2016 Volume 240 Issue 3 Pages 215-220
2016 Volume 240 Issue 3 Pages 215-220
The mechanisms of fetal semi-allograft acceptance by the mother’s immune system have been the target of many immunological studies. Early pregnancy factor (EPF) is a molecule present in the serum of pregnant mammals soon after conception that has been reported to have immunomodulatory effects. In the present study, we aimed to determine whether immune cells such as CD4+CD25+ regulatory T cells (Tregs) are involved in the suppressive mechanism of EPF. Accordingly, CD4+CD25– T cells were isolated from spleens of female C57BL/6 mice and stimulated with anti-CD3 antibody, anti-CD28 antibody and IL-2 in the presence or absence of EPF. Flow cytometry was used to analyze the differentiation of CD4+CD25– T cells to CD4+CD25+ Tregs. We thus found a remarkable rise in the Treg ratio in the EPF-treated cells. Higher mRNA and protein levels of fork head box P3 (Foxp3), a marker of the Treg lineage, were also observed in cells treated with EPF. Furthermore, the effect of EPF on Treg immunosuppressive capacity was evaluated. EPF treatment induced the expression of interleukin-10 and transforming growth factor β1 in Tregs. The suppressive capacity of Tregs was further measured by their capability to inhibit T cell receptor-mediated proliferation of CD4+CD25– T cells. We thus found that EPF exposure can enhance the immunosuppressive functions of Tregs. Overall, our data suggest that EPF induces the differentiation of Tregs and increases their immunosuppressive activities, which might be an important mechanism to inhibit immune responses during pregnancy.
Pregnancy constitutes an immunological paradox because the fetus, which is antigenically distinct from the mother, is not rejected by her immune system during gestation. Early pregnancy factor (EPF) is a biologically active protein first isolated in the serum of pregnant mammals, that was demonstrated to have immunosuppressive effects by the rosette inhibition test (Morton et al. 1974). The protein was named EPF because it appears early in maternal serum in all mammalian species that have been tested including mice (Morton et al. 1974), horses (Rabinowich et al. 1996), cows (Ghaffari Laleh et al. 2008) and humans (Morton et al. 1992). EPF is an extracellular form of the mitochondrial matrix protein chaperonin 10, and highly conserved between mice and humans (nearly 99% identical at the amino acid level). Immunolocalization of EPF has been reported in early equine embryos and reproductive tissues, i.e., ovary, oviduct and uterine tissue (Grosso et al. 2015). Nahhas and Bernea (1990) reported that trophoblast cells from human embryos from 7 to 9 weeks of pregnancy secreted significant amounts of EPF. Today, EPF is recognized to be an immunosuppressive agent that prevents a fetal-directed immune response during pregnancy. Several studies have also found that EPF treatment can induce immune tolerance in other contexts. For instance, EPF therapies decrease clinical signs of autoimmune diseases in experimental models (Athanasas-Platsis et al. 2003); in an allogeneic graft model, recombinant human EPF was demonstrated to prolong skin graft survival time (Morton et al. 2000). However, the mechanisms that mediate this immunosuppression require further investigation.
A subset of T cells, CD4+CD25+ regulatory T cells (Tregs), can suppress immune responses to both self and foreign antigens. Tregs can be generated in the thymus (tTregs) or differentiate from peripheral CD4+CD25– T cells (induced or iTregs). An increasing number of studies have demonstrated that Tregs are important for embryo implantation. For example, Aluvihare et al. (2004) found that Tregs mediate maternal tolerance to the fetus in the mouse and the depletion of Tregs leads to gestation failure. Two other studies have confirmed this role for Tregs in human pregnancy (Sasaki et al. 2004; Somerset et al. 2004).
The generation and suppressive functions of Tregs can be strongly affected by immunosuppressive drugs or cytokines. Moreover, there is systemic expansion of maternal Tregs during pregnancy (Chen et al. 2003; Demirkiran et al. 2008). However, there have been no reports on a possible association of this phenomenon with EPF expression. In this article, we present evidence demonstrating that EPF converts naïve CD4+CD25– T cells into CD4+CD25+ Tregs in vitro and show that this conversion involves the induction of the transcription factor fork head box P3 (Foxp3). Furthermore, EPF could enhance the suppressive effect of Tregs in vitro. Together, our data highlight novel roles for EPF in controlling the generation and function of Tregs.
Recombinant human EPF was purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-mouse CD3 antibody, anti-mouse CD28 antibody, and recombinant mouse interleukin 2 (IL-2) were obtained from eBioscience Inc. (San Diego, CA, USA). MagCellect mouse CD4+CD25+ Treg cell isolation kit, FITC-conjugated anti-CD4 antibody and PE-conjugated anti-CD25 antibody were purchased from R&D Systems (Minneapolis, MN, USA).
Cell preparationSpleens from female C57BL/6 mice were gently minced in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen-Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum, and then CD4+CD25+ Tregs were isolated using MagCellect mouse CD4+CD25+ Treg cell isolation kit following the manufacturer’s instructions. To isolate CD4+CD25– T cells, anti-CD25 antibody was added into the antibody cocktail and incubated with splenic cells before separation. To prepare antigen presenting cells (APCs), whole spleen cells obtained from nude mice were treated with mitomycin C (50 μg/mL) for 30 min at 37°C, and then cells were washed three times with PBS.
Flow cytometryFreshly isolated CD4+CD25– T cells were co-cultured with APCs and stimulated with 1 μg/mL anti-CD3 antibody, 1 μg/mL anti-CD28 antibody, and IL-2 for 3 days. Cells were fixed in 4% paraformaldehyde for 20 min, and washed three times with PBS. Subsequently, cells were stained with FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 antibodies for 1 h at 4°C protected from light. Next, cells were washed with PBS and directly analyzed by flow cytometry. Data analysis was performed using Flowjo7.6 software (Flowjo Inc., Ashland, OR, USA).
Cell culture and Treg cell suppression assaysPurified CD4+CD25– T cells were co-cultured with APCs in RPMI 1640 at 37°C under 5% CO2 with or without EPF, and stimulated with anti-CD3 antibody, anti-CD28 antibody, and IL-2 for 3 days. For the Treg suppression assay, CD4+CD25+ Tregs were treated with EPF for 24 h. Following treatment, cells were washed twice with PBS, and 2 × 104 Tregs were added to anti-CD3 antibody-coated plates containing soluble anti-CD28 antibody and 1 × 104 CD4+CD25– T cells. The ratio of Treg/effector T cells was 1:2. This ratio was chosen so that Tregs did not completely suppress the proliferation of the effector T cells and to allow for the delineation of the EPF effect on Tregs in this assay. Cell growth was measured by the Cell Counting Kit-8 (CCK-8) assay (Beyotime, Haimen, China) at different times (24, 48 and 72 h). Absorbance was read at 450 nm.
Enzyme-linked immunosorbent assay (ELISA) and real-time PCRCD4+CD25– T cells or Tregs were cultured in RPMI 1640, and after treatment, culture supernatants were collected. Subsequently, the concentrations of IL-10 and transforming growth factor (TGF)-β1 were measured with ELISA, following the manufacturer’s instructions. For real-time PCR assays, total RNA was extracted using TRIzol regent (Invitrogen). The reverse transcription reaction contained 1 μg total RNA and utilized the avian myeloblastosis virus reverse transcriptase (Toyobo, Osaka, Japan), and 1 μL of cDNA was subsequently used in a SYBR green PCR assay (Roche LightCycler 480, Basel, Switzerland). Each sample was assayed in triplicate, and mRNA levels were normalized to those of murine glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Specific oligonucleotide primers were designed with Primer Premier 5.0 (Premier Biosoft, Palo Alto, CA, USA) and are listed in Table 1.

Primers used in this study.
Purified CD4+CD25– T cells were co-cultured with APCs in RPMI 1640 at 37°C under 5% CO2 with or without EPF and stimulated with anti-CD3 antibody, anti-CD28 antibody, and IL-2. Three days later, cells were collected and lysed with lysis buffer (65 mM Tris-HCl, 4% sodium dodecyl sulfate, 3% DL-dithiothreitol, and 40% glycerol). Cells lysates were then analyzed for Foxp3 expression by western blot using a specific monoclonal antibody against murine Foxp3 (Cell Signal Technology, Beverly, MA, USA). β-actin was detected as a loading control.
Statistical analysisAll experiments were conducted at least three times to ensure that the results were reproducible. Data are presented as mean ± standard deviation (SD). A P-value less than 0.05 was considered significant, while a P-value less than 0.01 was considered highly significant. All animal experiments were performed in accordance with the ethical guidelines of Xuzhou Medical University.
Previous studies have focused on the direct effects of EPF in mediating immune suppression. In this study we investigated whether EPF could convert CD4+CD25– T cells to CD4+CD25+ Tregs, which then play a role in immunosuppression. To test this hypothesis, purified CD4+CD25– T cells from the spleens of C57BL/6 mice were cultured for 3 days with anti-CD3 antibody, anti-CD28 antibody, IL-2 and APCs in the presence or absence of EPF. The cells were then collected, fixed, and stained with FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 antibodies. Flow cytometry was used to analyze the differentiation of Tregs, and the results are shown in Fig. 1. We found that the addition of EPF promoted Treg generation in vitro compared with T cell receptor (TCR) activation alone in control cultures.
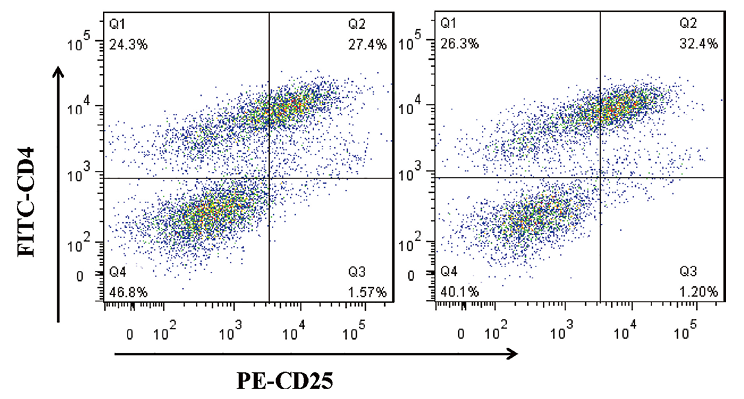
EPF promotes the conversion of naive CD4+CD25− T cells to CD4+CD25+ regulatory T cells.
Freshly isolated CD4+CD25− T cells were co-cultured with APCs and stimulated with anti-CD28 antibody, anti-CD3 antibody, and IL-2 with or without EPF. After 3 days, cells were fixed and stained with FITC-anti-CD4 and PE-anti-CD25 antibodies, and analyzed by flow cytometry. Data represent at least three independent experiments.
Although EPF converted naïve CD4+CD25– T cells into CD4+CD25+ Tregs, the molecular mechanism of this transition was unknown. Foxp3 is a critical gene for the development and function of Tregs. Chen et al. (2003) demonstrated that TGF-β1 induced Treg cell conversion from naïve T cells via Foxp3 upregulation. To determine whether EPF had an impact on Foxp3 expression in CD4+CD25– T cells, freshly purified CD4+CD25– T cells were stimulated with anti-CD3 antibody, anti-CD28 antibody, IL-2 and APCs in the presence or absence of EPF. Real-time PCR was performed to detect Foxp3 expression, and the results suggested that pre-incubation with EPF resulted in more than a 6-fold increase of Foxp3 mRNA expression (Fig. 2A). In addition, western blot analysis of Foxp3 protein expression was consistent with the real-time PCR results (Fig. 2B). The mechanism of Treg-mediated immunosuppression includes the secretion of inhibitory cytokines, such as IL-10 and TGF-β1, which in turn participate in the suppression of maternal alloresponses. To investigate whether EPF-induced Tregs were functionally active, we detected IL-10 and TGF-β1 expression by real-time PCR. As shown in Fig. 2C, compared to the CD4+CD25– T cells, higher expression was detected in EPF-induced Tregs.

EPF and TCR co-stimulation induces Foxp3 expression in naive CD4+CD25− T cells.
CD4+CD25− T cells were sorted from dissected spleens and cultured with anti-CD28 antibody, anti-CD3 antibody, IL-2 and APCs in the absence (control) or presence of EPF for 3 days. (A) Total cellular RNA was extracted from the cells and murine Foxp3 and GAPDH mRNA levels were evaluated by real-time PCR. (B) Cells were collected for western blot analysis using a monoclonal antibody against Foxp3. β-actin expression was used as a loading control. (C, D) CD4+CD25− T cells and EFP-induced Tregs were isolated, IL-10 and TGF-β1 expression were analyzed by real-time PCR. Data represent at least three independent experiments (**P < 0.01).
To extend our investigation into the mechanism of EPF-mediated immunosuppression, we further investigated whether EPF augmented the suppressive role of CD4+CD25+ Tregs in our co-culture system. Firstly, we investigated IL-10 and TGF-β1 expression in CD4+CD25+ Tregs by real-time PCR. The results showed that both IL-10 and TGF-β1 mRNA levels were significantly increased during treatment with EPF (Fig. 3A, B). ELISA was performed to evaluate IL-10 and TGF-β1 secretion into cell culture supernatants, and EPF-treated Tregs showed a significant increase of IL-10 and TGF-β1 secretion (Fig. 3C, D). In addition, the suppressive capacity of CD4+CD25+ Treg cell in the presence of EPF was measured by their capability to inhibit TCR-mediated proliferation of CD4+CD25– T cells. To that end, CD4+CD25+ Tregs were pretreated with EPF and co-cultured with CD4+CD25– T cells. Cell proliferation was measured by CCK-8 at the indicated time points. As shown in Fig. 3E, a higher proliferation rate was found in untreated cells. Taken together, the results suggested that EPF can enhance the immunosuppressive role of Tregs.

EPF promotes the suppressive activity of CD4+CD25+ Tregs.
CD4+CD25+ Tregs were sorted from dissected spleens and cultured with anti-CD28 antibody, anti-CD3 antibody, IL-2 and APCs in the absence (control) or presence of EPF for 3 days. (A, B) Cells were harvested and the expression of IL-10 and TGF-β1 were analyzed by real-time PCR; data are presented in values relative to GAPDH. (C, D) IL-10 and TGF-β1 levels in supernatants were analyzed by ELISA. (E) CD4+CD25− T cells and CD4+CD25+ Tregs were isolated from dissected spleens and co-cultured at a 2:1 ratio. CCK-8 was used to measure cell proliferation at different time points (24, 48 and 72 h). Data represent at least three independent experiments (*P < 0.05, **P < 0.01).
In a normal pregnancy, the maternal immune system must tolerate an allogenic fetus. EPF can be detected in serum 6 h after fertilization in mice and within 48 h in humans, and its expression is maintained virtually until the end of pregnancy (Morton et al. 1976; Morton et al. 1992). Its immunosuppressive role during pregnancy was first shown by Morton et al. (1974) using the rosette inhibition test. EPF is now recognized to be an immunosuppressive agent involved in developing the immune tolerance required for implantation. In addition, in a number of model systems, increased levels of EPF were shown to be correlated with down-regulated immune responses. For example, EPF can prolong allogeneic skin graft survival time in rats (Morton et al. 2000), and was found to play an important role in the reduction of disease signs in a murine model of experimental autoimmune encephalomyelitis (Athanasas-Platsis et al. 2003).
EPF might employ a number of mechanisms to influence immunosuppression. It has been demonstrated that EPF can suppress the immune system by reducing T-cell proliferation and cytokine production (Rabinowich et al. 1996; Reichert et al. 1998; Cheriyan et al. 2009). However, the contribution of EPF to Treg differentiation and function has not been previously described. The goal of this study was to explore the relationship between EPF and Tregs in vitro. By following the differentiation of CD4+CD25– T cells, we found that addition of EPF to the T cell co-culture system resulted in a significant increase in Treg percentage. The results indicate that CD4+ CD25– T cells can be induced to form CD4+CD25+ Tregs by EPF. Foxp3 has been shown to be the most reliable Treg cell marker (Geiger and Tauro 2012; Rowe et al. 2012) and is associated with the development of Treg function. Retroviral gene transfer of Foxp3 into naïve responder T cells converts them towards a Treg phenotype (Fontenot et al. 2003; Hori et al. 2003; Khattri et al. 2003). Here, we demonstrated that EPF exposure upregulated Foxp3 mRNA and protein levels in CD4+CD25– T cells. These results further demonstrated the role of EPF on Treg polarization. In addition, we have demonstrated that EPF-induced Tregs were functionally active. We conclude from the described data that induction of Treg cell polarization might be an important mechanism of EPF-mediated immunosuppression.
Further confirmation of another EPF activity in Tregs was provided by the analysis of IL-10 and TGF-β1 expression; namely, the expression of IL-10 and TGF-β1 was significantly enhanced by EPF. It is conceivable that IL-10 and TGF-β1, secreted by Tregs, may play an important role in immunosuppression. We thus analyzed the function of Tregs by performing an in vitro suppression assay, showing that EPF-treated Tregs were more potent in suppressing the proliferation of naïve T cells than control Tregs. Taken together, our findings uncover a new immunosuppressive mechanism of EPF; EPF regulates Treg development and function, which may be an important mechanism of fetal tolerance by the maternal immune system during pregnancy.
This research was financially supported by the Scientific Research Foundation of Xuzhou Medical University No. 53631319, Natural Science Foundation of Jiangsu province (grant number BK20151151), China Postdoctoral Science Foundation funded project (grant number 2016M590506) and Jiangsu Planned Projects For Postdoctoral Research Funds (grant number 1501010B). All the experiments in this article were completed in Research Center for Neurobiology of Xuzhou Medical University. We would also like to thank our colleagues at the institute for their support and help during the experiments.
The authors declare no conflict of interest.