2016 Volume 240 Issue 1 Pages 47-56
2016 Volume 240 Issue 1 Pages 47-56
The interleukin (IL)-17 family, consisting of six homodimeric cytokines IL-17A, IL-17B, IL-17C, IL-17D, IL-17E/IL-25, and IL-17F, mediates a variety of biological activities including regulation of chemokine secretion and angiogenesis. Among the IL-17 family members, IL-17A and IL-17E/IL-25 are angiogenesis stimulators, while IL-17B and IL-17F are angiogenesis inhibitors. Recently, IL-17A/F heterodimer, comprised of the IL-17A and IL-17F subunits, was found as another member of the IL-17 cytokine family. However, to date, it has been unknown whether IL-17A/F has biological actions to affect the angiogenesis-related vascular endothelial functions. Therefore, in this study, we investigated the biological effects of IL-17A/F on the growth, migration and capillary-like tube formation of vascular endothelial cells. Recombinant IL-17A/F protein had no direct effects on the growth of human dermal microvascular endothelial cells (HMVECs), whereas, after 4-hour incubation in a modified Boyden Chemotaxicell chamber, IL-17A/F significantly induced migration of HMVECs over a wide range of doses via the phosphatidylinositol-3 kinase (PI3K) signaling pathway. We further investigated the biological effect of IL-17A/F on capillary-like tube formation using a co-culture system of human umbilical vein endothelial cells (HUVECs) and human dermal fibroblasts (HDFs), which mimicked the in vivo microenvironment. In this co-culture system, IL-17A/F significantly promoted capillary-like endothelial tube formation in a dose-dependent fashion via the PI3K and extracellular signal-regulated kinase (ERK) signaling pathways. Additionally, IL-17A/F up-regulated secretion of angiogenic growth factors such as IL-8 and growth-related oncogene (GRO)-α by HDFs. These findings identify a novel biological function for IL-17A/F as an indirect angiogenic agent.
The IL-17 cytokine family is composed of six structurally related molecules, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E/IL-25 and IL-17F (Kolls and Lindén 2004). IL-17A, IL-17E/IL-25 and IL-17F are mainly produced by immune cells, especially T helper cell lineage Th2 or Th17 cells. The other IL-17 cytokine family members, IL-17B, IL-17C, and IL-17D, are mainly produced by a non-T cell source. Members of this cytokine family are secreted as disulfide-linked homodimers, with the exception of IL-17B, which is secreted as a non-covalent homodimer (Li et al. 2000). On the other hand, the IL-17 receptor family contains five receptor subunits, IL-17 receptor A (IL-17RA), IL-17RB, IL-17RC, IL-17RD and IL-17RE. Recently, another member of the IL-17 cytokine family, IL-17A/F heterodimer, consisting of IL-17A and IL-17F, was found to be also secreted by activated human CD4+ T cells (Wright et al. 2007) and mouse Th17 cells (Liang et al. 2007), which signals through the same heterodimeric receptor complex of IL-17RA and IL-17RC as IL-17A and IL-17F homodimers (Wright et al. 2008).
The IL-17 cytokine family members have been demonstrated to have similar or different kinds of biological actions. With regard to the common biologic characteristics, the IL-17 family of cytokines induces secretion of numerous cytokines and chemokines by a wide variety of cell types (Yao et al. 1995a, b; Fossiez et al. 1996; Kawaguchi et al. 2001; Laan et al. 2001; Jones and Chan 2002; Starnes et al. 2002; Prause et al. 2003; Inoue et al. 2006; Huang et al. 2007). For instance, IL-17A, the well-characterized molecule of this cytokine family, acts primarily on parenchymal cells such as fibroblasts, epithelial cells and endothelial cells, resulting in up-regulation of chemokine secretion such as GRO-α, and granulocyte-colony stimulating factor (G-CSF) (Yao et al. 1995a, b; Jones and Chan 2002; Numasaki et al. 2004a, b). IL-17F, sharing the highest amino acid sequence homology with IL-17A, also induces secretion of GRO-α and G-CSF in primary human bronchial epithelial cells (McAllister et al. 2005). Overexpression of IL-17F in mouse airways using expression vector carrying IL-17F resulted in up-regulated secretion of chemokines and neutrophil recruitment in lungs, similar to what have been observed for IL-17A (Oda et al. 2005). In addition, IL-17A/F has also been demonstrated to induce chemokine production and airway neutrophilia with intermediate potency between IL-17A and IL-17F (Liang et al. 2007). Neutralization of the IL-17A/F heterodimer showed intermediate efficacy in blocking airway inflammation mediated by adoptive transfer of ovalbumin-specific polarized Th17 cells and airway challenge with antigen, compared with the efficacy of neutralization of either IL-17A or IL-17F.
Our group and others have investigated the biological role of the IL-17 cytokine family members in angiogenesis and angiogenesis-related vascular endothelial cell functions. Numasaki et al. (2003) demonstrated that IL-17A is an angiogenesis mediator, which induces new capillary-like vessel development in a rat corneal micropocket assay for angiogenesis, and stimulates migration and capillary-like tube formation of vascular endothelial cells. Conversely, there have been reports demonstrating that although IL-17F binds to the same heterodimeric receptor complex IL-17RA and IL-17RC as IL-17A, IL-17F inhibits angiogenesis and tumor-associated angiogenesis (Starnes et al. 2001; Xie et al. 2010). In contrast, it is unknown whether IL-17A/F heterodimer, sharing the same receptor complex with IL-17A and IL-17F, may influence the angiogenesis-related functions of vascular endothelial cells or not. Therefore, in the current study, we assessed the potential of IL-17A/F to impact on the traits of vascular endothelial cells including growth, migration and capillary tube formation, closely associated with the process of angiogenesis. To the best of our knowledge, this is the first report to demonstrate that, like IL-17A, IL-17A/F has the biological properties to stimulate migration and capillary-like tube formation of human vascular endothelial cells. These findings revealed a novel biological function for IL-17A/F as an indirect angiogenesis mediator.
Recombinant human IL-17A homodimer, human IL-17A/F heterodimer, human basic fibroblast growth factor (bFGF), mouse anti-human IL-17RA monoclonal antibody (mAb) and goat anti-human IL-17RC polyclonal antibody (Ab) were purchased from R&D Systems (Minneapolis, MN, USA). Recombinant human vascular endothelial growth factor (VEGF) and Suramin were purchased from KURABO (Osaka, Japan). Specific chemical inhibitors LY294002, PD98059 and SP600125 were purchased from Cell Signaling Technology (Danvers, MA, USA). Primary HMVECs were purchased from KURABO (Osaka, Japan), and maintained in HuMedia-EB2 with 10 ng/mL epidermal growth factor (EGF), 1 μg/mL hydrocortisone, 5 ng/mL bFGF, 10 μg/mL heparin, 39.3 μg/mL dbcAMP, 50 μg/mL gentamycin, 50 ng/mL amphotericin-B and 5% fetal calf serum (FCS) (all from KURABO, Osaka, Japan). Cells were grown at 37°C in an atmosphere of 5% CO2 and used between passages 4 and 5. Primary human dermal fibroblasts were purchased from Takara Bio (Kusatsu, Shiga, Japan), and maintained in Fibroblast Basal Medium supplemented with 1 ng/mL bFGF, 5 μg/mL Insulin and 2% FCS (Takara Bio).
In vitro cell growth assayTo evaluate the effects of IL-17A/F heterodimer on the proliferation of vascular endothelial cells, HMVECs were suspended in HuMedia-EB2 with 50 μg/mL gentamycin, 50 ng/mL amphotericin-B and 2% FCS, and plated at 1.5 × 105 cells in 10 cm culture dish coated with collagen (Becton Dickinson, Bedford, MA). After 18 hours, medium was then replaced with HuMedia-EB2 containing with 50 μg/mL gentamycin, 50 ng/mL amphotericin-B and 2% FCS medium with or without cytokine (IL-17A/F at 0.5, 1, 10, 50, or 100 ng/mL or bFGF at 10 ng/mL) (on day 0). On days 3 and 5, the medium was replaced with fresh HuMedia-EB2 containing 50 μg/mL gentamycin, 50 ng/mL amphotericin-B and 2% FCS with or without cytokine (IL-17A/F at 0.5, 1, 10, 50, or 100 ng/mL or bFGF at 10 ng/mL). The number of cells was counted on days 3, 5 and 7 using TC20™ Automated Cell Counter (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Migration assayCell migration activity was evaluated using a modified Boyden Chemotaxicell chamber (KURABO), as described previously (Numasaki et al. 2003). Briefly, cytokine (IL-17A/F at 0, 0.5, 1, 10, 50, or 100 ng/mL or IL-17A at 10 ng/mL in HuMedia-EB2 with 1% FCS) was applied in the wells of 24-well culture plates (Corning Costar, Corning, NY, USA). Then, Chemotaxicell chamber containing polycarbonate filter (5-μm pore size) coated with 10 μg/mL fibronectin (Sigma-Aldrich, St. Louis, MO, USA) was inserted into each well of 24-well culture plates. Before use, HMVECs were cultured in HuMedia-EB2 with 2% FCS and no growth factors for 12 hours. Then, HMVECs suspended in HuMedia-EB2 with 1% FCS were seeded at 12 × 104 cells/cm2 onto the polycarbonate filter of Chemotaxicell chambers. Thereafter, the plates inserted with Chemotaxicell chambers were incubated for 4 hours at 37°C. For inhibitory assay, HMVECs were preincubated with mouse anti-human IL-17RA mAb (10 μg/mL) and goat anti-human IL-17RC polyclonal Abs (10 μg/mL) for 1 hour. Then, cells were further incubated for 4 hours in the presence of mouse anti-human IL-17RA mAb (10 μg/mL) and goat anti-human IL-17RC polyclonal Abs (10 μg/mL) in both lower and upper chambers. To define which signaling pathway(s) is involved in IL-17A/F-induced HMVEC chemotaxis, HMVECs, preincubated with specific inhibitors to phosphatidylinositol-3 kinase (PI3K) (LY294002; 20 μM), extracellular signal-regulated kinase (ERK) (PD98059; 20 μM), c-Jun N-terminal kinase (JNK) (SP600125; 10 μM) or DMSO for 45 min, were plated at 12 × 104 cells/cm2 onto the polycarbonate filter of Chemotaxicell chambers in the presence or absence of respective inhibitor. The cells passing through the membrane pores were fixed and stained with Diff-Quick (Harleco, Gibbstown, NJ, USA), and the number of cells migrating through the membrane was quantified by counting cells in 5 randomly selected microscopic fields (×200) in each chamber.
In vitro capillary-like endothelial tube formation assayIn vitro capillary-like endothelial tube formation assay was performed using commercially available Angiogenesis assay kit according to the manufacturer’s instructions (KURABO). Briefly, HUVECs and HDFs were admixed and seeded into 24-well culture plates in medium with or without cytokine (IL-17A/F at 1, 10, 50, or 100 ng/mL, IL-17A at 50 ng/mL, VEGF at 10 ng/mL or Suramin at 100 μM). Cells were incubated for up to 11 days, and the medium was replaced with fresh every 3 days. On day 11, cells were washed and directly fixed with 70% ice-cold ethanol for 30 min in the wells. The fixed cells were serially incubated with 1% bovine serum albumin in buffer, and stained with mouse mAb against human CD31. The evaluation of CD31-positive tubes was made by computerized image analysis of the number of pixels occupied by the endothelial tubes in a total of 9 random areas from these separate wells.
To determine whether the heterodimeric receptor complex IL-17RA and IL-17RC is involved in IL-17A/F-mediated capillary-like tube formation in a co-culture system of HUVECs and HDFs, mouse anti-human IL-17RA mAb (10 μg/mL) and goat anti-human IL-17RC polyclonal Abs (10 μg/mL) were added to the culture medium. In addition, to define which signaling pathway(s) is involved in IL-17A/F-induced capillary-like endothelial tube formation in a co-culture system of HUVECs and HDFs, specific inhibitor to PI3K (LY294002), ERK (PD98059) or JNK (SP600125) (10.0 μM for the first 3 days, followed by 2.0 μM until the end of the 11-day culture period) was added (Saito et al. 2003; Corrigan et al. 2011).
Angiogenic growth factor assaysFor the measurement of the concentrations of several angiogenic growth factors in cell culture supernatants, HDFs (1 × 105/mL) were seeded into collagen-coated 24-well flat-bottomed plates (Becton Dickinson, Bedford, MA, USA), and cultured overnight in Fibroblast Basal Medium with 2% FCS, 50 μg/mL gentamycin and 50 ng/mL amphotericin-B. Cells were washed with Fibroblast Basal Medium twice, and cultured in Fibroblast Basal Medium containing 2% FCS, 50 μg/mL gentamycin and 50 ng/mL amphotericin-B with or without cytokine (IL-17A at 1, 10, or 100 ng/mL or IL-17A/F at 1, 10, or 100 ng/mL) for 48 hours. Cell-free supernatants were collected and stored at −70°C until use. Concentration of angiogenic growth factor was measured using commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
Statistical analysisStatistical analysis was performed using an unpaired 2-tailed Student t test with a confirmation by parametric and F tests. Difference was considered significant with a P value less than 0.05.
Our previous study and others demonstrated that vascular endothelial cells including HMVECs and HUVECs express IL-17RA and IL-17RC on the cell surface (Moseley et al. 2003; Pickens et al. 2010; Fujie et al. 2012). Thus, we examined the biological effects of IL-17A/F on in vitro angiogenesis-related functions of HMVECs. We first evaluated the effects of IL-17A/F on the in vitro growth of HMVECs. In clear contrast to bFGF as a positive control, a wide range of doses of recombinant human IL-17A/F protein (0.5-100 ng/mL) had no direct effects on the proliferation of HMVECs (Fig. 1).

IL-17A/F has no direct biological effects on the growth of HMVECs.
HMVECs were allowed to grow over 3, 5 or 7 days in the presence or absence of either increasing concentrations of recombinant human IL-17A/F or 10 ng/mL bFGF. Bars represent mean cell number ± standard deviation (SD) (n = 3). The result is a representative of 2 independent experiments (Day 3: control vs. 10 ng/mL bFGF, *p < 0.002; Day 5: control vs. 10 ng/mL bFGF, **p < 0.0005; Day 7: control vs. 10 ng/mL bFGF, ***p < 0.0006).
We next examined whether IL-17A/F is chemotactic for vascular endothelial cells. For this purpose, migration assay was performed using a modified Boyden Chemotaxicell chamber (KURABO), in which we tested various concentrations of recombinant human IL-17A/F as well as 10 ng/mL IL-17A as a positive control. In our previous experiments, HMVECs exhibited greater chemotactic responsiveness to IL-17A than did HUVECs (our unpublished data). Therefore, we used HMVECs for the migration assay. Like IL-17A, we observed a dose-dependent chemotactic response of HMVECs toward IL-17A/F (Fig. 2C-F). The maximal chemotactic response occurred around 10 ng/mL IL-17A/F (Fig. 2D, H). It seems that the effect of IL-17A/F to stimulate migration of HMVECs is slightly lower than that of IL-17A. Furthermore, the specificity of this chemotactic response was determined by using anti-human IL-17RA mAb and anti-human IL-17RC polyclonal Abs. IL-17A/F-stimulated migration of HMVECs was significantly inhibited when cells were treated with Abs to IL-17RA and IL-17RC (Fig. 2G, H). In addition, to define which intracellular signaling pathways are involved in IL-17A/F-induced HMVEC chemotaxis, HMVECs were preincubated with specific inhibitor to PI3K (20 μM LY294002), ERK (20 μM PD98059), or JNK (10 μM SP600125) for 45 min, and further incubated with respective inhibitor for 4 hours in the modified Boyden Chemotaxicell chamber with IL-17A/F (10 ng/mL). Inhibition of ERK or JNK was ineffective in suppressing IL-17A/F-mediated HMVEC migration. In contrast, inhibition of PI3K significantly reduced migration (Fig. 2I).
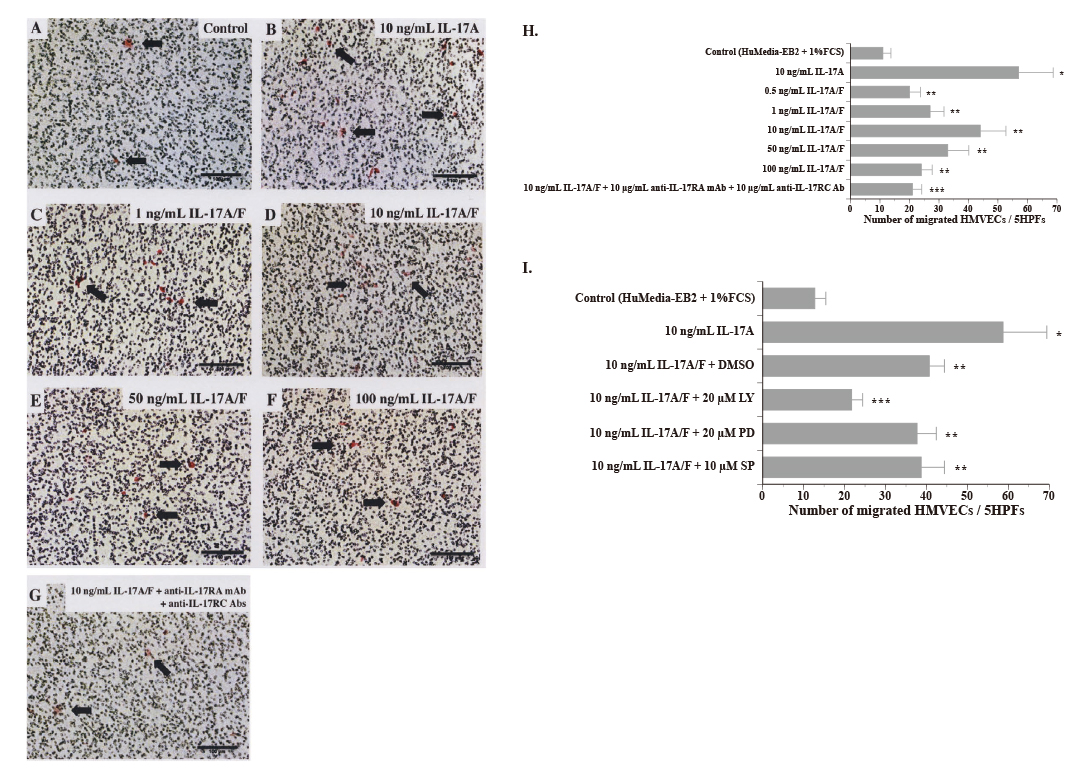
Dose-dependent migration of HMVECs following stimulation with IL-17A/F.
HMVEC chemotaxis was performed in a Chemotaxicell chamber with or without varying concentrations of IL-17A/F or 10 ng/mL IL-17A. Representative photograph of migrated HMVECs stimulated with (A) medium alone, (B) 10 ng/mL IL-17A, (C) 1 ng/mL IL-17A/F, (D) 10 ng/mL IL-17A/F, (E) 50 ng/mL IL-17A/F, (F) 100 ng/mL IL-17A/F, or (G) 10 ng/mL IL-17A/F + 10 μg/mL anti-IL-17RA mAb + 10 μg/mL anti-IL-17RC Ab. (Scale bar = 100 µm) (H) Dose-response curve of IL-17A/F-mediated HMVEC chemotaxis. A wide range of doses of IL-17A/F significantly stimulated the migration of HMVECs. IL-17A (10 ng/mL) also markedly stimulated the migration of HMVECs. Bars represent the mean number of migrated cells ± SD per 5 high power fields (HPFs) (× 200) (n = 4). The result is a representative of 2 independent experiments. (control vs. 10 ng/mL IL-17A, *p < 0.003; control vs. 0.5-100 ng/mL IL-17A/F, *p < 0.03; 10 ng/mL IL-17A/F vs. 10 ng/mL IL-17A/F + 10 μg/mL anti-IL-17AR mAb + 10 μg/mL anti-IL-17RC Ab, ***p < 0.02). (I) To determine intracellular signaling pathways associated with IL-17A/F-induced migration of HMVECs, cells were treated with the chemical inhibitor for PI3K (LY294002), ERK (PD98059) or JNK (SP600125). Inhibition of PI3K only down-regulated HMVEC migration induced by IL-17A/F. Bars represent the mean number of migrated cells ± SD per 5 high power fields (HPFs) (× 200) (n = 3). The result is a representative of 2 independent experiments. (control vs. 10 ng/mL VEGF, *p < 0.003; control vs. 10 ng/mL IL-17A/F + DMSO, control vs. 10 ng/mL IL-17A/F + PD98059 or control vs. 10 ng/mL IL-17A/F + SP600125, **p < 0.002; 10 ng/mL IL-17A/F + DMSO vs. 10 ng/mL IL-17A/F + LY294002, ***p < 0.002).
We further examined the effects of IL-17A/F on vascular endothelial cell capillary-like tube formation using Angiogenesis assay kit (KURABO). Control culture developed small amounts of capillary-like vessel formation (Fig. 3A). VEGF (10 ng/mL) and IL-17A (50 ng/mL) as positive controls markedly increased vessel-like structure (Fig. 3B, H). Suramin as a negative control markedly inhibited the capillary-like tube formation (Fig. 3C). The addition of a wide range of doses of recombinant human IL-17A/F protein at the start of assay and with each medium change resulted in a significantly enhanced development of structures resembling a microvasculature bed in a concentration-dependent fashion (Fig. 3D-G). For example, the capillary-like vessels induced by 10 ng/mL IL-17A/F exhibited a significant increase that was more than three-fold compared to the control (Fig. 3E). Ten ng/mL of IL-17A/F developed most abundant microvessel-like structures in this assay system.
To delineate the role of IL-17RA and IL-17RC, we evaluated whether Abs to IL-17RA and IL-17RC could block IL-17A/F-mediated capillary-like tube formation. HUVECs and HDFs were co-cultured with 10 ng/mL IL-17A/F in the presence or absence of Abs to IL-17RA and IL-17RC. As shown in Fig. 3H, IL-17A/F-dependent capillary formation was significantly decreased when the cells were treated with both anti-human IL-17RA mAb and anti-human IL-17RC Abs. In addition, to examine the contribution of the PI3K, ERK or JNK pathway to the IL-17A/F-induced capillary-like vessel formation in a co-culture system of HUVECs and HDFs, we used a panel of commercially available pharmaceutical inhibitor LY294002, PD98059, or SP600125. As shown in Fig. 3H, IL-17A/F-promoted tube formation was significantly attenuated by PI3K inhibitor LY294002 and ERK inhibitor PD98059, but not JNK inhibitor SP600125. These findings indicated that IL-17A/F promotes capillary-like vessel formation in this co-culture system through the PI3K and ERK signaling pathways.

IL-17A/F promotes capillary-like endothelial tube formation.
An in vitro angiogenesis assay was performed using a 2-dimensional co-culture system of HUVECs and HDFs, which were stimulated with a wide range of doses of IL-17A/F, 50 ng/mL IL-17A, 10 ng/mL VEGF or 100 μM suramin for 11 days. (A) CD31-stained microvessels developed by medium alone. (B) CD31-stained microvessels developed by 10 ng/mL VEGF. (C) CD31-stained microvessels developed by 100 μM suramin. (D-G) CD31-stained microvessels developed by 1-100 ng/mL IL-17A/F. (H) CD31-stained microvessels developed by 10 ng/mL IL-17A. (Scale bar = 500 µm) (I) 1-100 ng/mL IL-17A/F significantly promotes endothelial capillary tube formation. Both 10 ng/mL IL-17A and 10 ng/mL VEGF also significantly enhanced development of microvessel-like structures. Suramin inhibited the development of microvasculature. Bars represent the mean number of pixels ± SD per 9 HPFs (× 40) (n = 3). The result is a representative of 2 independent experiments. (control vs. 10 ng/mL VEGF, *p < 0.002; control vs. 100 μM suramin, **p < 0.009; control vs. 1-100 ng/mL IL-17A/F, ***p < 0.008; control vs. 50 ng/mL IL-17A, ****p < 0.002) (J) Effect of specific inhibitor PI3K (LY294002), ERK (PD98059) or JNK (SP600125) on IL-17A/F-induced capillary-like endothelial tube formation in a co-culture system of HUVECs and HDFs. Inhibition of either PI3K or ERK significantly attenuated the capillary-like endothelial tube formation induced by IL-17A/F. Bars represent the mean number of pixels ± SD per 9 HPFs (× 40) (n = 3). The result is a representative of 2 independent experiments. (control vs. 10 ng/mL VEGF, *p < 0.0002; control vs. 10 ng/mL IL-17A/F + DMSO or SP600125, **p < 0.0008; 10 ng/mL IL-17A/F + DMSO vs. 10 ng/mL IL-17A/F + LY294002, ***p < 0.0003; 10 ng/mL IL-17A/F + DMSO vs. 10 ng/mL IL-17A/F + PD PD98059, ****p < 0.002; 10 ng/mL IL-17A/F + DMSO vs. 10 ng/mL IL-17A/F + 10 µg/mL anti-IL-17AR mAb + 10 µg/mL anti-IL-17RC Ab, *****p < 0.00005).
In our previous experiments using IL-17A, we found that, among various kinds of angiogenic mediators tested, IL-17A selectively and markedly up-regulated secretion of several angiogenic chemokines IL-8, GRO-α and epithelial-derived neutrophil-activating peptide (ENA)-78 and angiogenic growth factor VEGF-A (Numasaki et al. 2004a, 2005). Therefore, we next tested whether IL-17A/F might up-regulate secretion of IL-8, GRO-α, ENA-78 or VEGF-A by HDFs. IL-17A/F significantly increased secretion of IL-8 and GRO-α by HDFs in a dose-dependent manner (Fig. 4A, B). In addition, to a lesser degree, 100 ng/mL IL-17A/F slightly increased secretion of VEGF-A (Fig. 4C). On the other hand, IL-17A/F-induced up-regulated secretion of ENA-78 was not observed (Fig. 4D).

IL-17A/F up-regulates secretion of angiogenic chemokines GRO-α and IL-8 and an angiogenic stimulator VEGF by dermal fibroblasts.
Dermal fibroblasts were cultured with or without cytokine (1, 10 or 100 ng/mL IL-17A or 1, 10, or 100 ng/mL IL-17A/F). After 48 hours of culture, VEGF, GRO-α, IL-8 and ENA-78 levels in the supernatants were measured using an ELISA. Results are expressed as the mean ± SD of triplicate cultures. The data are representative of two independent experiments. (VEGF: control vs. 10 or 100 ng/mL IL-17A, *p < 0.02; control vs. 100 ng/mL IL-17A/F, **p < 0.05), (GRO-α: control vs. 1-100 ng/mL IL-17A, *p < 0.0002; control vs. 10 or 100 ng/mL IL-17A/F, **p < 0.002), (IL-8: control vs. 1-100 ng/mL IL-17A, *p < 0.03; control vs. 10 or 100 ng/mL IL-17A/F, **p < 0.03) (ENA-78: control vs. 100 ng/mL IL-17A, *p < 0.002).
In the present study, we investigated the biological effects of IL-17A/F on angiogenesis-related vascular endothelial cell functions such as growth, migration and capillary-like tube formation. Our results demonstrated that although IL-17A/F is not mitogenic for vascular endothelial cells, this cytokine promotes migration and capillary-like tube formation of vascular endothelial cells in a dose-dependent manner, which is mostly due to its ligation to heterodimeric receptor IL-17RA and IL-17RC. Our results also indicated that the PI3-kinase signaling pathway is involved in IL-17A/F-induced migration and capillary-like tube formation of vascular endothelial cells. Moreover, ERK pathway is also involved in capillary-like tube formation induced by IL-17A/F in a co-culture system of HUVECs and HDFs. In addition, IL-17A/F significantly increased secretion of angiogenic chemokines IL-8 and GRO-α and proangiogenic factor VEGF-A by HDFs. These findings demonstrate a novel biological function for IL-17A/F as an indirect mediator of angiogenesis.
We previously described that IL-17A is an indirect angiogenic mediator, which elicits the formation of new capillaries from pre-existing blood vessels in rat cornea assay, and stimulates migration and capillary-like cord formation of vascular endothelial cells (Numasaki et al. 2003). As is the case with IL-17A, IL-17A/F significantly promotes migration and capillary-like structure, but not growth, of vascular endothelial cells. On the other hand, IL-17E/IL-25 is also an angiogenic stimulator, which signals through heterodimeric receptor complex composed of IL-17RA and IL-17RB. Unlike IL-17A and IL-17A/F, IL-17E/IL-25 has the ability to promote the growth of vascular endothelial cells in a concentration-dependent manner (Corrigan et al. 2011). Conversely, IL-17B and IL-17F negatively regulates angiogenesis through inhibition of migration and/or capillary tube formation of vascular endothelial cells (Sanders et al. 2010). It is of particular interest that IL-17F, which signals through the same heterodimeric receptor as IL-17A, negatively regulates angiogenesis-related vascular endothelial cell functions. This functional difference might be due to the different binding affinity of IL-17A and IL-17F to the heterodimeric receptor subunit IL-17RA (Toy et al. 2006; Kuestner et al. 2007). Taken together, the IL-17 cytokine family members seem to regulate angiogenesis-related vascular endothelial functions positively or negatively through distinct mechanisms.
Cytokines and chemokines, which failed to demonstrate mitogenic activity for vascular endothelial cells, but promoted migration and capillary-like endothelial tube formation, have been termed indirect angiogenic factors (Fràter-Schröder et al. 1987; Phillips et al. 1993; Brogi et al. 1994). Investigations of the mechanisms responsible for the above-mentioned properties of indirect angiogenic agents indicated that indirect angiogenic stimulators mediate new blood vessel formation via either induction of endothelial cell mitogen(s) such as VEGF-A and bFGF by a variety of cell types including vascular smooth muscle cells and interstitial mesenchymal cells (Terrell and Swain 1991; Phillips et al. 1993; Brogi et al. 1994; Cohen et al. 1996; Pola et al. 2001) or recruitment of inflammatory cells such as macrophages, which release the angiogenic growth factors at the site of neovascularization (Fràter-Schröder et al. 1987; Pola et al. 2001). We found that, like IL-17A, IL-17A/F has the biological action to stimulate secretion of angiogenic growth factors such as IL-8, GRO-α and VEGF by HDFs. Therefore, there might be a possibility for IL-17A/F to up-regulate secretion of several angiogenic growth factors, which, in turn, stimulate the growth of vascular endothelial cells in physiological and pathological angiogenesis-related microenvironmental conditions.
Inhibition of IL-17A/F-activated signaling pathways in HMVECs demonstrated that PI3K signaling pathway is critically involved in IL-17A/F-mediated chemotaxis. Therefore, IL-17A/F uses PI3K signaling pathway to stimulate the migration of vascular endothelial cells, as is the same as IL-17A (Pickens et al. 2010). Similar finding has been reported that the PI3K signaling plays an important role in VEGF-A-mediated migration of endothelial cells (Hayashi et al. 2009). Furthermore, Lai et al. (2011) demonstrated that PI3K signaling pathway plays a vital role in IL-8-induced endothelial cell migration. Recent studies also suggest that PI3K signaling plays a critical role in cytokine secretion and regulation of endothelial cell migration (Graupera et al. 2008; Choi et al. 2010). Taken together, these cumulative findings suggest that PI3K signaling pathway is essentially involved in the mediation of angiogenesis-related vascular cell function by various inflammatory stimulators.
In the current experiment, we utilized a co-culture system of HUVECs and HDFs as a model of in vivo angiogenesis. Culture conditions in this system are more similar to the microenvironment in vivo, and capillary-like structures are more stable than those in the frequently utilized extracellular matrix culture system. In a co-culture of HUVECs and HDFs, we found that, unlike migration assay, inhibition of ERK as well as PI3K with the pharmacological inhibitor PD98059 or LY294002 significantly attenuated IL-17A/F-induced capillary-like tube formation. In this co-culture system, even without any supplemented angiogenesis stimulators, capillary-like structures are formed to some extent as a background. Saito et al. (2003) previously reported that addition of anti-VEGF-A neutralizing mAb to this co-culture of HUVECs and HDFs completely blocked the background tube formation. This reported finding strongly suggested that most of basal tube formation in this co-culture system is due to the endogenous VEGF-A, which is mostly secreted by dermal fibroblasts. In addition, IL-17A-induced capillary-like tube formation is significantly inhibited by the addition of a neutralizing mAb against either VEGF-A or IL-8 to this co-culture system (our unpublished observations). Taken together, in addition to the possible direct effects on HUVECs, IL-17A/F might indirectly promote capillary-like tube formation through up-regulation of angiogenic stimulators by fibroblasts in this co-culture system. Actually, in this study, we verified that IL-17A/F significantly increases secretion of IL-8, GRO-α and VEGF-A in dermal fibroblasts. Therefore, one possible explanation for the observed inhibitory effect of ERK inhibitor PD98059, in addition to PI3K inhibitor LY294002, on capillary-like tube formation induced by IL-17A/F is that ERK inhibitor PD98059 could suppress IL-17A/F-induced secretion of angiogenic stimulators in fibroblasts, which, in turn, decreases capillary tube development in a co-culture of HUVECs and HDFs.
It is well known that angiogenesis is critically involved in the pathogenesis of psoriasis and rheumatoid arthritis. IL-17A has been demonstrated to participate in the pathological angiogenesis in rheumatoid arthritis, psoriasis and tumors (Numasaki et al. 2005; Kim et al. 2013; Varricchi et al. 2015). In addition, IL-17A/F is expressed at the lesion of psoriasis, chronic obstructive pulmonary disease and rheumatoid arthritis (Chang et al. 2014; Kakeda et al. 2014; Sarkar et al. 2014). Although the biological activities of IL-17A/F to promote angiogenesis-related endothelial cell functions seem to be slightly lower than those of IL-17A, IL-17A/F may also contribute to the pathological angiogenesis characterized in rheumatoid arthritis and psoriasis.
In conclusion, our findings illustrate a novel biological function for the IL-17 cytokine family member IL-17A/F, mainly produced by Th17 cells and γδ T cells, as an indirect angiogenesis stimulator, which promotes migration and capillary-like tube formation of vascular endothelial cells and up-regulate secretion of proangiogenic growth factors by dermal fibroblasts. Further analyses are needed to elucidate the precise molecular mechanisms of IL-17A/F to promote angiogenesis-related vascular endothelial functions.
We thank Prof. Kazunori Kato, Dr. Jun Kobayashi and Dr. Keizo Kasono for their excellent technical assistance and useful discussions in carrying out this study. Dr. Ohrui was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Culture, Sports, Science and Technology (26460900).
The authors declare no conflict of interest.