascia elasticity at ovulation, balance would be impaired. Previous studies have shown that there is more laxness at the ankle at ovulation versus other periods of the menstrual cycle (Shultz et al. 2012). This would support the fact that other connective tissues in the foot also had 17-β estradiol receptors. More laxness at the knee and foot should lead to poorer balance. Here, balance for the 3 most difficult balance tests was reduced at ovulation. Further, the increase in tremor in both bands (10 Hz physiological tremor and 24 Hz central reflex tremor (McAuley et al. 1997)) shows that more motor control was needed to stabilize the body at ovulation probably due to laxness at the foot and knee. While muscle activity was not measured here, it would have been interesting to see if more muscle activity was needed to stabilize the body at ovulation due to laxness in ligaments; further research is needed as was seen during female runners (Khowailed et al. 2015a, b). Previous studies show differences in muscle strength and neuromuscular control with the menstrual cycle (Sarwar et al. 1996). While some have attributed the increase incidence of knee and ankle injuries to a tighter ACL during menstruation. This is an open chain system and certainly instability at the foot and ankle may also lead to knee injuries.
Of additional interest would be to study the birth control pill and ovulation since these should both have a dynamic effect on laxness and balance. By leveling estrogen, there should be no differences in elasticity in the foot and balance.
In conclusion, plantar fascia elasticity increased during ovulation compared to menstruation during the menstrual cycle in young healthy women. Associated with this increase in plantar fascia elasticity at ovulation, there was poorer balance with more tremor when estrogen was greatest in the serum. Reduction in balance could be a potential risk factor for falling. Therefore, health professional should be aware of these physiological effects in young healthy women.
We thank Seokcheon Ham for helping with data collection and data entry.
The authors declare no conflict of interest.