2016 Volume 239 Issue 1 Pages 57-66
2016 Volume 239 Issue 1 Pages 57-66
The fragile X mental retardation 1 (FMR1) gene contains a highly polymorphic trinucleotide (CGG) repeat and consists of various allelic forms. Traditionally, 55-200 repeats and over 200 CGG repeats have been highlighted to be associated with ovarian dysfunction and neuro-psychiatric risks. However, previous studies had paid little attention to the allelic forms of 5-55 CGG repeats. Herein, we sought to evaluate the pathological features of FMR1 allelic category with a range of 5-55 CGG repeats. We further classified the spectrum of CGG sizes (5-55 repeats) into three sub-groups as low numbers of CGG repeat (< 26 repeats), normal CGG count (26-34 repeats), and small CGG expansion (35-54 repeats). Our systematic review documented that low numbers of CGG repeat (< 26 repeats) revealed a close relationship with premature ovarian failure. Correspondingly, the meta-analysis showed that small CGG expansion, involving allelic sizes with 35-54 (n = 8, OR = 1.22, 95% CI: 0.75-2.00, P > 0.05) and 41-54 (n = 7, OR = 1.62, 95% CI: 1.14-2.30, P < 0.05), was both linked to the risk of ovarian dysfunction. Additionally, small CGG expansion exerts significant influence on male Parkinsonism cohorts (OR = 2.17, 95% CI: 1.50-3.14, P < 0.05), mental retardation, and repeat instability. Our data provide evidence that the CGG-repeat numbers below 26 or above 34 of FMR1 gene are also associated with disease risks and thus should be regarded as pathological genotypes for a routine test.
The fragile X mental retardation 1 (FMR1) gene carries unstable CGG expansion in its 5’untranslated region that is associated with a large number of disorders (Groh et al. 2014). Based on the CGG repeat numbers, traditional expansion of the FMR1 gene is classified as a normal range (CGG: 5-55), premutation (CGG: 55-200) and full mutation (CGG > 200). One of the most common diseases is fragile X syndrome (FXS) caused by CGG full mutation, and the absence of fragile X mental retardation protein (FMRP) contributes to the clinical phenotypes of FXS, such as cognitive deficits, behavioral problems and connective tissue disorders (Garber et al. 2008). Besides, our knowledge of clinical involvement in premutation carriers is also prevalent. Premutation patients showed a broad range of neurological, neurocognitive, endocrine and psychiatric problems related to RNA toxicity (Hagerman and Hagerman 2013; Kraan et al. 2013), and they could present two other obvious disorders: fragile X-related primary ovarian insufficiency (FXPOI) and fragile X-associated tremor/ataxia syndrome (FXTAS) (Tassone et al. 2012; Hagerman and Hagerman 2013).
However, apart from full mutation and premutation, other CGG expansion ranges are also involved in disease phenotypes and cause severe clinical symptoms. Since Fu et al. (1991) had demonstrated that the distribution pattern for triple CGG repeat in a general population between 29 and 30 was ubiquitous, many researchers further analyzed the CGG expansion numbers by detecting the scale outside the range of 26-34, and found that CGG repeat under 26 could be associated with reproductive diseases. Gleicher and his colleagues indicated females with the low numbers of CGG repeat were predisposed to early ovarian aging (Gleicher et al. 2009, 2010b, 2011). This allelic form was also correlated with BRCA1/2 mutations, which may reflect potential increased cancer risks and embryo-lethality (Weghofer et al. 2012). In addition, the significance of CGG numbers below 26 had been implicated in neuropsychiatric biology. Nagamani et al. (2012) reported that the loss of FMR1 copy counts could cause pathological neurodevelopment phenotypes. Mailick et al. (2014) compared adults with the low numbers of CGG repeat to normal age-peers, discovering that both men and women with CGG counts < 26 had higher risks of cognition disability and mental health.
Other studies also offered clues about potential negative effects of small CGG expansion in reproduction. Small CGG expansion was known as intermediate alleles and gray zone as well. The earliest papers had presented that a high risk factor for premature ovarian failure can be observed from small CGG expansion (CGG: 35-54) to premutation status (CGG: 55-200) (Fu et al. 1991; Conway et al. 1998; Bretherick et al. 2005; Allen et al. 2007; Wittenberger et al. 2007). Recent studies also indicated that intermediate alleles should be responsible for ovarian dysfunction (Pastore et al. 2012; Barasoain et al. 2013; De Geyter et al. 2014; Guo et al. 2014). Furthermore, the increased transcriptional activity was found in population with small CGG expansion relative to individuals with normal CGG repeat (Loesch et al. 2007), in combination with the transmission instability of CGG repeat close to 55 (Fernandez-Carvajal et al. 2009). Thus, there may be undesirable phenotypes correlated with the destructive ‘‘gain-of-function’’ effect of small CGG expansion.
These arguments suggest that some allelic forms of CGG repeat length, deviating from 26 to 34, may have pathological significance. Therefore, more attention should be focused on the other end of the spectrum, namely low numbers of CGG repeat (CGG < 26) and small CGG expansion (35-54). Normal CGG range was here defined as 26-34 (median 30). The research primarily continued to refine our understanding the significance of the low numbers of CGG repeat with a systematic review and of small CGG expansion with a meta-analysis in phenotypic associations. We revealed a broad range of overlapping pathological phenotypes observed in affected individuals, which will illuminate the mechanisms of underlying disorders with abnormal CGG expansion and provide therapeutic approaches.
A systematic review and meta-analysis were carried out according to the flowchart of PRISMA statement (Preferred Reporting Items for Systematic reviews and Meta-Analysis) guidelines (Hutton et al. 2015). Official institutional review board approval was not necessary due to this analysis that consisted of mass of published studies.
Eligibility criteriaStudies were selected based on the following criteria: 1, the articles were incorporated if they analyzed the association between low numbers of CGG repeat (< 26) and disorder phenotypes, or between small CGG expansion (35-54) and ovarian dysfunction. Primary ovarian insufficiency (POI) is a comprehensive clinical spectrum related to early aging of the ovaries. Diminished ovarian reserve and premature ovarian failure both belong to the definition of POI (Pastore et al. 2012). They are all defined as ovarian dysfunction. 2. Diminished ovarian reserve is defined as the concentration of FSH > 10 m IU/ml for 2-5 days; or taking 100 mg clomiphene citrate medicine, with FSH > 12 m IU/ml after 5 days. Whereas premature ovarian failure is diagnosed as the cessation of the menstrual cycle for 4 months before the age of 40 years, with a high concentration of FSH > 40 IU/L, and detects on average twice 1-month interval. The diagnosis of POI is accorded with diminished ovarian reserve and premature ovarian failure.
Search and selection strategy and study quality assessmentThe literatures were retrieved to determine studies on the value of the low numbers of CGG repeat and small CGG expansion in disorders phenotypic associations. We performed searches in PubMed, Medline, Google Scholar, and Web of Science, using combination of the following keywords: CGG repeat, CGG expansion, CGG counts, low repeat, small expansion, intermediate alleles, gray zone, premature ovarian senescence, primary ovarian failure, POI, ovarian dysfunction, diminished ovarian reserve, infertility, or premature menopause. The included studies were limited to July 2015. We assessed methodological quality by Strengthening the Reporting of Observational studies in Epidemiology (STROBE), which provided items on observational studies such as cohort studies, case controls and cross sectional studies (Vandenbroucke et al. 2014). Any studies that could probably exhibit the association between the low numbers of CGG repeat or small CGG expansion and phenotypic associations were preselected. The references of the selected articles were necessary to identify additional possible relevant studies.
The studies of low numbers of CGG repeat describing the disease phenotypic associations were retrieved by the search read, and from which the relevant first author, publication year, ethnicity, and pathological phenotypes were extracted (Table 1). While first author, publication year, location, sample size, disorders form, and alleles size were extracted from each study with small CGG expansion (Table 2). If data were vacant in published articles, complementary information was necessary to contact with the corresponding authors. The meta-analysis was conducted when three or more studies were related with disorder phenotypes.

The association between low numbers of CGG repeat and phenotypic disorders.

Characteristics of small CGG expansion with ovarian dysfunction.
POI, primary ovarian insufficiency; DOR, diminished ovarian reserve; POF, premature ovarian failure.
As the low numbers of CGG repeat with the disorder phenotypes were considerable heterogeneity in statistical methodology used, we considered that it was inappropriate to collect the data into a meta-analysis, and presented it in summary form instead (Table 1). Statistics were analyzed using Review Manager 5.0. The association between small CGG expansion and the risk of ovarian dysfunction was expressed using odds ratios (OR) and 95% confidence intervals (CI). The statistical implication of combined OR was assessed using Z-test. The meta-analysis was stratified by allelic sizes. A test of heterogeneity among the articles was conducted using a Q-test that was based on X2 (Zamora et al. 2006). Statistical heterogeneity was evaluated using I2, and I2 > 50% was regarded as considerable heterogeneity (Cochrane Collaboration. 2011). Considering existent heterogeneity was detected, the random effects model was applied rather than a fixed effects model. The significance analysis was performed by t-test, and P < 0.05 was considered significant.
The systematic literature retrieval through PubMed, Medline, and Google Scholar yielded 1,088 articles. Among them, 1,010 articles were removed by scanning the titles or abstracts, and 78 articles were evaluated fully for eligibility (Fig. 1). Of these, 29 studies were related with the low numbers of CGG repeat, while 58 studies were associated with small CGG expansion. There were 9 duplicates among them. After screening full-text articles, 9 of the 29 studies described the association between low numbers of CGG repeat and disorder phenotypes. Out of the 58 studies with small CGG expansion, 10 eligible studies related to ovarian dysfunction were selected and subjected to a meta-analysis. The characteristics of the incorporated studies are shown in Table 2. As shown, diminished ovarian reserve and premature ovarian failure, defined as POI, are all associated with ovarian dysfunction (Pastore et al. 2012). Therefore, we primarily analyzed the association between different allelic sizes of small CGG expansion and ovarian dysfunction. Consequently, studies were classified into allelic size with 35-54 (n = 8) and 41-54 (n = 7), and there were 5 articles are duplicates among them because of the extraction of various allelic sizes in one literature as possible (Fig. 1). In addition, we performed a subgroup analysis and categorized the allelic size with 35-54 into 2 groups: one group comprised 4 studies of premature ovarian failure, the other group comprised 4 studies of POI (Fig. 1).

Study flow chart. There are 5 articles that are duplicates among allelic size with 35-54 and 41-54 because of the extraction of various allelic sizes in one literature as possible. POF, premature ovarian failure; POI, primary ovarian insufficiency.
The 5 of 9 retrieved papers emphasized on relationship between FMR1 low genotypes and BRCA1/2 mutations. Weghofer et al. (2012) investigated 99 BRCA1/2 mutation-positive women and 410 female controls to explore the distribution of FMR1 genotypes. The results showed that women with heterozygous genotypes, in which one allele is normal and the other is below the normal range, also known as low FMR1 sub-genotypes, were correlated with BRCA1/2 mutations. FMR1 low genotypes could reflect potential increased cancer risks and embryo-lethality (Weghofer et al. 2012). However, the other 4 studies were discordant with the observation.
Other literatures discussed diverse pathological phenotypes of the low numbers of CGG repeat. The 3 studies identified that CGG counts closed to the distribution of normal range (26-34) can imply lifelong normal ovarian function, while deviation from the range indicated elevated risks towards ovarian diseases. Among them, Gleicher and his colleagues, with 316 consecutive infertility patients, specified that CGG repeat counts < 28 would increase the likelihood of diminished ovarian reserve (Gleicher et al. 2009). Then they further assessed the ovarian reserve by anti-mullerian hormone, and the results presented abnormal heterozygous and homozygous CGG numbers, especially FMR1 low genotypes, would reduce ovarian reserve at younger ages (Gleicher et al. 2010a). Moreover, a cross-sectional study explored the association between CGG expansion and premature ovarian senescence, showing that CGG counts < 28 may be both the molecular-genetic and autoimmune factors of premature ovarian failure (Shamilova et al. 2013).
A new study also investigated individuals with the normal and the low numbers of CGG repeat, respectively, and compared the outcome variables in cognition, mental health, and cancer domains (Mailick et al. 2014). In the end, the authors concluded that people with the low numbers of CGG repeat apparently had more difficulty in solving day to day problems with their memory and ability. And the women, in particular those with the low numbers of CGG repeat of both alleles, had significantly increased odds of feeling that they were necessary to drink more to obtain the same capacity as previously. Still, more risks in having breast cancer and uterine cancer were also increased, the odds of which were even extended to two and one-half times or four times. More severely, both men and women with CGG counts < 26 will be more likely to have a disabled child with a mental retardation or developmental disability (Mailick et al. 2014).
Ultimately, we presented 5 articles to clarify the association between the low numbers of CGG repeat and disorder phenotypes (Table 1). In these studies, “low numbers” were defined as CGG numbers below 26, and over 34 as ‘high alleles’. Genotypes were classified as following: normal (both alleles were in 26-34), heterozygous (one allele was not in normal range) and homozygous (both alleles were not in normal range).
Small CGG expansion with a meta-analysisAllelic size with 35-54: The 8 studies investigated the prevalence of small CGG expansion (35-54) in ovarian dysfunction cohorts and general cohorts, including 1,190 cases and 4,144 controls. The integral diagnostic OR for the incidence of FMR1 small CGG expansion between ovarian dysfunction cases and control subjects was 1.22 (95% CI: 0.75-2.00, P > 0.05). And, the meta-analysis was categorized the allelic size into POF and POI groups due to a significant heterogeneity among the studies. In both populations, a little difference was found between the patients and controls (premature ovarian failure: 1.32, 95% CI: 0.57-3.07, P > 0.05 and POI: 1.21, 95% CI: 0.55-2.66, P > 0.05) (Fig. 2).
Allelic size with 41-54: The 7 papers mentioned the relationship between the prevalence of FMR1 small CGG expansion (41-54) in ovarian dysfunction cohorts and general cohorts, which contained 1,572 cases and 4,203 controls. A certain difference was found between the patients with ovarian dysfunction and controls (1.62, 95% CI: 1.14-2.30, P < 0.05). We found no heterogeneity among the studies because of the estimated I-squared was 0% (Fig. 3).
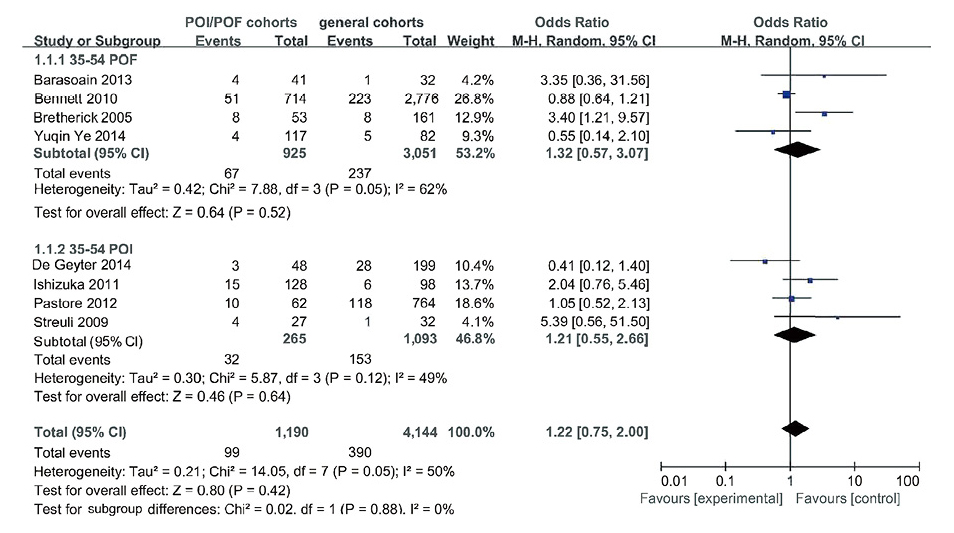
Association between the prevalence of small CGG expansion (35-54) and risk of ovarian dysfunction. Forest plot illustrated the overall and subgroup meta-analysis in ovarian dysfunction cohorts and general cohorts. The area of the squares reflects the study-specific weight. The diamond represents the summary OR and 95% CI. POF, premature ovarian failure; POI, primary ovarian insufficiency.

Association between the prevalence of small CGG expansion (41-54) and risk of ovarian dysfunction. The area of the squares reflects the study-specific weight. The diamond represents the summary OR and 95% CI. POF, premature ovarian failure; POI, primary ovarian insufficiency.
This systematic review and meta-analysis concentrated on the correlation between different expanded CGG numbers and the risk of phenotypic associations. We primarily classified into 2 groups to analyze: the low numbers of CGG repeat with disease phenotypes and small CGG expansion with ovarian dysfunction.
Low numbers of CGG repeatThe FMR1 gene plays a significant role in mental development and function. Many studies of FMR1 CGG repeat had illustrated a wide normal range, with the expanded numbers being 5-55, but little attention was focused on the low numbers of CGG repeat. In this study, we have presumed that participants with the low numbers of CGG repeat may be associated with terrible disorder outcomes.
Fu et al. (1991) initially showed the distribution patterns of triple CGG counts in general population, and then some researchers proposed that CGG counts should be defined as the normal range close to 30. Chen et al. (2003) also reported CGG numbers of 30 switched the translation effect of FMR1 gene product. It is common knowledge that this gene product FMRP, indeed, plays a key role in fragile X spectrum disorders, especially happening to synaptic plasticity which is one of the common reasons lead to mental retardation and developmental disability (Rousseau et al. 2011). The low numbers of CGG repeat may result in inefficient translation due to few transcripts; thus, individuals with the low numbers of CGG repeat are more likely to suffer from poor cognitive functions.
The symptoms of females with the low numbers of CGG repeat have also been implicated in research on reproductive biology. Premature ovarian failure, one of the prevalent disorders in the reproductive system, is associated with fertility issues and causes an earlier onset of menopause. In fact, low numbers of CGG repeat also play a role in premature ovarian failure and diminished ovarian reserve. Recently, it has been reported that the pathogenesis of premature ovarian failure may not only be correlative with genetic influence, but also depend on autoimmune factors (La Marca et al. 2009). In this context, Shamilova et al. (2013) found CGG counts < 28 were significantly associated with the anti-ovarian antibodies. The autoimmune-driven factor is more moderate in preservation of follicular original pool compared to the genetic factor of premature ovarian failure, and thus ovarian dysfunction patients with the low numbers of CGG repeat may be more promising in therapeutic approaches. The genetic mechanism of premature ovarian failure, which always depends upon the count of triple CGG repeats, has been confirmed by many investigators. However, the distinction between autoimmune factor and genetic influence may be a matter of treatment.
Weghofer et al. (2012) demonstrated that women with low FMR1 sub-genotypes would increase cancer risks associated with BRCA1/2 mutations. However, there were 4 studies inconsistent with the observation. Gleicher and coworkers presented ovarian cancer patients with BRCA1/2 mutations had no relative increased low FMR1 alleles compared to the patients without BRCA1/2 mutations (Gleicher et al. 2014). Ricci et al. (2014) also illustrated FMR1 genetic testing was inappropriate to prescreen the related BRCA mutations. Dagan et al. (2014) strengthened the evidence by the parallel epidemiological studies. Laitman et al. (2014) also explored the distribution of FMR1 CGG repeat sizes. Although they believed that the lower FMR1 alleles indeed had a discrepancy between BRCA mutation carriers and general population, it was still unlikely to serve as a regulator of breast cancer in associated BRCA mutations, nor as a prediction of diminished ovarian reserve (Laitman et al. 2014). The above-mentioned papers can not be determined the association between the low numbers of FMR1 CGG repeat and BRCA1/2-associated cancers. The distinct differences among these studies may be attributed to diverse research methods and groups. Therefore, the information needs to be further validated through amounts of samples available in the same occasion. Maybe the low numbers of CGG repeat would be one of opportunities for screening BRCA1/2-associated cancers in a specific circumstance.
In the aggregate, the small sample of studies with the low numbers of CGG repeat, at least in part, may have pathological implications. Many investigators classified the CGG expansion among 5 to 55 as a normal range all the time. In fact, more attention should be focused on the low numbers of CGG repeat (< 26). If people can track the distribution of expanded CGG numbers below 26, it will be valuable for finding the pathogenesis of ovarian diseases and providing therapeutic approaches. If necessary, further epidemiological researches as well as based biological investigations are required in next step.
Small CGG expansionMany population studies had investigated the association between FMR1 permutation (55-200) alleles and premature ovarian failure. A meta-analysis had proved that female premutation carriers had an increased risk of premature ovarian failure, in particular to European descent (Tosh et al. 2014). Actually in general population, small CGG expansion (35-55) was more prevalent than premutation alleles (Bennett et al. 2010). Moreover, publications presented a statistically significant correlation between ovarian dysfunction and CGG expansion numbers among 35 to 55. On the contrary, some investigators, based on the background of demographic characteristics, demonstrated no significant difference in populations. In our meta-analysis, the main reason for summarizing previous studies was to validate if there were certain mechanisms of small CGG expansion involvement in ovarian dysfunction. The presented paper for the first time illustrated CGG repeat numbers close to 55 had an elevated risk in ovarian dysfunction.
In this meta-analysis, 10 independent case-control studies were included to make a more accurate estimation of the association between FMR1 small CGG expansion with ovarian dysfunction. We extracted all allelic sizes available and divided them into 2 groups, and no heterogeneity was found in our analysis. The general results showed CGG expansion in 41-54 range had a significant difference in ovarian dysfunction cases and control subjects.
A paper described the carriers, confirmed no family history of FXS, with CGG repeat numbers among 35 to 55 may be liable to repeat instability. This allelic size was susceptible to become the full mutation alleles in two generations, and 59 CGG numbers were the smallest alleles expanded to a full mutation range in a single generation (Nolin et al. 2003; Fernandez-Carvajal et al. 2009). These observations underscored affected males with even smaller CGG alleles might lead to the FXS phenotype. AGG interruptions also affected the CGG expansion stability of FMR1 gene during parental transmission (Yrigollen et al. 2012). The AGG sequence varied largely between populations, but upon most occasions, inherited generations will not be changed (Yrigollen et al. 2012; Nolin et al. 2013). Despite the negative influence of small CGG expansion instability, we could use total CGG and AGG length to predict the risk of the expansion to a full mutation.
Small CGG expansion was also connected with Parkinson. We extracted the data from Zhang’s study (Zhang et al. 2012) and studies in PubMed, which described FMR1 expanded alleles in different populations with Parkinsonism, and performed a meta-analysis. The purpose with a statistical methodology is to estimate whether small CGG expansion should be considered in Parkinson (Table 3, Fig. 4). Finally, the 6 papers investigated the relationship between the prevalence of CGG repeat numbers among 41 to 54 in male Parkinsonism cohorts and general cohorts, including 1,780 cases and 2,407 controls, were found a certain significant difference (OR = 2.17, 95% CI: 1.50-3.14, P < 0.05) (Hedrich et al. 2005; Kurz et al. 2007; Loesch et al. 2009; Hall et al. 2011; Zhang et al. 2012; Tassone et al. 2012). Besides, FMR1 small CGG expansion was even broadened to the definition of FXTAS, and some intermediated alleles (35-54) carriers with neurological signs may be account for the increased levels of FMR1 mRNA, similar to the changes presented in premutation carriers with FXTAS (Hall et al. 2012). Other parallel studies indicated the CGG numbers of this range could contribute to mental disability. A set of data was analyzed about the distribution of CGG repeat numbers in individuals with developmental disabilities, and a milder learning disability was found in carriers with intermediated alleles (35-54). FMR1 small CGG expansion also could cause to learning difficulties in a population, and potential negative effects of this range were discovered in intellectual functioning (Murray et al. 1996; Patsalis et al. 1999). Maybe, there were still some unknown diseases associated with small CGG expansion, and more information needs to be further researched. The underlying biological mechanisms of small CGG expansion in the pathogenesis of ovarian dysfunction and mental disorders have become clearer.
The presented study has also several limitations. Firstly, we retrieved a small number of studies with the low numbers of CGG repeat because of little information in database, as well as the small sample sizes with small CGG expansion for meta-analysis. Secondly, some studies were not eligible for meta-analysis due to the various study types, which caused the loss of certain worth papers. Thirdly, there were different models of the statistical data in collecting the numbers of small CGG expansion, either the direct acquirement of single allelic count, or acquirement of biallelic mean count. Despite these limitations, the results of this meta-analysis confirm a significant association between small CGG expansion and ovarian dysfunction.

Characteristics of small CGG expansion with male parkinson.

Association between the prevalence of small CGG expansion (41-54) and risk of Male Parkinson. The area of the squares reflects the study-specific weight. The diamond represents the summary OR and 95% CI.
In summary, our study has revealed that the low numbers of CGG repeat have pathological implications, especially leading to premature ovarian failure. The pathogenesis may be attributed to the few transcripts or autoimmune factors. Correspondingly, the meta-analysis has suggested that small CGG expansion of FMR1 gene is closely correlated with ovarian dysfunction, and a significant influence on male Parkinsonism and mental retardation are also observed in this allelic form. Besides, the instability of small CGG expansion could cause the phenotype of FXS. Therefore, we propose that the AGG and CGG allelic sizes through a PCR-based assay should be a routine test for carriers, which would be extremely valuable for finding etiology and pathogenesis of disorders with CGG repeat numbers below 26 or above 34. However, elucidation of mechanisms of these diseases still has challenges to the scientific community.
The authors thank Zhihong Wang (Department of Clinical Genetics and Experimental Medicine, Fuzong General Hospital, Fuzhou, Fujian, China) for help in performing the searches, screening and data extraction, Duo Zhang for analytical input and editorial support, and editorial support in collating authors’ comments and preparing the final manuscript for submission.
The authors declare no conflict of interest.