2016 年 239 巻 4 号 p. 269-278
2016 年 239 巻 4 号 p. 269-278
Glioma has a poor prognosis due to its rapid overgrowth, diffuse invasion, and chemotherapy resistance. The improvements in clinical outcome are still limited and the identification of novel biomarkers involved in the progression of gliomas is still under critical demands. Amino acid ADP-ribosyltransferase 1 (ART1) is an enzyme that catalyzes the mono-ADP-ribosylation, a reversible post-translational modification. For example, the mono-ADP-ribosylation of transcription factors can affect their binding to target gene promoters. However, the functional significance of ART1 in glioma has not been reported. We collected 107 glioma cases from Qianfoshan Hospital and Yidu Central Hospital of Weifang between April 2008 and September 2015 to analyze the prognosis value of ART1 in gliomas. RT-qPCR analysis showed that the expression level of ART1 mRNA in glioma tissues was 4-fold higher than that in normal brain tissues. According to the immunohistochemical staining results, 44 patients (41.1%) were categorized as ART1 positive (≥ 20% of stained glioma cells), while the other 63 patients (58.9%) categorized as ART1 negative (< 20% of stained glioma cells). Moreover, the mean percentage of ART1-positive cells was 43.7%, 53.6% and 64.2% in WHO grade II, III and IV specimens, respectively. Through univariate and multivariate analyses, we identified ART1 as an independent prognostic factor. We also found that ART1 overexpression in U251 glioblastoma cells could significantly decrease the susceptibility to vincristine, one of tubulin-targeted drugs, which is widely used in clinical treatment for glioma. Taken together, we propose that up-regulation of ART1 expression is associated with the aggressiveness of glioma.
Glioma accounts for about 30% of all primary tumors in central nervous system (Mamelak and Jacoby 2007; Khasraw et al. 2014) and can be graded into four grades (I-IV) by the histopathologic evaluation according to World Health Organization (WHO) grading criteria (Louis et al. 2007). At least 70% of them can be classified as malignant gliomas (grade III and IV) (Dolecek et al. 2012; Ostrom et al. 2013). Glioma has a poor prognosis due to its characteristic progressive overgrowth, diffuse invasion and high level of chemotherapy resistance (Kesari 2011). For example, the glioblastoma (WHO grade IV), which makes up approximately 60-70% of all malignant glioma, is the most malignant intrinsic brain neoplasm in adults with a median survival time of less than two years (Stupp et al. 2009). Despite that attempts are being made in clarifying the pathogenesis and molecular mechanisms of glioma, the improvement in clinical outcome is still limited (Wen and Kesari 2008). Therefore, identification of novel biomarkers participating in the development of glioma remains under critical demands.
Mono-ADP-ribosylation is a type of protein post-translational modification (PTM), which can transfer a single ADP-ribose from nicotinamide adenine dinucleotide (NAD+) to the specific amino-acids residues of target proteins (Zolkiewska et al. 1992; Moss et al. 1999). PTM plays an important role in various physiological and pathophysiological processes, such as DNA repair, transcription, cell cycle regulation, signal transduction, necrosis and apoptosis (Hassa et al. 2006). Amino acid ADP-ribosyltransferases (ARTs) are the important enzymes participating in this specific PTM process. The ART protein family contains seven members, ART1-7; ART1-4 are mainly expressed on the plasma membrane, while ART5-7 can be secreted extracellularly (Okazaki et al. 1994; Glowacki et al. 2002). Among them, ART1 is an arginine-specific transferase, which has been reported to mediate ADP-ribosylation in human cells (Zolkiewska 2005), thus inhibiting the activity of its substrates such as defensin and integrin (Corda and Di Girolamo 2002, 2003). Integrin α7β1 is involved in the adhesion and invasion of cancer cells (Zolkiewska and Moss 1995; Zhao et al. 2005; Friedrich et al. 2008), indicating the potential role of ART1 in tumor progression. Moreover, the ART1 expression was reported to be up-regulated in colon carcinoma tissues, while silencing of ART1 can significantly inhibit the tumor development (Xiao et al. 2013; Kuang et al. 2014; Tang et al. 2015).
However, the expression of ART1 and its implication in glioma have not been reported. In the present study, we investigated the expression pattern of ART1 in glioma tissues and glioblastoma cell lines. We also demonstrated the role of ART1 in the regulation of chemotherapy resistance.
This study was approved by the Research Ethics Committees of Qianfoshan Hospital and Yidu Central Hospital of Weifang. Written informed consents were obtained from all patients.
All samples were collected from Qianfoshan Hospital and Yidu Central Hospital of Weifang between April 2008 and September 2015, and all specimens were handled according to ethical and legal standards. Normal brain tissues were obtained from thirteen patients during surgical treatment of epilepsy (Eleven frontier temporal tissues from patients underwent anterior temporal lobectomy, two hippocampus tissues from patients underwent anteriomedial temporal lobectomy). Ethical approval and informed contents were obtained from our hospital and patients. Disease diagnosis and classification were made by pathological examination based on the WHO classification system. The clinicopathological characteristics of all glioma patients are shown in Table 1. A total of 107 glioma cases were enrolled in our study: 26 diffuse astrocytomas (WHO grade II), 33 anaplastic astrocytomas (WHO grade III), and 48 glioblastomas (WHO grade IV). The median age of all the patients at the time of diagnosis was 46 years (range 27-67 years). Among the 107 patients, 21 (19.6%) cases were treated with VCR alone, while other patients were treated with combined regimen: 49 (45.8%) cases received vincristine plus carboplatin and 37 patients (34.6%) received procarbazine, lomustine and vincristine after surgery.

Clinicopathological characteristics and correlations with ART1 expression in glioma patients.
*Statistically significant.
ART1, ADP-ribosyltransferase 1; KPS, Karnofsky; GTR, gross total resection; STR, subtotal resection; PR, partial resection.
Total RNA from frozen glioma and normal brain tissues was extracted with Trizol reagent (Invitrogen, USA) and then reversely transcribed to cDNA using the PrimerScript 1st Strand cDNA Synthesis Kit (Takara, China). Quantitative PCR was carried out using SYBR green master mix (Roche, USA). The relative mRNA expression levels were calculated with the 2-ΔΔct method, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard. The primer sequences were as followed:
ART1:
Forward: 5′-AGGCCTAGATGAGGAAACTGAGACC-3′
Reverse: 5′-GCACACATGGCCAACATCCTCAGGA-3′
(Yadollahi-Farsani et al. 1999)
GAPDH:
Forward: 5′-GAAGGTCGGAGTCAACGGATTT-3′
Reverse: 5′-CCTGGAAGATGGTGATGGGATT-3′
Immunohistochemical stainingImmunohistochemical (IHC) staining was performed on formalin-fixed, paraffin-embedded tissues. After deparaffinization and rehydration, 4-μm-thick sections were incubated in 0.3% H2O2 for 30 min and performed antigen retrieval in sodium citrate buffer pH 6.0. IHC analysis for ART1 was performed using a goat polyclonal antibody (S-15, Santa Cruz Biotechnology, 1:100) at 4°C overnight after blocked and then detected the immunoreactivity using the DAB staining kit (Tiangen, China) according to the manufacturer’s instructions. 5% fetal bovine serum (FBS) was used as negative control for the staining.
Evaluation of immunostainingAll IHC results were scored by two independent pathologists following the criterions of double-blind trials. The degree of immunoreactivity was evaluated according to the percentage of immune-positive cells (0-19%, negative; 20-50%, weak-positive; 51-75%, moderate-positive; 76-100%, strong-positive) from three visual areas.
Cell culture and transient transfectionHuman glioblastoma cell lines U87 and U251 were purchased from American Type Culture Collection (ATCC, USA), and were cultured in DMEM supplemented with 10% FBS, 100 μg/mL streptomycin and 100 U/mL penicillin in 5% CO2 at 37°C. The pcDNA3.1-ART1 plasmid was provided by the Beijing Genomics Institute (China) while the siRNAs were synthesized by Sigma-aldrich (USA) with the following sequences: siRNA-ART1: 5′-GCAGUUUGGUGAGGACACCUUCUUC-3′ (Balducci et al. 2007); siRNA-negative control (NC-siRNA): 5′-UUCUCCGAACGUGUCACGU-3′.
U87 cells were transfected with siRNAs while the U251 cells were transfected with the pcDNA3.1-ART1 plasmid, both using Lipotransfectamine 3000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Western blot analysisCell samples were homogenized in lysis buffer containing 1 M Tris-HCl pH 7.5, 10% sodium dodecyl sulfate (SDS), 1% NP-40, 1% Triton X-100, 0.5 M EDTA and proteinase inhibitors (0.5% sodium deoxycholate, 1 mM PMSF, 10 μg/ml aprotinin and 10 μg/ml leupeptin); then centrifuged at 13,000 rpm for 30 min to collect the supernatant and SDS loading buffer was added to denature the proteins for 10 min at 100°C. An equivalent amount of proteins (20 μg) from each sample was electrophoresed by 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA). After being blocked with 5% non-fat milk for 1 h, the membranes were incubated with primary antibodies overnight at 4°C. The membranes were then incubated with horseradish peroxidase-linked IgG as the secondary antibody for 2 h after washing with TBST for three times. The immunoreactivity was detected by the ECL detection systems (Pierce, Rockford, USA). The intensity was evaluated using NIH ImageJ analysis system (Wayne Rasband, USA). All experiments were carried out at least three times.
Cell proliferation assayThe basal cell proliferation assay was carried out with the Cell Counting Kit-8 (CCK-8, Dojindo, Japan). U87 and U251 cells were plated in 96-well plates with three replicates. Then the numbers of cells were detected by the absorbance (450 nm) of reduced WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-isulfophen-yl)-2H-tetrazolium, monosodium salt) according to the manufacturer’s instructions at the designated time points.
Chemosensitivity testingSince all the patients enrolled in our study were treated with VCR, a tubulin-targeted drug which is widely used in clinical treatment for gliomas. We further tested the chemosensitivity of glioma cells to VCR in vitro. U87 and U251 cells in the logarithmic growth phase were seeded into the 96-well plates at 3 × 103 cells/well triplicate. After culturing for 16 h, the vincristine sulfate (VCR, Sigma, USA) was added in with different concentrations, followed by incubation for another 72 h at 37°C. The number of cells was then detected with the CCK-8 assay. Each experiment was performed at least three times independently.
Statistical analysisThe differences between groups were measured with the nonparametric test (Mann-Whitney U test) while categorical data were evaluated using Chi-square test. Kaplan-Meier method was employed to calculate the overall survival rate. The multivariate analysis was carried out by the Cox proportional hazards model. Statistical analysis was performed using SPSS 21.0 (SPSS, Chicago, IL, USA). All the P values were two-sided, and the differences were considered to be statistically significant with P values less than 0.05.
The clinicopathological characteristics of all the glioma patients (n = 107) are summarized in Table 1. There were 64 (59.8%) male and 43 (40.2%) female patients. Of the 107 included samples, 58 patients (54.2%) underwent gross total resection (GTR), 26 patients (24.3%) underwent subtotal resection (STR) and the other 23 patients (21.5%) under partial resection (PR) or biopsy. As for the WHO classification, there were 26 patients (24.3%) with grade II, 33 patients (30.8%) with grade III, and 48 patients (44.9%) with grade IV. Tumor diameter varied from 0.7 to 13.0 cm (mean, 4.4 cm): 61 cases (57.0%) with the size of smaller than 5.0 cm and the other 46 cases (43.0%) larger than 5.0 cm.
Correlations of ART1 expression with clinicopathologic featuresRT-qPCR results demonstrated that the mRNA expression of ART1 in gliomas was much higher than that in normal brain tissues (Fig. 1A). Through IHC analysis of 107 glioma tissues, we found that the positive staining of the ART1 protein was mainly located in the cell membrane and cytoplasm of the tumor cells (Fig. 1). The positive threshold was set at 20% (≥ 20%) after distribution analysis (Table 2). Accordingly, 44 patients (41.1%) were classified into ART1-positive group and the other 63 patients (58.9%) as ART1-negative expression. The results of IHC staining of ART1 in glioma tissues and its correlations with clinicopatholgical parameters are shown in Table 1. In addition, there was a difference of positive staining percentage among different WHO grade groups (Fig. 1B). The mean percentage of positive cells was 43.7%, 53.6% and 64.2% in WHO grade II, III and IV group, respectively. The ART1 expression was higher in gliomas with more advanced WHO grade (P = 0.028).

Expression of ART1 in gliomas and normal brain tissues.
(A) RT-qPCR showed higher mRNA expression of ART1 in gliomas than that in normal brain tissues (NBT). (B) Distribution of ART1 positive IHC staining intensity according to WHO grade. Representative IHC images in WHO grade IV glioma tissues from temporal lobe showed negative expression (C, 0%), weak-positive expression (D, 20-50%), moderate-positive expression (E, 51-75%) and strong-positive expression (F, 76-100%) of ART1, respectively. Arrows pointed the strong IHC staining.
Scale bar: 50 μm.
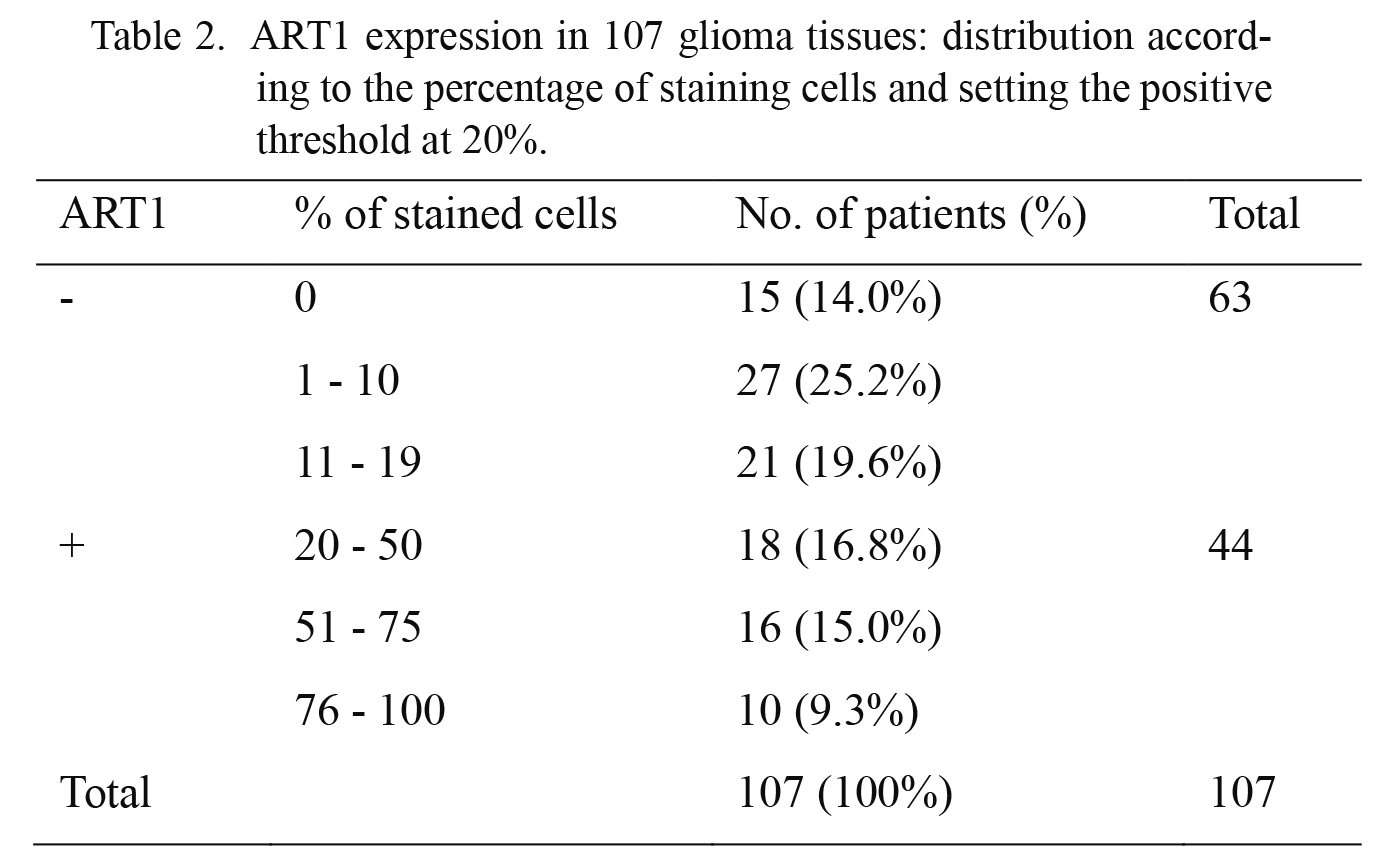
ART1 expression in 107 glioma tissues: distribution according to the percentage of staining cells and setting the positive threshold at 20%.
ART1, ADP-ribosyltransferase 1.
The median follow-up time was 32 months (range, 1-79 months), 83 patients (77.6%) died during this period. The median overall survival (OS) time of patients with WHO grade II was 62.0 months, while only 31.0 months and 11.0 months for the patients with WHO grade III and IV, respectively.
According to univariate survival analysis, conventional prognostic parameters, including WHO grade and operation methods can significantly affect the OS (P < 0.001 and P = 0.031, respectively) (Fig. 2A, B, Table 3). In addition, ART1 expression can affect the OS of glioma patients; patients with negative ART1 expression had significantly longer OS than those with positive ART1 expression (median survival time 49.0 months vs. 11.0 months, P < 0.001) (Fig. 2C, Table 3).
To further evaluate whether ART1 expression in glioma was an independent predictor of OS, a multivariate analysis using the Cox proportional hazard regression model was performed with the following variables: age, sex, tumor size, WHO grade, Karnofsky score (KPS score), surgery and ART1 expression. Multivariate analysis revealed that higher WHO grade (HR = 3.288, 95% confidence interval [CI] = 2.20-4.91, P < 0.001) and positive ART1 expression (HR = 1.691, 95% CI = 1.05-2.71, P = 0.029) (Table 4) were independent risk factors for poor OS of glioma patients.

Survival analysis of glioma patients.
WHO grade (A) and surgery procedure (B) can affect the overall survival of glioma patients. (C) ART1 expression was negatively correlated with patients’ overall survival.

Univariate analysis of prognostic factors for OS of glioma patients.
*Statistically significant.
ART1, ADP-ribosyltransferase 1; KPS, Karnofsky; GTR, gross total resection; STR, subtotal resection; PR, partial resection.

Cox proportional hazards analysis of the OS of glioma patients.
*Statistically significant.
KPS, Karnofsky; ART1, ADP-ribosyltransferase 1.
To elucidate the underlying mechanisms of ART1’s function in glioma, we tested the expression pattern of two glioblastoma cell lines, the U87 and U251 cells. The basal expression of ART1 in U87 cells was approximately 1.6-fold higher than that of U251 cells (P < 0.05, Fig. 3A). We thus chose the U87 cells to carry out the knock-down experiments and U251 cells to perform the overexpression experiments. The siRNA and overexpression efficiency were confirmed by western blot analysis (Fig. 3B). The expression of ART1 showed no noticeable effect on the basal proliferation of U87 and U251 cells (Fig. 3C, D), but when cells were treated with VCR, the higher expression level of ART1 significantly increased the chemoresistance of the cells (Fig. 3E, F).
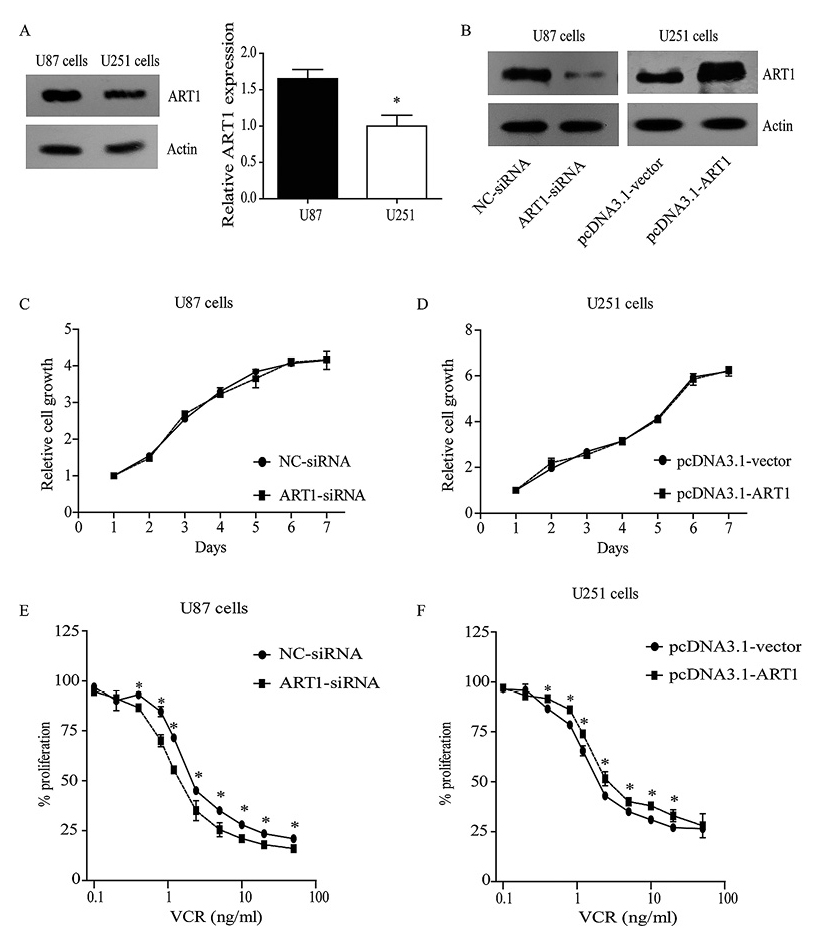
Expression of ART1 in two glioma cell lines and its potential role for vincristine resistance.
(A) Western blot analysis of the protein levels of ART1 expression in U87 and U251 human glioma cell lines. (B) Efficiency of ART1-siRNA in U87 cells (left panel) and overexpression in U251 cells (right panel) were evaluated by Western blot analysis. CCK-8 results showed basal proliferation (C) and cell survival under VCR stimulation (E) of U87 cells with ART1 knock-down. The basal proliferation (D) and VCR resistance (F) of U251 cells with ART1-overexpression were also tested.
* indicates significant difference with P < 0.05.
Several studies reported that TUBB3 was involved in the resistance of VCR in gastric cancer and lung cancer (Nardone et al. 2004; Gan et al. 2007; Zhang et al. 2009, 2013; Fetisova et al. 2010; Li et al. 2015). We thus analyzed the expression of TUBB3 in our experiments. The expression level of TUBB3 was decreased by about 30% in U87 cells transfected with the ART1 siRNA, compared with the level in cells transfected with negative control siRNA (P < 0.05, Fig. 4A). In contrast, upon overexpression of ART1 in the U251 cells, the TUBB3 expression level increased by about 27% (P < 0.05, Fig. 4B). These results suggest that ART1 may regulate the expression of TUBB3 protein.
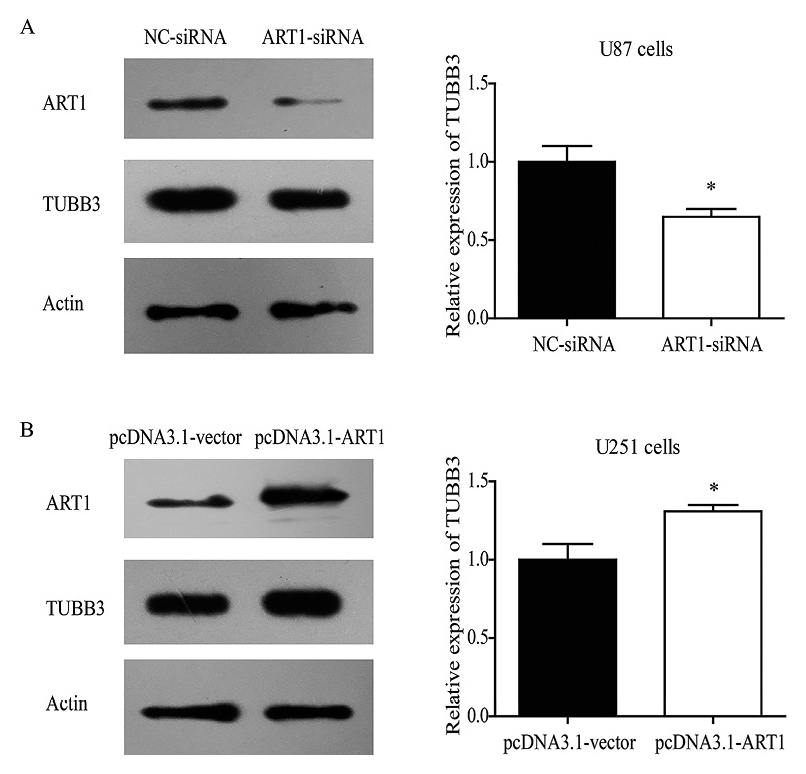
The protein level of TUBB3 is regulated by ART1.
TUBB3 expression level was decreased by 30% with ART1 knock-down in U87 cells (A), while it can be up-regulated by 27% with ART1 overexpression in U251 cells.
* indicates significant difference with P < 0.05; experiments were repeated three times.
ART1, which exists in humans, mice and rats (Laing et al. 2011), was reported to catalyze the mono-ADP-ribosylation of substrate proteins, such as fibroblast growth factor-2 (FGF-2), integrin and platelet-derived growth factor-BB (PDGF-BB) (Zolkiewska and Moss 1993). These substrate proteins are involved in cell proliferation, angiogenesis, and inflammation (Zolkiewska and Moss 1995; Jones and Baird 1997; Saxty et al. 2001; Paone et al. 2002). Moreover, the ART1 expression has been reported to be up-regulated in human colorectal carcinoma and hepatocellular carcinoma, and silencing ART1 in CT26 colon adenocarcinoma cells can significantly inhibit cell proliferation and promote cell apoptosis (Tang et al. 2013; Xiao et al. 2013; Su et al. 2014). The functions of ART1 may include various pathways including the ERK and AKT signaling, as well as the integrin and poly (ADP-ribose) polymerase (PARP-1) signaling (Tang et al. 2013, 2015; Xiao et al. 2013, 2015).
In our studies, RT-qPCR analysis indicated that the mRNA expression level of ART1 in glioma was higher than that in normal brain tissues, and we found that the positive expression of ART1 was closely correlated with higher-grade of glioma and can serve as an independent predictor for the poorer prognosis of glioma patients. To our knowledge, this is the first report demonstrating the expression and function of ART1 in glioma.
In addition, we tested the difference of ART1 expression between two glioblastoma cell lines, showing U87 has a relative higher ART1 expression level compared with U251 cells. To further explore the oncogenic role of ART1, we used knock-down and overexpression methods to verify its effect. Interestingly, we found that ART1 has no effect on the basal proliferation capacity of glioma cells; however, overexpression of ART1 can significantly increase the resistance of U251 cells to apoptosis induced by the chemotherapeutic agent VCR, while the knock-down of ART1 can decrease the drug-resistance of U87 cells. The differences between ART1’s effects on basal proliferation and drug-induced apoptosis may result from the fact that under basal conditions, the glioma cell proliferation capacity is much stronger than cell apoptosis. It is possible that under basal condition, the ART1, which can regulate the activity of Bcl-2 and Bcl-xl through inflammation signaling pathways (Xiao et al. 2013), may have little function in the whole cell cycle and proliferation. Kuang’s study also found that, with the stimulation of cisplatin, which will cause the accumulation of apoptosis bodies in CT26 cells (colon cancer cell line), the expression and function of ART1 can be regulated, indicating that ART1 may have more significant functions under the cell apoptosis stress (Kuang et al. 2014). Corresponding to our results, the in vivo study by Xiao et al. (2015) also demonstrated that the proliferation effect of ART1 was more obvious under cisplatin stimulation compared with no stimulation. Similarly, several studies illustrated that TUBB3, which can regulate the cell sensitivity to tubulin-binding agents such as VCR, plays important roles in the drug-induced cell apoptosis and efficiency of chemotherapy in pancreatic cancer, breast cancer, and lung cancer (Stengel et al. 2010; Tamura et al. 2013; Li et al. 2014; McCarroll et al. 2015). Therefore, we performed Western blot to test the TUBB3 expression level and found it can be positively regulated by ART1, indicating that TUBB3 may participate in the ART1-enhanced VCR resistance of glioma cells.
In conclusion, we have demonstrated that ART1 upregulation is associated with the aggressiveness of glioma. Moreover, ART1 may mediate the VCR resistance.
The authors have no conflict of interest.