2016 Volume 239 Issue 1 Pages 39-45
2016 Volume 239 Issue 1 Pages 39-45
Central sleep apnea (CSA) is characterized by recurring cycles of crescendo-decrescendo ventilation during sleep, and enhances sympathetic nerve activity. Thus CSA has a prognostic impact in patients with chronic heart failure (CHF). Although nocturnal oxygen (O2) therapy decreases frequency of CSA and improves functional exercise capacity, it is also known that some non-responders to the therapy exist. We thus aimed to identify predictors of responders to nocturnal O2 therapy in CHF patients with CSA. In 12 CHF patients with CSA hospitalized at our department, sleep study was performed at 2 consecutive nights. Patients nasally inhaled O2 at either the first or second night in a randomized manner. To predict the percentage reduction in apnea-hypopnea index (%ΔAHI) in response to the nocturnal O2 therapy, we performed multiple regression analysis with a stepwise method with variables including age, brain-natriuretic peptide, circulation time, baseline AHI, hypercapnic ventilatory response and end-tidal carbon dioxide tension (PETCO2). Nocturnal O2 therapy significantly decreased AHI (from 32 ± 13 /h to 12 ± 10 /h, P < 0.0001). Among the possible predictors, PETCO2 was the only variable that is predictive of % changes in AHI. Receiver operating characteristics analysis determined 4.25% as the optimal cutoff PETCO2 level to identify responder to nocturnal O2 therapy (> 50% reduction of AHI), with 88.9% of sensitivity and 66.7% of specificity. In conclusion, PETCO2 is useful to predict the efficacy of O2 therapy in CHF patients with CSA, providing important information to the current nocturnal O2 therapy.
Central sleep apnea (CSA) is frequently observed in 30-40% of patients with chronic heart failure (CHF) (Bradley and Floras 2003). It has been reported that CSA adversely affects cardiac function through enhancement of neurohumoral factors and sympathetic nerve activity (Bradley and Floras 1996), which worsens morbidity and mortality of patients with CHF (Ancoli-Israel et al. 1994). It is well known that low setpoint of partial pressure of arterial carbon dioxide (PaCO2) plays an important role in the pathogenesis of CSA in CHF (Naughton et al. 1993). In patients with CHF, stimulation of pulmonary vagal irritant receptors by pulmonary congestion induces hyperventilation and decreases a setpoint of PaCO2 (Lorenzi-Filho et al. 2002). In addition, enhanced chemosensitivity in patients with CHF and CSA unstabilizes the control system of ventilation by respiratory center (Javaheri 1999). These mechanisms induce destabilized normal breading, leading to respiratory instability and the rhythmic pattern of breathing referred to as Cheyne-Stokes respiration (Hanly et al. 1989; Bradley and Phillipson 1992). CSA is usually initiated during sleep by further acute increase in ventilation and reduction in PaCO2 (Naughton et al. 1993). When PaCO2 falls below the threshold level that is required to stimulate breathing, CSA occurs and persists until PaCO2 rises above the threshold (Bradley and Phillipson 1992). Nocturnal oxygen (O2) supplementation has been shown to abolish apnea-related hypoxia, alleviate CSA, decrease nocturnal norepinephrine levels over periods of one night to one month, and then improve left ventricular (LV) function and exercise tolerance (Hanly et al. 1989; Sasayama et al. 2006). However, it is also known that some non-responders to O2 therapy exist (Staniforth et al. 1998; Javaheri et al. 1999). In this study, we thus aimed to elucidate predictor(s) for responders to the O2 therapy in CHF patients with CSA.
We confirmed the principles outlined in the Declaration of Helsinki, and the ethical committees of Tohoku University Hospital approved the study protocol (No. 2001-022). All patients provided written informed consent.
Study subjectsWe prospectively enrolled 12 patients with CHF who were referred to our hospital between August 2000 and November 2006. This study included ambulatory patients with persistent impairment of systolic LV function evaluated by echocardiography (LVEF ≤ 50%), who had at least one previous episode of hospitalization due to exacerbation of CHF and central sleep breathing disorder diagnosed by type-1 or type-2 polysomnography previously.
All patients were treated with optimal medical therapies and were in stable clinical condition for at least 3 months. All patients did not complain of snoring or daytime sleepiness. Patients were excluded when they had the following conditions; unstable angina, acute myocardial infarction, congenital heart disease, significant renal, neurological or respiratory disease, and marked obesity (body weight > 100 kg). At the entry of the present study, we obtained demographic data, New York Heart Association (NYHA) functional class, morbidity period, medications (angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blocker (ARB), and β-blockers), and echocardiographic parameters including LV end-diastolic diameter (LVDd), left atrial (LA) diameter, and LVEF. In the present study, hypertension was defined as the use of antihypertensive agents and/or systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 80 mmHg, diabetes mellitus as the use of anti-diabetic agents, fasting glucose ≥ 110 mg/dl and/or glucose ≥ 200 mg/dl 2 hours after a 75 g oral glucose tolerance test, and dyslipidemia as the use of lipid-lowering agents, plasma low-density lipoprotein (LDL) cholesterol ≥ 140 mg/dl, triglycerides ≥ 150 mg/dl and/or high-density lipoprotein (HDL) < 40 mg/dl. We performed venous and arterial blood tests for measurement of brain-natriuretic peptide (BNP) and arterial-blood gases before first sleep study, echocardiography, sleep study, and ventilatory response tests to carbon dioxide (CO2).
Sleep studyThe protocol was designed as a crossover style. All 12 Patients were assigned to the sleep study with and without overnight O2 inhalation at consecutive 2 nights in a randomized order. Flow rate of O2 therapy was 3 L/min via nasal cannula. All patients underwent overnight sleep study with type 3 portable monitor that recorded cardiotachography, norsal-oral air flow, chest and abdominal effort and pulse oximetry (Morpheus, Teijin Ltd, Japan) (Chesson et al. 2003). Oronasal airflow was measured by thermister. Thoraco-abdominal movement was assessed using calibrated respiratory effort bands. Oxygen saturation pulse (SpO2) was recorded via a finger electrode. Two-channel electrocardiograms (CM5 and NASA) digitalized with a 16-bit were recorded with a sampling frequency of 256 Hz. Apnea was defined as cessation of airflow lasting at least for 10 sec. Central apnea was defined as the absence of flow and thoraco-abdominal movement and obstructive apnea as the absence of airflow in presence of thoracoabdominal movements. Hypopnea was defined as more than 50% decrease in the sum of thoraco-abdominal movements lasting for more than 10 sec, followed by a reduction in SpO2 of at least 3%. The apnea-hypopnea index (AHI) is expressed as a total number of apnea and hypopnea divided by total sleep time per hour. The number of episodes of obstructive apnea and hypopnea per hour is referred to as the obstructive AHI, and the number of episodes of central apnea and hypopnea per hour is referred to as the central AHI. We accepted a threshold of AHI ≧ 5/h as diagnostic for sleep-disorder breathing, and defined AHI ≥ 10 as the guidance to introduce the respiratory therapy (American Academy of Sleep Medicine Task Force 1999). CSA was defined as AHI > 10/h, whereby 75% of all apnea-hypopnea events were central origin. Number of event times of ≥ 3% fall in SpO2 per hour was defined as oxygen desaturation index (3% O2 desaturation index). We also measured the circulation time, which was defined as the time from the end of apnea to the nadir of SpO2 on the recordings of sleep study (Kwon et al. 2014). To estimate urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 pm to 6:00 am during sleep study was collected using containers acidified with 20 ml of 6 M hydrochloric acid.
EchocardiographyTwo-dimensional echocardiography with Doppler ultrasound examination was performed by standard techniques following the American Society of Echocardiography guidelines (Sin et al. 1999). LVEF was measured according to the Teichholz’s or modified Simpson’s method. LA diameter and LVDd were also measured by M-mode and corrected by 2D echocardiography.
Ventilatory response tests to carbon dioxideBefore the sleep study, the ventilatory tests were performed (Tun et al. 2000). Measurements were started several minutes after a steady state had been achieved, as evidenced by the finding of a stable end-tidal partial CO2 pressure. The end-tidal CO2 tension (PETCO2) was defined as the mean of 10 consecutive measurements with a mass spectrometer (WSMR-100, Westron, Chiba), before hypercapnic ventilatory response test. The hyperoxic hypercapnic ventilatory response was determined by Read’s rebreathing method (Read 1967). Arterial O2 saturation was monitored continuously with a finger pulse oximeter. The ventilatory response to hypercapnia was expressed as the regression line of ventilation against the end-tidal CO2 (ΔVE/ΔPETCO2).
Statistical analysisAll data are expressed as means ± SD unless otherwise mentioned. Among the sleep study data before and after O2 therapy, a paired t-test was used as post hoc test. To predict the percentage reduction in AHI (%ΔAHI) after O2 therapy, multiple regression analysis with a stepwise method was performed with variables of age, BNP, circulation time, baseline AHI, hypercapnic ventilatory response and PETCO2, all of which are known to be associated with the development of CSA and the severity of sleep disordered breathing (SDB) and CHF (Bradley and Floras 2003). The relation between %ΔAHI and variables was examined by using the Pearson correlation test. A two-tailed P value < 0.05 was considered as statistically significant. We defined the patient with > 50% reduction in AHI after O2 therapy as the responder. The diagnostic accuracy of the responder was evaluated using the receiver operating characteristics (ROC) curve. Area under the curve was calculated and cut-off diagnostic threshold value was chosen that yielded the highest combined sensitivity and specificity for the value of variables predicting the responder. All analyses were performed with JMP Pro 11.0.0 (SAS Institute Inc., Cary, NC, USA).
The characteristics of the 12 patients are shown in Table 1. The underlying cause of CHF was dilated cardiomyopathy in 6 patients, coronary artery disease in 3, valvular heart disease in 2 and hypertrophic cardiomyopathy in one. They were treated with optimal medical therapy, including β-blockers (72%) and ACE inhibitors/ARB (91%). All patients had impaired EF (mean 33.6%). The mean values for awake PaO2 and PaCO2 were within normal range.
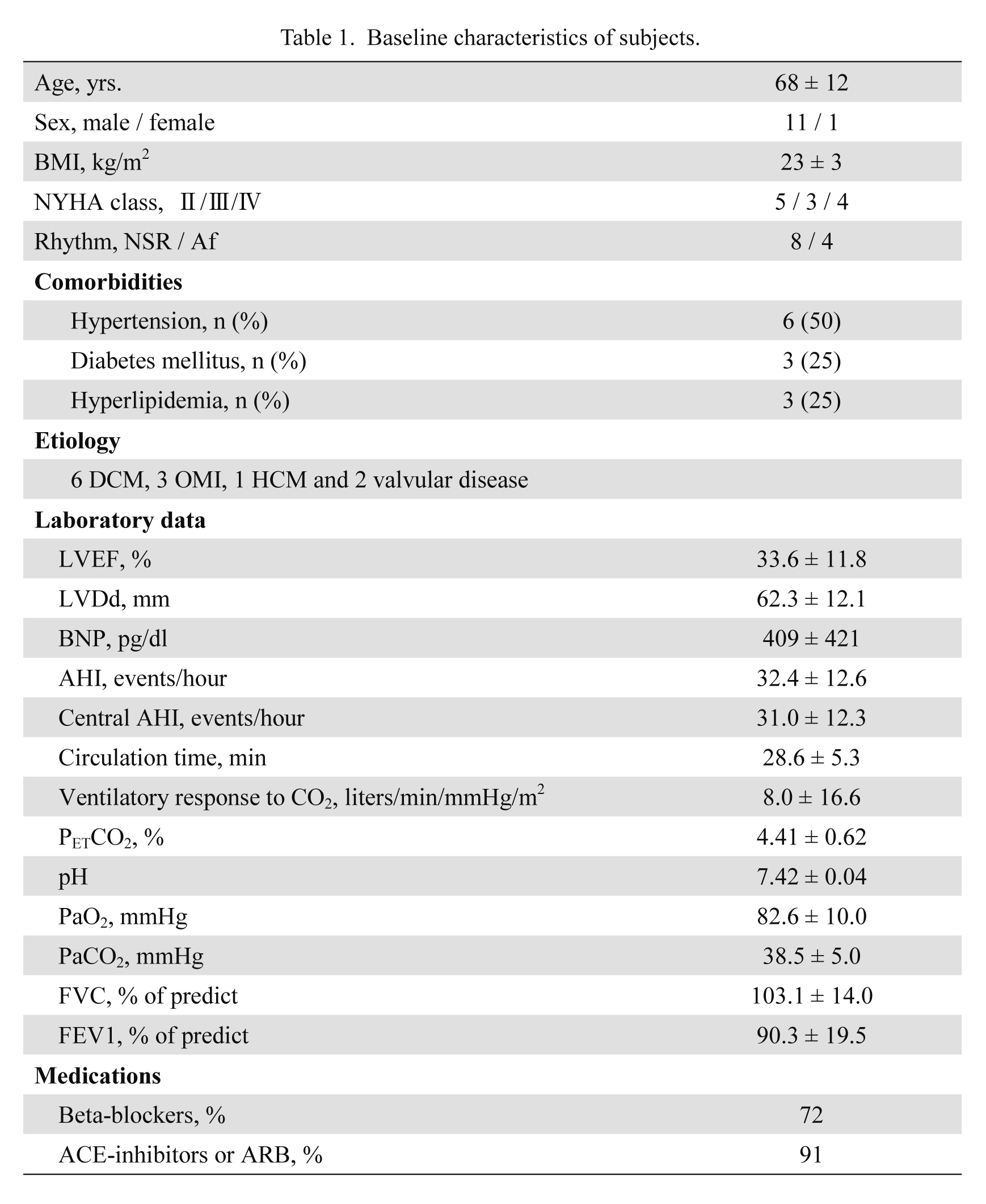
Baseline characteristics of subjects.
Results are shown as mean ± SD.
DCM, dilated cardiomyopathy; OMI, old myocardial infarction; HCM, hypertrophic cardiomyopathy; NSR, normal sinus rhythm; Af, atrial fibrillation; BMI, body mass index; LVEF, left ventricular ejection fraction; LVDd, left ventricular end-diastolic dimension; BNP, brain natriuretic peptide; AHI, apnea hypopnea index; PETCO2, end-tidal carbon dioxide tension; PaO2, partial pressure of O2 in arterial blood; PaCO2, partial pressure of CO2 in arterial blood; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
The sleep study data are shown in Table 2. In the present study, the patients had relatively severe breathing disorder during sleep with an average AHI of 32 per hour, mainly consisted of CSA. The O2 therapy significantly improved AHI (P < 0.001), central AHI (P < 0.001) (Fig. 1), 3% O2 desaturation index (P < 0.001) and % time < SpO2 90%/total sleep time (P < 0.05) in all patients without exacerbating OSA. The O2 therapy also significantly reduced heart rate and overnight urinary excretion of norepinephrine, but not that of epinephrine.
All patients were treated with nocturnal O2 therapy and visited our outpatient clinic every 4-8 weeks for follow-up (median follow-up period of 5.7 years). There were 10 deaths during the follow-up, including progressive HF death in 6, pneumonia in 2, and lung cancer in 2. We followed 2 survivors in our hospital; one was the responder, and the other non-responder. There was no significant difference in the long-term prognosis between the responder and the non-responder groups in the present study.
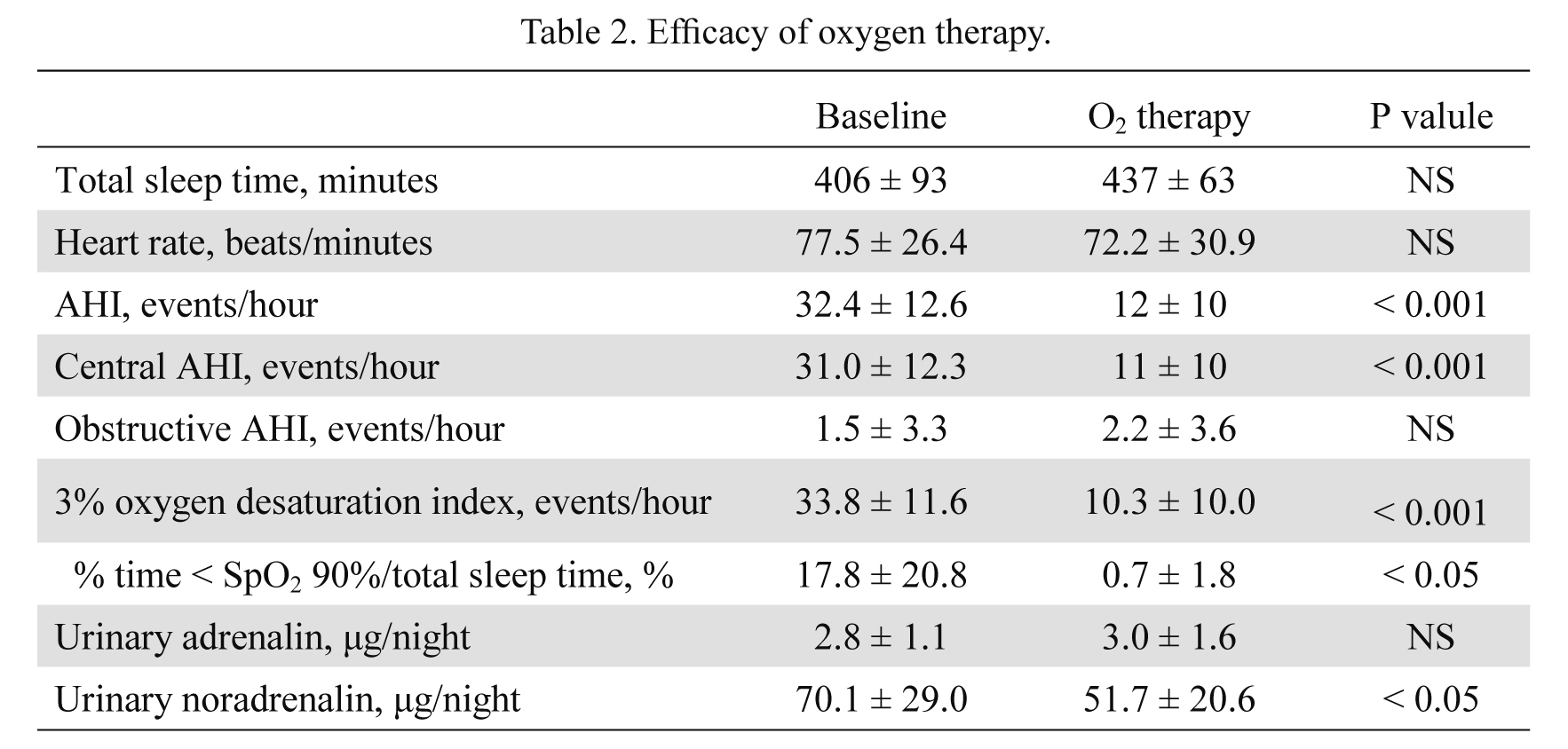
Efficacy of oxygen therapy.
All results given as mean ± SD.
AHI, apnea hypopnea index; NS, not significant.
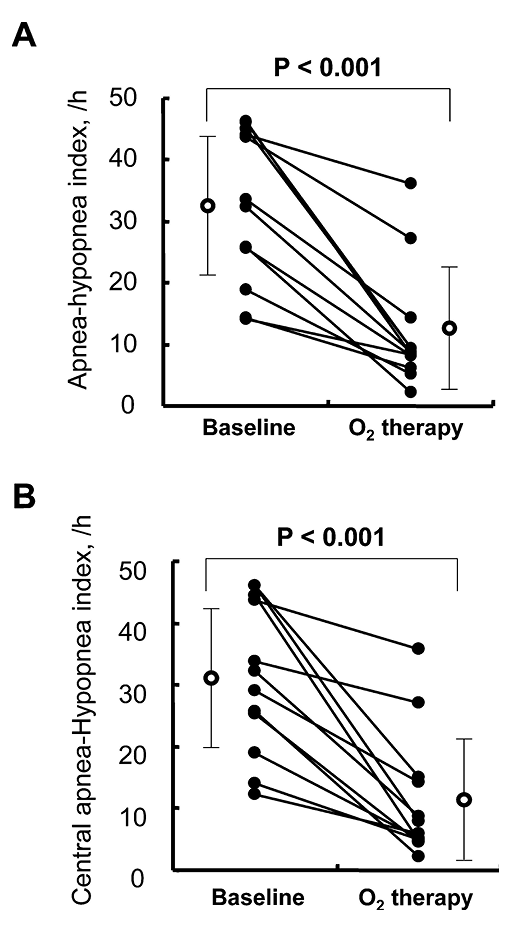
Effects of nocturnal O2 therapy in CHF patients with CSA.
The O2 therapy significantly improved the apnea-hypopnea index (A) and the central apnea-hypopnea index (B).
Multivariate regression analysis with stepwise method showed that among the variables examined, only PETCO2 significantly contributed to the variance of %ΔAHI (Table 3). There was a significant correlation between %ΔAHI and PETCO2 (Fig. 2). The ROC analysis of PETCO2 for 50% reduction in AHI showed the greatest area under the curve of 0.833 (P < 0.05), with an optimal cut-off value of 4.25%, yielding a sensitivity of 88.9% and a specificity of 66.7% (Fig. 3).
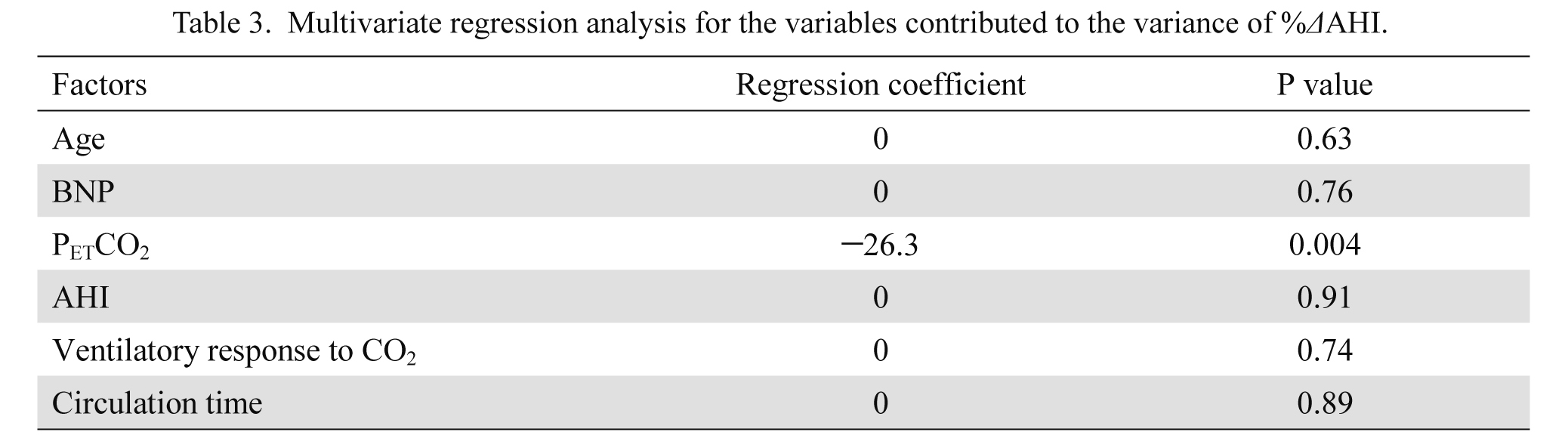
Multivariate regression analysis for the variables contributed to the variance of %ΔAHI.
BNP, brain natriuretic peptide; PETCO2, end-tidal carbon dioxide tension; AHI, apnea hypopnea index; CO2, carbon dioxide.
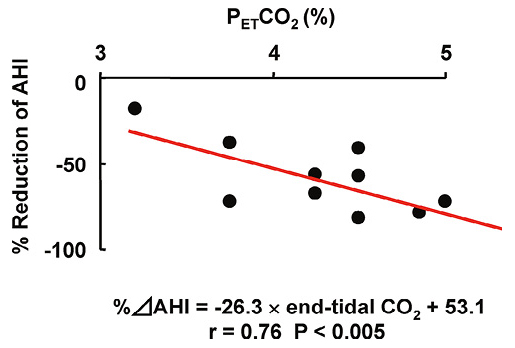
Correlation between %ΔAHI and end-tidal CO2 tension.
%ΔAHI significantly correlated with end-tidal CO2 (r = 0.76, P < 0.005). The relationship between %ΔAHI and end-tidal CO2 was expressed as %ΔAHI = −26.3 × end-tidal CO2 + 53.1.
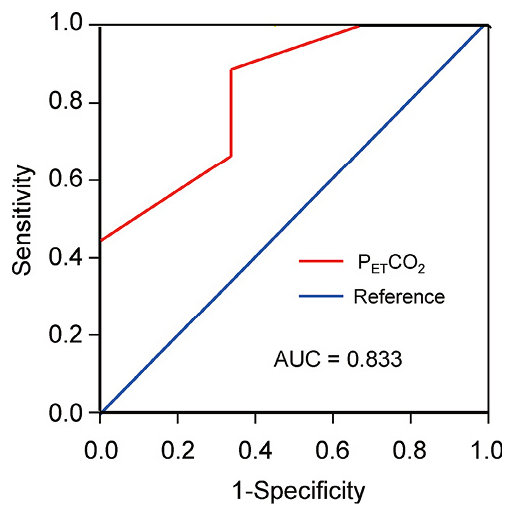
Receiver operating characteristics curves of end-tidal CO2 levels in detecting effect of nocturnal O2 therapy.
The receiver operating characteristics (ROC) analysis of end-tidal CO2 levels in predicting %ΔAHI showed an area under the curve (AUC) of 0.833.
AUC, area under the curve.
The novel finding of the present study is that end-tidal CO2 is predictive of the individual response to nocturnal O2 therapy in CHF patients with CSA. In the present study, one night O2 therapy decreased AHI in those patients. %ΔAHI, which represents the value of effectiveness of O2 therapy, was inversely correlated with awake end-tidal CO2. These results suggest that the set points of PaCO2 may determine the responsiveness to the O2 therapy in CHF patients with CSA.
O2 therapy for CSAIt was reported that CSA, unlike OSA, might not have adverse hemodynamics effects in patients with CHF (Yumino et al. 2013). Meanwhile nearly half of CHF patients have evidence of CSA, which was reported to be contributed to increased mortality in those patients through enhancement of neurohumoral factors and sympathetic nerve activity (Bradley and Floras 1996; Sin et al. 1999). In patients with CHF, nocturnal O2 therapy improves not only CSA (Javaheri et al. 1999) but cardiac function and quality of life, and then decreases sympathetic nerve activity (Sasayama et al. 2006). In the CANPAP study, a randomized, continuous positive airway pressure (CPAP) had no effect on heart transplant-free survival of CHF patients with CSA (Bradley et al. 2005). The subsequent analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) study showed that the prognosis was improved in patients who achieved AHI < 15 in response to CPAP (Arzt et al. 2007). Although we did not examine the therapeutic effect of O2 therapy on CSA between different concentrations in this study, O2 therapy at a flow rate of 3 L/min via nasal cannula decreased AHI and urinary catecholamine excretion in patients with CHF and CSA. However, 2 patients did not achieve AHI < 15, and 3 patients did not achieve %ΔAHI < −50%. It was also reported that in 61% of CHF patients, CSR was not fully improved by nocturnal O2 therapy (Javaheri et al. 1999). Thus, it is important to elucidate the predictor of the responsiveness to O2 therapy in CHF patients with CSA. There was no significant difference in the long-term prognosis between the responder and the non-responder groups in the present study.
Predictors of response to O2 therapyIn patients with CHF, three main factors are currently considered to interact and lead to respiratory instability, including hyperventilation, circulatory delay and cerebrovascular reactivity (Bradley and Floras 2003). In the present study, we performed multiple regression analyses to examine the relationship between %ΔAHI and cardiac function (BNP, EF and circulation time) and cerebrovascular reactivity to CO2 (hypercapnic ventilatory response). However, our results showed that there was no significant relationship between %ΔAHI and, cardiac function or cerebrovascular reactivity to CO2, suggesting that these indices may not have definitive influence on the response to O2 therapy in CHF patients with CSA.
PETCO2The level of PaCO2 is an important factor in the pathogenesis of CSA in patients with CHF (Sin et al. 1999). The chronic hyperventilation observed in CHF patients with CSA has been proposed to drive the PaCO2 toward the hypocapnic apnea threshold, thereby predisposing the patients to periodic breathing (Naughton et al. 1993). In this study, PaCO2 was not associated with %ΔAHI, a consistent finding with the previous report that there were no significant correlation between PaCO2 and change in AHI in O2 therapy (Javaheri et al. 1999). However, in the present study, we found a significant correlation between %ΔAHI and PETCO2. Although PETCO2 usually is correlated with PaCO2, it is difficult to accurately measure PaCO2 at one point even in the daytime because of instability of ventilation in patients with severe CHF. In the present study, we measured PETCO2 non-invasively at 10 times in stable breathing and used the average value to examine the relationship with O2 therapy. Thus, PETCO2 might reflect the CO2 stimulation of central chemoreceptors in CHF patients compared with PaCO2.
In addition, we measured PETCO2 in the daytime and did not examine the change in PETCO2 from wakefulness to sleep. It was reported that CHF patients with CSA showed no rise in PETCO2 from wakefulness to sleep unlike patients without CSA whose PETCO2 increases during sleep (Xie et al. 2002). This means the narrowed proximity of the eupneic PaCO2 to the apnea threshold, which may be a crucial determinant of the susceptibility to apnea. On the other hand, hypercapnia in the daytime was reported to be an important determinant of CSA in CHF patients (Naughton et al. 1993).
Study limitationsSeveral limitations should be mentioned for the present study. First, our diagnosis and classification of SDB were based on type 3 cardiorespiratory polygraphy, but not on polysomnography that is considered as the gold standard to diagnose SDB. Second, the present study had a relatively small number of patients in a single center nearly 10 years ago with a time span for the patient enrollment. Recently, the confirmatory, multicenter, randomized, controlled study of adaptive servo-ventilation (ASV) in patients with chronic heart failure (SAVIOR-C) showed that ASV therapy was not superior to guideline-directed medical therapy in the cardiac function-improving effect, and an interim analysis of the SERVE-HF Study found a statistically significant 2.5% increase in annual risk of cardiovascular mortality for those with LVEF ≤ 45% compared with the control group (Cowie et al. 2015). In response to the results, we thought that nocturnal O2 therapy became more important in the management of CHF with SDB. Third, the impact of O2 therapy for prognosis of patients with CHF and SDB is very important topic. However, the long-term prognosis remains to be examined because of insufficient statistical power in this study. Thus, the present findings need to be confirmed in future multi-center studies with a large number of patients.
In conclusion, in the present study, we are able to demonstrate that PETCO2 may identify responders to O2 therapy in CHF patients with CSA, providing important information on the current nocturnal O2 therapy.
This work was supported in part by the Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (No. 09470158).
The authors declare no conflict of interest.