2016 Volume 238 Issue 2 Pages 165-177
2016 Volume 238 Issue 2 Pages 165-177
Oral squamous cell carcinoma (OSCC) is the most common oral and maxillofacial malignancy, and its morbidity and mortality rates are still high in most countries. Oral potentially malignant disorders (PMDs) are used to refer to a heterogeneous group of conditions that are characterized by increased risk for malignant transformation to OSCC. Currently identified oral PMDs include leukoplakia, erythroplakia, palatal lesions associated with reverse smoking, oral lichen planus, oral submucous fibrosis, actinic keratosis, and discoid lupus erythematosus. The early detection and diagnosis of these lesions are important for cancer prevention and disease management. In recent years, there has been a growing and persistent demand for new non-invasive, practical diagnostic techniques that might facilitate the early detection of oral PMDs. The non-invasive detection techniques evaluated in this review are divided into four categories: vital staining with a solution that can be used as a mouth rinse or applied onto a suspected area of the mouth, light-based detection systems, optical diagnostic technologies that employ returned optical signals to reflect structural and morphological changes within tissues, and salivary biomarkers. Most of these techniques have shown great potential for screening and monitoring oral PMDs. In this review article, the authors critically assess these non-invasive detection techniques for oral PMDs. We also provide a summary of the sensitivity and specificity of each technique in detecting oral PMDs and oral cancer, as well as their advantages, disadvantages, clinical applications, and indications.
Oral squamous cell carcinoma (OSCC) is the most common oral and maxillofacial malignancy (Lambert et al. 2011). Currently, the morbidity and mortality rates of OSCC are still high in most countries. The reported 5-year survival rate is around 50-63% (Mehrotra et al. 2012; Messadi 2013). OSCC is usually preceded by premalignant lesions (Warnakulasuriya et al. 2007). In 2005, the World Health Organization recommended the use of the term oral “potentially malignant disorders” (PMDs) instead of precancerous lesions/disorders. However, a definition of oral PMDs had not been proposed at that time. Sarode et al. (2012) attempted to propose a definition for oral PMDs; namely, it is a group of disorders of varying etiologies, usually tobacco, characterized by mutagen associated, spontaneous or hereditary alterations or mutations in the genetic material of oral epithelial cells with or without clinical and histomorphological alterations that may lead to oral squamous cell carcinoma transformation. Currently identified oral PMDs include leukoplakia, erythroplakia, palatal lesions associated with reverse smoking, oral lichen planus, oral submucous fibrosis, actinic keratosis and discoid lupus erythematosus (Table 1) (Warnakulasuriya et al. 2007). These lesions have greater potential for malignant transformation than other oral lesions. The early detection and diagnosis of oral PMDs allow clinicians to monitor and treat oral carcinogenesis at intraepithelial stages, including mild, moderate, and severe dysplasia, as well as carcinoma in situ. This is crucial for improving the survival rate and reducing morbidity, disfigurement, function loss, treatment duration, and hospital costs of OSCC patients.
Conventional oral examinations, including visual inspection and palpation, are the routine methods for the screening of oral lesions. However, subtle lesions may pass undetected, and it is difficult to make a distinction among benign, premalignant, and malignant lesions. It has been reported that dysplasia or micro-invasive carcinoma can occur in clinically normal-appearing mucosa (Thomson 2002).
Currently, oral biopsy with histological assessment remains the gold standard for the diagnosis of oral PMDs. Nevertheless, as it is invasive, some patients may not accept this test, especially when the lesion appears “normal”. Moreover, subjectivity and observer variability (intra- and interobserver variability) are very common in the histologic diagnosis of oral PMDs and oral cancer (Karabulut et al. 1995; Brothwell et al. 2003; Fischer et al. 2004). The results could be affected by the size and depth of the biopsy, the quality of the specimen, fixation and freezing techniques, and the pathologist’s experience (Seoane et al. 2002, 2004; Mercadante et al. 2012).
Accordingly, non-invasive detection techniques for oral PMDs are needed. In the last few decades, wide ranges of non-invasive techniques have been developed for the detection of oral PMDs. However, it is difficult for the clinicians to decide which diagnostic tool is most appropriate and useful for oral PMD screening. The aim of this review is to critically assess these non-invasive techniques and to present the available evidence concerning their efficacy in the early detection and diagnosis of oral PMDs.
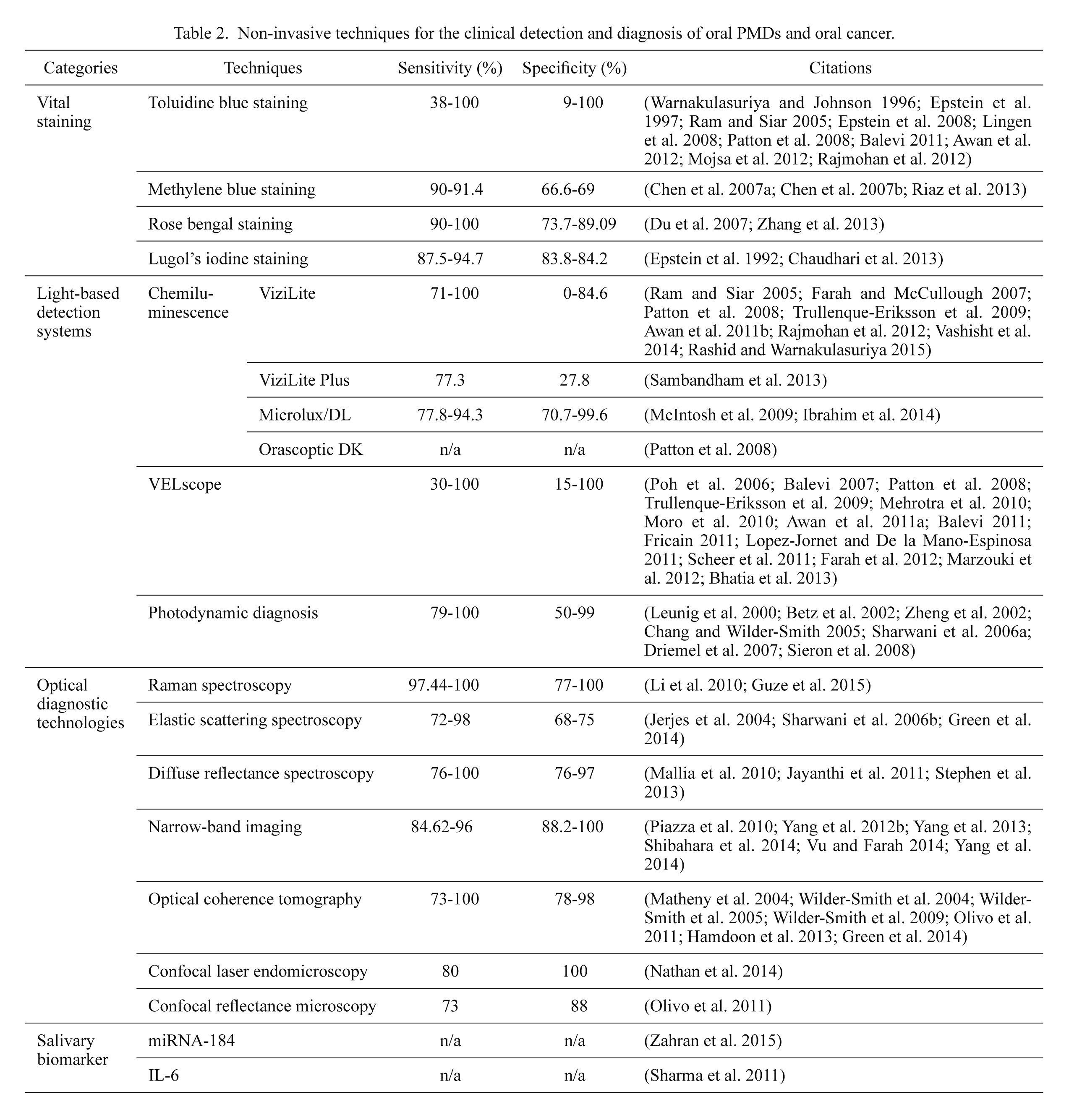
In this review, the non-invasive detection techniques employed for oral PMD detection are divided into four categories: vital staining with the solution that can be used as a mouth rinse or applied directly onto a suspected area, light-based detection systems, optical diagnostic technologies that employ returned optical signals to reflect structural and morphological changes within tissues, and salivary biomarkers (Table 2).

Oral potentially malignant disorders and their clinical features.
Non-invasive techniques for the clinical detection and diagnosis of oral PMDs and oral cancer.
Vital staining is usually simple, cheap, sensitive, and efficient. It can be used and interpreted by clinicians by the chair. Vital staining can enhance lesion characteristics, identify satellite or clinically non-apparent lesion sites, and assist in the choice of site and the timing of a biopsy.
Toluidine blue stainingToluidine blue (TB) is a basic thiazine metachromatic dye with a high affinity for acidic tissue components; therefore, it stains tissues rich in nucleic acids. Dysplastic and neoplastic cells contain more nucleic acids than normal cells. Moreover, malignant epithelium may contain intracellular canals that are wider than those of normal epithelium, facilitating penetration of the dye (Epstein et al. 1992). TB-stained tissue may appear dark or pale royal blue. Dark royal blue stain is considered positive (Fig. 1), pale royal blue stain is doubtful, and when no color is observed it is interpreted as negative. It is still controversial whether pale blue staining should be considered as positive. Several studies suggested that only dark royal blue staining should be regarded as positive (Gandolfo et al. 2006; Pallagatti et al. 2013). Digital color analysis may be helpful to observers in the color interpretation of TB-stained oral lesions, especially when the blue tone is intermediate (Guneri et al. 2013).
TB staining has been used for many years as an aid for the screening and post-surgical management of oral PMDs and oral cancer (Epstein et al. 2007; Patton et al. 2008; Epstein and Guneri 2009; Awan et al. 2012). It has been claimed that TB staining has a higher detection rate of oral PMDs and could further reduce the incidence of oral cancer compared to conventional visual inspection (Su et al. 2010). However, TB staining can yield a high percentage of false positive results. Some benign hyperplasia and inflammatory lesions can be stained, as they contain large quantities of nucleic acids (Driemel et al. 2007). Traumatic benign lesions can also be stained; however, they differ from malignant lesions in that the coloration often surrounds the ulceration and does not last long. In order to reduce the false positive rate, further assessment of a non-healing wound after at least 15 days is highly recommended (Mercadante et al. 2012).
Most studies of TB staining had significant limitations and biases (Lingen et al. 2008): (1) very few were randomized controlled trials; (2) histological diagnosis was not used as gold standard; and (3) there was variability in the staining techniques and color interpretation.

TB staining and VELscope images of an erythroplakia lesion. (a) Erythroplakia lesion on the buccal mucosa of a 62-year-old man. (b) Same lesion with TB staining. TB was retained in some part of the lesion. (c) Same lesion viewed through VELscope. The arrows indicate areas with loss of fluorescence. (d) The lesion was histologically diagnosed as erythroplakia with severe dysplasia (H&E staining, 100×).
Methylene blue was first used in detecting oral mucosa lesions in 2007 (Chen et al. 2007a, b). Similarly to TB, methylene blue also stains tissue with large quantities of nucleic acids (Riaz et al. 2013). Methylene blue staining is useful for screening oral cancer in high-risk individuals, and shows high sensitivity in detecting oral PMDs (see Table 1).
Rose bengal stainingRose bengal (RB) is the 4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodo-derivative of fluorescein. RB staining is widely used to diagnose ocular surface disorders (Rodrigues et al. 2009; Murube 2014). It stains desquamated ocular epithelial cells, dead or degenerated cells, but not healthy epithelial cells (Mittal et al. 2012). Two studies demonstrated that RB staining may be a valuable diagnostic technique in the detection of oral PMDs and oral cancer (Du et al. 2007; Mittal et al. 2012). In one study, authors investigated the efficacy of RB staining in the detection of oral PMDs and oral cancer in 132 patients, and concluded that RB staining seemed promising for the detection of epithelial dysplasia in oral leukoplakia, lichen planus, and leukokeratosis (Du et al. 2007). Zhang et al. (2013) combined fluorescence spectroscopic techniques with RB staining to detect oral PMDs in an animal model in vivo and reported that the method showed excellent sensitivity. Recently, a rose-bengal-conjugated gold nanorod (RB-GNR) platform was developed for the optical detection of cancer cells (Wang et al. 2014). Studies have demonstrated that the RB-GNR platform has positive significance in detecting cancer cells, but it has not yet been used in a clinical setting.
Lugol’s iodine stainingThe mechanism of Lugol’s iodine staining is that iodine reacts with glycogen in the cytoplasm. The reaction, known as the iodine-starch reaction, is visualized by a color change. The loss of cellular differentiation and the enhanced glycolysis in cancer cells do not promote the iodine-starch reaction. When it is applied on the suspicious lesions, normal mucosa stains brown or mahogany due to its high glycogen content, while dysplastic and cancer lesions do not stain, and appear pale compared with the surrounding tissue (Petruzzi et al. 2010).
Chaudhari et al. (2013) conducted a study on male inmates, and suggested that Lugol’s iodine was highly effective as a screening tool for oral PMDs and oral cancer in inmate populations. Lugol’s iodine staining during surgery can determine surgical resection margins, reduce local recurrence and improve survival in patients with epithelial dysphasia or malignant lesions (Umeda et al. 2011; Kurita et al. 2012). Maeda et al. (2010) applied colorimetric analysis to analyze the color of unstained lesions in relation to the histopathologic findings, they made a color chart and proposed a possible histological diagnosis based on the measured colors of the unstained lesions. Epstein et al. (1992) conducted a comparative study, in which TB and Lugol’s iodine were used both in combination and separately in 59 patients. The sensitivity and specificity of TB were 0.925 and 0.632, respectively, while the sensitivity and specificity of Lugol’s iodine were 0.875 and 0.842, respectively. When combined, the two stains showed a sensitivity of 0.850 and a specificity of 0.895. The authors concluded that Lugol’s iodine had less sensitivity in identifying oral malignant and dysplastic lesions but it had greater specificity. Moreover, in another study, 30 subjects with clinically suspicious oral PMDs and 30 subjects with clinically suspicious malignant lesions were stained consecutively with TB and Lugol’s iodine, the overall sensitivity in detecting dysplasia or malignant lesions was 92.7% while the specificity was 60% (Nagaraju et al. 2010). Most of the studies consulted found the Lugol’s iodine might have great potential for oral PMDs and oral cancer screening.
In a recent meta-analysis on the application of vital staining in detecting oral PMD and oral cancer (Macey et al. 2015), 14 studies were included (12 using TB, one using TB and/or Lugol’s iodine and one using RB). The estimated sensitivity was 0.84 (95% CI 0.74 to 0.90), and the specificity was 0.70 (0.59 to 0.79).
A number of light-based detection systems have been developed for the identification of oral PMDs and oral cancer at their earliest stage. Mucosal tissues undergoing abnormal metabolic or structural changes have different absorbance and reflectance profiles when exposed to various forms of light sources, enabling the identification of oral mucosal abnormalities (Awan et al. 2011a).
ChemiluminescenceCommercially available chemiluminescence techniques include ViziLite, ViziLite Plus, Microlux/DL and Orascoptic DK. The main difference between these techniques is that ViziLite and ViziLite Plus involve a single-use chemiluminescent stick, while Microlux/DL and Orascoptic DX provide a blue-white light-emitting diode (LED) fiber-optic light.
The ViziLite kit contains a vial of 1% acetic acid solution, a capsule, a retractor and the manufacturer’s instructions (Sambandham et al. 2013). The capsule has an outer shell of flexible plastic and an inner vial of fragile glass. To activate it, the capsule is bent to break the glass vial, so that the chemical products react with each other and produce bluish-white light with a wave length of 430-580 nm that lasts for around 10 min. The specific wavelength is absorbed by normal cells and reflected by abnormal cells that have a higher nuclear-cytoplasmic ratio. The presence of an “ace to white” lesion after a one-minute rinse with 1% acetic acid solution is considered as positive. The absence of such findings is considered as negative (Rajmohan et al. 2012). ViziLite positive lesions are considered to be abnormal oral mucosa.
ViziLite shows high sensitivity in detecting oral PMDs and oral cancer. However, these studies often involved only patients with mucosal lesions that were previously visualized under conventional light (Patton et al. 2008). Some studies found that ViziLite could not differentiate between keratotic or inflammatory oral PMDs and oral cancer (Farah and McCullough 2007; Ram and Siar 2005). It preferentially detected leukoplakia but might fail to spot red patches (Epstein et al. 2006; Kerr et al. 2006; Awan et al. 2011b; Rashid and Warnakulasuriya 2015). In short, the evidence supporting the use of ViziLite for the early detection of oral PMDs is actually quite insufficient. Some studies compared the efficacies of ViziLite and TB staining for the diagnosis of oral PMDs and oral cancer, reporting that ViziLite was relatively more reliable compared to TB staining (Rajmohan et al. 2012; Vashisht et al. 2014).
The manufacturer of ViziLite is now marketing an updated product called ViziLite Plus. The only difference between the Plus and the earlier version is that the latter contains a TB staining solution (Patton et al. 2008). TB can further delineate ViziLite-positive lesions, thus improving the specificity (Rashid and Warnakulasuriya 2015).
Microlux/DL shares the basic principles of ViziLite. The oral cavity is examined with a battery-powered LED fiber-optic source that emits a blue-white light (Patton et al. 2008; Ibrahim et al. 2014). Few studies have been published on its effectiveness in oral PMD diagnosis (McIntosh et al. 2009). In a study of 599 tobacco users, using biopsy as the gold standard, the sensitivity and specificity of Microlux/DL in detecting oral PMDs were 100% and 32.4%, which indicated that it seemed useful for enhancing lesion visibility, but couldn’t discriminate between benign and malignant lesions (Ibrahim et al. 2014). Microlux/DL is also a poor discriminator for inflammatory, traumatic, and malignant lesions (McIntosh et al. 2009).
The Orascoptic DK system also requires an acetic acid rinse and a three-in-one, battery-operated, hand-held LED instrument to improve the visualization of oral lesions (Patton et al. 2008). There is no published evidence regarding its utility in oral PMD detection.
VELscopeThe VELscope is a hand held device that can enhance the visibility of oral mucosal abnormalities by activating tissue autofluorescence. Autofluorescence is due to the presence of endogenous fluorophores in cells, which produce a fluorescent emission when exposed to light of a specific wavelength (Farah et al. 2012). Within the oral mucosa, the most relevant fluorophores are nicotinamide adenine dinucleotide and flavin adenine dinucleotide in the epithelium, and collagen cross-links in the stroma (Pavlova et al. 2008). Mucosal abnormalities can alter the absorption and scattering properties of light as a result of changes in tissue architecture and concentrations of fluorophores (Bhatia et al. 2013). At these excitation wavelengths, normal cells show a pale green fluorescence when viewed through a filter, while abnormal cells show a loss of autofluorescence and appear dark (Fig. 1) (Balevi 2007).
Some studies found that the VELscope can assist in screening for oral PMDs and oral cancer in populations at risk (Moro et al. 2010; Scheer et al. 2011). However, there is no evidence that it can distinguish between them (Balevi 2007), and no published studies have assessed VELscope as a diagnostic adjunct in general populations. In addition, several commonly occurring conditions, such as mucosal pigmentations, ulcerations, irritations, and gingivitis showed a loss of fluorescence under VELscope (Huber 2009). Blood hemoglobin can also reduce fluorescence. If a dark area appears during direct fluorescence visualization, the oral lesion must be considered as suspicious and clinical examination should be repeated by applying some pressure to remove blood from the target area. If normal green fluorescence returns after this pressure, the lesion is likely to have an inflammatory component (Mercadante et al. 2012). Moreover, Kois and Truelove (2006) proposed that positive lesions need to be followed up with great caution; if they do not resolve within two weeks, further assessment and biopsy are generally recommended. In short, the results should be interpreted with caution, bearing in mind the frequent occurrence of false positive results.
Photodynamic diagnosisPhotodynamic diagnosis (PDD) is based on the fluorescence generated by administration of an exogenous photoactivated compound that accumulates in cells with malignant potential, followed by appropriate photoirradiation (Uekusa et al. 2010). One of the most promising photosensitizers for oral PMDs and oral cancer diagnosis is 5-aminolevulinic acid (ALA), which does not fluoresce itself, but can induce protoporphyrin IX (PPIX) fluorescence in tissue. PDD in the oral cavity can be performed simply by rinsing with a 0.4% ALA solution for 20 min (Zenk et al. 1999). Topical application of excessive 5-ALA stimulates the production and intracellular accumulation of highly fluorescent PPIX in dysplastic and cancerous tissues, which is excited by brief exposure to light with a wavelength of 405 nm (Chang and Wilder-Smith 2005). Fluorescent tissues are considered suspicious for malignant transformation and biopsy should be considered (Driemel et al. 2007).
A high sensitivity but limited specificity of PDD in the detection of oral PMDs and oral cancer has been shown in several studies (Leunig et al. 2000; Sharwani et al. 2006b). The false-positive rate is especially high in patients who have a history of radiotherapy (Zenk et al. 1999). Given that various bacterial strains in the oral cavity can also produce PPIX under incubation of 5-ALA (Zenk et al. 1999), professional hygiene measures must be employed before PDD in order to increase the specificity. The need for an interval of about one hour between hygiene measures and the application of photosensitizing 5-ALA, as well as about three hours between the application of 5-ALA and the formation of a sufficient level of PPIX for fluorescence measurement, limits the use of this method in outpatient cancer prevention and aftercare. Moreover, even low levels of glucose can influence PPIX production; thus, patients must fast during the four hours necessary for examination, which requires strict patient management.
To improve the diagnostic specificity, a 5-ALA-mediated digitized fluorescence endoscopic imaging system has been built (Zheng et al. 2002). When PPIX fluorescence endoscopic images are quantified, the red-to-green and red-to-blue intensity ratios of dysplasia and malignancy are larger than those of benign tissues (Zheng et al. 2002; Bigio and Bown 2004). Zheng et al. (2004) quantified the red-to-blue intensity ratio in the ALA-induced PPIX fluorescence endoscopic images, and showed that it could provide good differentiation between benign dysplasia and cancer. Wang et al. (2009) found that oral submucous fibrosis mucosa had the lowest red-to-blue intensity ratio, and their results indicated that ALA-induced PPIX fluorescence spectroscopy could be used to identify oral submucous fibrosis. Moreover, in a study of 71 patients with clinically suspicious oral leukoplakia, Sharwani et al. (2006b) found that ALA-induced PPIX fluorescence spectroscopy had great potential for identifying dysplastic changes at an early stage and might be useful as a screening tool for the detection of subsequent primary lesions.
In a recent meta-analysis on the application of light-based detection systems in detecting oral PMD and oral cancer, 11 studies were included. The estimated sensitivity was 0.91 (95% CI 0.77 to 0.97) and the specificity was 0.58 (0.22 to 0.87) (Macey et al. 2015).
Raman spectroscopy is a vibrational spectroscopic technique that relies on the inelastic scattering of light, usually from a laser in the visible, near-infrared, or near-ultraviolet range. The vibrational changes in tissue parallel the variations in chemical characterization and molecular structure in the sample (Krishnakumar et al. 2013). Raman spectroscopy performs vibrational spectroscopy of the tissue content, thus providing immediate real-time histology.
Raman spectroscopy has been successful in differentiating normal tissue from premalignant and malignant tissue in a range of non-oral tumor types, including brain, breast, lower gastrointestinal tract, nasopharynx, skin, lung, and cervix (Guze et al. 2015). In vivo Raman spectroscopy has shown efficacy in the detection of normal tissue, PMDs, cancer, and even of early changes such as cancer field-effects or malignancy-associated changes in the oral cavity (Singh et al. 2012; Krishna et al. 2014). However, the clinical applications of Raman spectroscopy have been limited by both the difficulty of capturing inherently weak tissue Raman signals and the relatively slow speed of spectrum acquisitions (Guze et al. 2009). Because of the technological limitations, research using Raman spectroscopy has mostly been confined to either ex vivo studies of tissue samples or in vivo studies in animal models.
Elastic scattering spectroscopyElastic scattering spectroscopy (ESS) is an emerging technique for generating a wavelength spectrum that reflects structural and morphological changes within tissues (Bigio and Mourant 1997; Green et al. 2014). ESS has been shown to be sensitive to nuclear size, chromatin content, and nuclear/cytoplasmic ratio—all of which characteristics are of interest to the histopathologist who is screening for malignancy within tissue (Wallace and Van Dam 2000). ESS has rarely been used in studies of premalignant and malignant oral tissue. Sharwani et al. (2006b) compared the findings of ESS with the histopathology of oral leukoplakia in 25 patients, reporting a sensitivity of 72% and a specificity of 75%.
Diffuse reflectance spectroscopyIn diffuse reflectance spectroscopy (DRS), white light entering the tissue undergoes a combination of multiple elastic scattering and absorption. The diffusely reflected emanating light is greatly affected by tissue morphology, such as nuclear size, distribution, epithelial thickness, collagen content, and the amount of oxy- and deoxy-hemoglobin in the exposed tissue, all of which can vary during carcinogenesis of epithelial tissue (Schwarz et al. 2008; Jayanthi et al. 2011). A current DRS system typically consists of an imaging device for recording diffuse reflectance images and a fiber-optic probe for relaying light to and from the instrument (Yu et al. 2014).
Some studies have showed that information about tissue transformation, obtained in vivo with the aid of the oxygenated hemoglobin spectral ratio (R545/R575) algorithm, was able to differentiate normal mucosa, hyperplasia, dysplasia, and OSCC with high sensitivity and specificity (Table 1) (Mallia et al. 2008, 2010; Jayanthi et al. 2011). Moreover, Stephen et al. (2013) suggested that DRS could be very effective as a screening tool for the location of oral PMDs.
Narrow-band imagingNarrow-band imaging (NBI) is based on the depth of light penetration. The narrow-band blue light with a short wavelength (415 nm) penetrates into the mucosa and highlights the superficial capillaries as brown in color, while another wavelength (540 nm) identifies prominent vessels in the submucosal layer as cyan (Tan et al. 2012). The reflected light is captured by a monochromatic charge-coupled device located at the tip of the endoscope; a colored composite image is then created by the image processor, which is displayed on a high-definition video screen (Piazza et al. 2008). Potentially malignant and malignant lesions have distinct microvascular morphologies, as angiogenesis occurs at an early stage of carcinogenesis (Fujii et al. 2010). Under NBI, these lesions appear as scattered spots with well-demarcated borders. Moreover, the regular capillary arrangement is lost (Tan et al. 2012; Green et al. 2014). NBI highlights abnormalities in the superficial vasculature of mucosal lesions so that precancerous or cancerous lesions can be identified more easily (Tan et al. 2012).
Yang et al. (2012a, b; 2013) showed that NBI is a promising non-invasive tool for the evaluation and management of oral leukoplakia. NBI can capture the twisted elongation of intrapapillary capillary loops (IPCL). IPCL pattern destruction can be a useful indicator of the severity of oral leukoplakia. Nguyen et al. (2013) conducted a prospective study involving 73 patients with head and neck cancer and found that the sensitivity for detecting moderate dysplasia or worse was 96% with NBI—better than white light, which achieved only 38%. However, chronic inflammation and postoperative radiotherapy may lead to false positivity, and this may be exacerbated if the operator lacks experience in recognizing the different IPCL changes associated with inflammatory and neoplastic lesions (Bhatia et al. 2013).
Optical coherence tomographyOptical coherence tomography (OCT) is an evolving optical technology that produces cross-sectional images of tissue with a high spatial resolution of 10-20 µm (Fercher et al. 1993). It has most often been compared with ultrasonic imaging. Both technologies employ back-scattered signals reflected from different layers within the tissue to reconstruct structural images, with the latter measuring sound rather than light. The high spatial resolution of OCT enables “optical biopsy” and provides immediate and localized diagnostic information.
This technique is capable of imaging tissue depths of up to 1-2 mm and is thus considered suitable for imaging oral mucosal lesions (Fujimoto et al. 1995), as the normal human oral mucosa is very thin, ranging from 0.2-1 mm (Wilder-Smith et al. 2004, 2009). In a study of 50 patients with suspicious lesions, including oral leukoplakia and erythroplakia, the sensitivity and specificity of OCT were 93-97%, showing the excellent capability of in vivo OCT for the detection and diagnosis of oral PMDs and oral cancer (Wilder-Smith et al. 2009). In another ex vivo study, Jerjes et al. (2010) confirmed the feasibility of using OCT to identify architectural changes in an abnormal lesion area; unfortunately, it was unable to provide a diagnosis or to differentiate between lesions. Moreover, there are still limitations regarding the application of OCT: (1) a histopathologist is needed to interpret the result, which is subjective, as OCT does not provide quantitative information; (2) only a small area can be examined at a time, because of the small size of the OCT probe (Olivo et al. 2011).
Several types of particulate contrast agent, such as air-filled microbubbles, engineered microspheres, and gold nanostructures, have been developed to improve the OCT image by enhancing the intensity of backscattered light from the tissue (Barton et al. 2002). In addition, an OCT system can be readily combined with nonlinear optical modalities, such as two-photon excited fluorescence and second-harmonic generation. These combined techniques have been found to yield increased sensitivity and specificity in the diagnosis of oral dysplasia and cancer (Wilder-Smith et al. 2005).
Confocal laser endomicroscopyConfocal laser endomicroscopy (CLE) is an adaptation of white-light microscopy in which tissue and cellular structures are visualized by stimulating the emission of light using a low-power laser beam after the application of fluorescent contrast agents (Wright and Wright 2002). The image of the scanned region can be constructed and digitized by measuring the light returning to the detector. A series of confocal images can be used to observe fine cellular or subcellular structures, and three-dimensional structures in the specimen can also be reconstructed—thus CLE provides a tool for “optical biopsy” in the clinic (De Palma 2009).
CLE holds promise for image-guided surgery by aiding the assessment of lesion margins during surgical procedures (Nguyen et al. 2009; Pogorzelski et al. 2012). Recently, Nathan et al. (2014) conducted a prospective study of 21 patients with leukoplakia and cancerous head and neck lesions; the results showed that the diagnosis based on CLE correlated well with histopathology.
Confocal reflectance microscopyConfocal reflectance microscopy (CRM) is a new optical imaging method that can provide detailed images of the tissue structure and cellular morphology of living tissue in real time. CRM is based on the mechanism of backscattering of light. This is primarily determined by natural differences in the refractive indices of organelles and other subcellular structures within the tissues (White et al. 2004). Basically, the CRM set consists of a light source, a condenser, an objective lens, and a detector. A tightly focused beam of light is focused into a small spot at a chosen depth within a sample. Only the light from the chosen spot at the chosen depth is in focus at the pinhole and is able to pass through it unimpeded (Gerger et al. 2006). Theoretically, in vivo confocal imaging resembles histological tissue evaluation, except that three-dimensional subcellular resolution can be achieved non-invasively without stains (Maitland et al. 2008).
Parameters extracted from confocal images were used to discriminate high-grade cervical pre-cancers with a sensitivity of 100% and a specificity of 91% in a study of 25 samples (Collier et al. 2002). In vivo CRM has already been applied to oral mucosa. The researchers suggested the potential of CRM in the clinical evaluation of oral lesions, early diagnosis of oral PMDs and oral cancer, real-time identification of tumor margins, and monitoring of the response to therapeutic treatment (Clark et al. 2003; Anuthama et al. 2010). However, in an pilot study, when the researchers examined the microscopic anatomy of freshly excised head and neck surgical specimens using CRM, and compared the findings with those generated by conventional histologic analysis, they found that there were fewer microscopic details visible in the CRM images than in paraffin-embedded histologic sections (White et al. 2004).
Advanced research exploring molecular markers for diagnosis and risk predictions appears to be promising. Salivary biomarker test might be a non-invasive, rapid adjunctive aid for revealing malignant transformation in oral PMDs. Zahran et al. (2015) found that miRNA-184 could distinguish OSCC from oral PMD with dysplasia. Sharma et al. (2011) assessed salivary interleukin-6 (IL-6) as a marker for malignant transformation of leukoplakia. IL-6 level was found to increase with the severity of dysplasia, but periodontitis and tobacco use also contributed to an elevated IL-6 level.
In this review article, the authors have critically described the non-invasive detection techniques for oral PMDs and summarized the range of sensitivity and specificity of each technique in detecting oral PMDs and oral cancer (Table 2). A summary of the advantages, disadvantages and clinical applications/indications of each technique is also provided (Table 3). Latest meta-analysis showed that vital staining had less sensitivity but greater specificity in identifying oral PMDs and oral cancer compared with light-based detection systems. However, given that the studies evaluating optical diagnostic technologies and salivary biomarker were too few, the integral evaluation was not done.
Physicians or dental practitioners at local community medical centers are often encountered with patients with oral PMDs. Early identification of these patients is necessary for cancer prevention and disease management. As oral medicine specialists, we believe that it would be helpful if the doctors at community medical centers could do some screening of these patients. Currently, there is no widely accepted strategy for the application of non-invasive techniques in the detection and diagnosis of oral PMDs. Considering sensitivity, specificity, expenses and feasibility of the techniques mentioned above, we propose a protocol for the detection and diagnosis of oral PMDs at local community hospitals/medical centers (Fig. 2).

Summary of the advantages, disadvantages, and clinical applications/indications of non-invasive detection techniques in the detection and diagnosis of oral PMDs.
TB staining, Toluidine blue staining; RB staining, Rose bengal staining; PDD, Photodynamic diagnosis; ESS, Elastic scattering spectroscopy; DRS, Diffuse reflectance spectroscopy; NBI, Narrow-band imaging; OCT, Optical coherence tomography; CLE, Confocal laser endomicroscopy; CRM, Confocal reflectance microscopy.

Diagnostic protocol of oral potentially malignant disorders (PMDs) proposed for community hospitals/medical centers.
Most of the non-invasive detection techniques evaluated in this review showed great potential for screening and monitoring oral PMDs. But there is insufficient evidence to recommend for or against the standalone use of any method for identifying oral PMDs. Besides, there is no site-specific study and few studies about the combination of plural techniques for the clinical detection and diagnosis of oral PMDs, which could be further explored in the future. Also, well-designed prospective studies with large sample sizes are still needed.
This work was supported by grants from the National Natural Science Foundation of China (No. 81321002, 81472533, 81200791), the Fok Ying Tung Education Foundation (No. 132028) and the Doctoral Program of the Ministry of Education of China (No. 20120181120011).
The authors declare no conflict of interest.