2017 Volume 241 Issue 2 Pages 149-153
2017 Volume 241 Issue 2 Pages 149-153
Infants with Down Syndrome (DS) are at risk of developing a transient abnormal myelopoiesis (TAM). TAM is characterised by increased circulating blast cells but usually self-limiting. DS patients with TAM sometimes show fetal hydrops and effusion in body cavities, but the mechanism remains unclear. We report here a case of infant with DS who had pericardial effusion, TAM, and eosinophilia. In her pericardial effusion, white blood cell count was 6.0 × 103/µL, 41% of which were eosinophils. After administration of prednisolone, pericardial effusion gradually decreased, and TAM and eosinophilia improved. In order to elucidate the immunological mechanism, we measured the levels of 17 cytokines in her pericardial effusion fluid and serum. In her pericardial fluid, there were high levels of 12 cytokines, and they were higher than those in her serum. In particular, IL-6 (44,573 pg/mL), IL-8 (4,865 pg/mL), and IL-13 (579.41 pg/mL) were at extremely high levels in her pericardial fluid. After administration of prednisolone, the levels of 8 of the 12 elevated cytokines in her pericardial fluid decreased and all of the elevated cytokines decreased in her serum. Corticosteroids can be effective to reduce cytokine levels and the amount of effusion in patients with DS. It is presumed that effusion seen in DS with TAM could be related to an abnormal production of cytokines at the effusion site.
Infants with Down Syndrome (DS) are at risk of developing a transient myeloproliferative disorder (TMD) in the neonatal period that is also known as transient abnormal myelopoiesis (TAM) or transient leukemia (TL) (Roy et al. 2012). TAM is estimated to occur in 4-10% of neonates with DS (Zipursky 2003; Pine et al. 2007). TAM is characterised by increased circulating blast cells that harbor acquired N-terminal truncating mutations in the key hematopoietic transcription factor gene GATA1 (Wechsler et al. 2002; Groet et al. 2003; Rainis et al. 2003; Bhatnagar et al. 2016). Furthermore, the interaction between DS, GATA1 mutations and TAM was reported, recently (Banno et al. 2016). TAM is usually self-limiting and undergoes spontaneous regression, but 10-20% of neonates with TAM subsequently develop myeloid leukemia in the first 5 years of life. Fetal hydrops and effusion in body cavities are sometimes seen in patients with DS. Although fetal hydrops in DS may be associated with TAM (Zipursky et al. 1999), the mechanism remains unclear.
We encountered a patient with DS who had pericardial effusion, TAM, and eosinophilia. Her pericardial effusion improved by administration of a corticosteroid.
In order to elucidate her immunological state, we examined the cytokine profile of her sera and pericardial effusion fluid. We found extremely high levels of several cytokines in her pericardial fluid compared to those in her sera. It is thought that effusion in DS with TAM could be related to an abnormal production of cytokines at the effusion site.
The patient was a female neonate with DS. Her mother was 37 years old, and it was a natural conception. In fetal echograph at 36 weeks of gestation, the patient was found to have pericardial effusion. Her mother delivered by scheduled caesarean section at 37 weeks of gestation because hepatomegaly was seen and progression of TAM was suspected. Her birth weight was 2,413 g and her Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. She showed a particular appearance of DS and had a hepatomegaly (3 cm below the costal margin), but she did not show any respiratory or circulatory disturbance at birth. Cardiomegaly appeared on X-ray examination. Pericardial effusion was confirmed with ultrasonography, but cardiac anomaly, ascites or pleural effusion could not be identified. Her platelet count was 298 × 103/µL, and her white blood cell count was 36.8 × 103/µL. The percentages of blasts and eosinophils were 19% and 32% of white blood cells in her peripheral blood, respectively. Her serum chemistry values on admission showed a direct bilirubin level of 1.7 mg/dL, aspartate aminotransferase (AST) level of 24 U/L, alanine aminotransferase (ALT) level of 30 U/L, typeⅣ collagen levels of 30.1 ng/mL and hyaluronic acid levels of 173.6 ng/mL. Her serum level of C-reactive protein was 0.03 mg/dL. Her karyotype was 47, XX, +21, and she had GATA1 mutation of c.49C>T (p.Q17X). This nonsense mutation has been reported in previous genetic analysis for TAM as a cause of premature termination codon which induces short form of GATA1 (GATA1s) (Kanezaki et al. 2010). We diagnosed her as DS with TAM. We started to administrate oxygen shortly after birth because she showed pulmonary hypertension. Her pericardial effusion increased and her respiratory condition worsened. On the 6th day of life, she underwent pericardial fenestration and a chest drain tube was placed in her left pleural cavity in order to decrease the amount of effusion. In her pericardial effusion fluid, triglyceride level was 15 mg/dL and white blood cell count was 6.0 × 103/µL (41% were eosinophils but no blasts). Bacterial culture of the pericardial fluid was negative, and the results of pathology of the removed epicardium revealed no malignancy and no inflammation at the site.
As shown in Fig. 1, pericardial effusion was drained at 40 ml per day on the 6th day of life and 36 ml per day on the 10th day of life. We presumed that the abnormal immune reaction might have been related to the pericardial effusion, and so we started administration of a corticosteroid (prednisolone 2 mg/kg per day) from the 10th day of life. Pericardial effusion gradually decreased and almost disappeared by the 20th day of life, and her respiratory condition gradually improved. We removed the chest drain tube on the 22th day of life and we tapered the dose of prednisolone. White blood cell count was 6.0 × 103/µL in her peripheral blood, and the percentage of blasts and eosinophils of the white blood cells had decreased to 2.5% and 1.5%, respectively, on the 45th day of life. The patient was discharged from the hospital on the 49th day of life. Although prednisolone was discontinued on the 51th day of life, pericardial effusion did not appear again. Our investigation was approved by Research Ethics Committee of our Institution. The parents of infant were informed of the study design, and written informed consent was obtained.
In order to elucidate the immunological pathophysiology of her pericardial effusion, we measured cytokine levels in her pericardial effusion fluid and serum several times during her clinical course. We used the BioPlex beads suspension array (BioRad, Hercules, CA) and Luminex 100 (Mirai Bio, Alameda, CA) system, as described previously (Takahashi et al. 2009). The BioPlex human cytokine 17-plex panel was used. Data are shown in Table 1 and Fig. 1. In her serum before administration of prednisolone, levels of pro-inflammatory cytokines (IL-6) were elevated, but the levels of Th1 cytokines (IFN- and IL-2) were not at detectable levels. Among Th2 cytokines, IL-13 was elevated, but IL-4, IL-5, and IL-10 were at comparable levels to those of serum controls. The level of IL-17 was also high. The level of the chemokine macrophage inflammatory protein 1 β (MIP-1β) was elevated, but IL-8 and MCP-1 were not elevated. In her pericardial fluid before administration of prednisolone, tumor necrosis factor-α (TNF-α) and IL-6 were at very high levels, and especially the level of IL-6 was at an extremely high of 44,573 pg/mL. The levels of IFN-γ and IL-12 were also high. IL-13 was also at a very high level. The levels of IL-17 and growth factors, such as IL-7, GM-CSF, and G-CSF, were at moderately high levels compared to those of the serum controls. The levels of chemokines IL-8, MCP-1, and MIP-1β were also at high levels, and especially the level of IL-8 was extremely high at 4,865 pg/ml. Except for IL-17 and GM-CSF, cytokine levels were higher in pericardial fluid than in the serum. After administration of prednisolone, the levels of all of the elevated cytokines in the serum and 8 cytokines among the 12 elevated cytokines in the pericardial fluid gradually decreased.
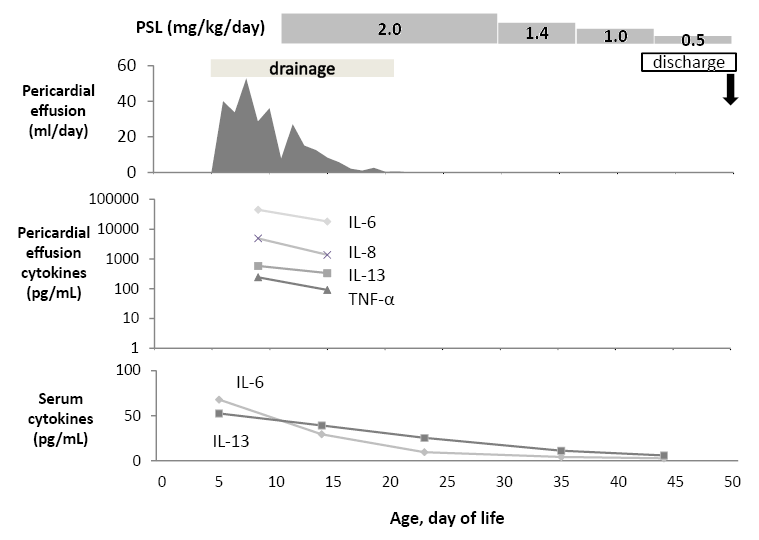
The clinical course of our patient.
PSL, prednisolone; WBC, white blood cells; Eosino, Eosinophils; TNF-α, tumor necrosis factor α.

Concentrations of cytokines, chemokines, and growth factors in our patient’s serum and pericardial effusion fluid and in neonatal controls with other complications.
DOL, day of life; TNF-α,tumor necrosis factor α; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony stimulating factor; MCP-1, monocyte chemotactic protein 1; MIP-1β, macrophage inflammatory protein 1β.
aThe unit of concentration of cytokines, chemokines and growth factors is all pg/mL in this Table.
bThe control group included 8 newborn patients who were admitted to the NICU with various risks. Among them, 5 had slightly low birth weight, 2 were infants of diabetic mothers, and another had hyperbilirubinemia caused by breastfeeding. Peripheral blood was taken from patients in the control group between their 4th and 8th days of life with informed consent from their parents. The control data are shown as mean ± 1 SD.
Some characteristics of cytokine production in patients with DS have been described (Shimada et al. 2007; Trotta et al. 2011; Nateghi Rostami et al. 2012). Shimada et al. (2007) reported higher levels of IL-1β, TNF-α, and IFN-γ in DS patients with TAM and concluded that abnormal cytokinemia may play a role in the pathophysiology of TAM. Trotta et al. (2011) reported a spontaneous increase in the production of IFN-γ, TNF-α, and IL-10 in adult patients with DS. Nateghi Rostami et al. (2012) found high levels of TNF-α and IFN-γ and low levels of IL-10 in patients with DS. Transforming growth factor (TGF)-β1 is a likely candidate involved in liver fibrogenesis of TAM (Arai et al. 1999). And recently Maeda reported that IL-8 was associated with TAM with liver fibrosis (Maeda et al. 2016). But these reports did not refer about the association between DS and pericardial effusion.
There have been some case reports on DS with pericardial effusion (Kusanagi et al. 1998; Hirashima et al. 2000; Shenoy et al. 2008; Buyukkale et al. 2012). Kusanagi et al. (1998) reported a DS patient who had hypereosinophilic syndrome, TAM and pericardial effusion. The patient had 28% eosinophils and 27.5% blasts in her peripheral white blood cells. They found a large number of eosinophils in her pericardial fluid and it was controlled by prednisolone. Hirashima et al. (2000) reported a patient who had TAM and isolated pericardial effusion. The patient had 49% blast cells of peripheral white blood cells, but they did not mention about eosinophils. Shenoy et al. (2008) reported a patient who had TAM and eosinophilic pericardial effusion. They found 29% eosinophils but no blasts in her pericardial effusion fluid, and her effusion was controlled by administration of prednisolone. Buyukkale et al. (2012) reported a patient who had TAM associated pericardial tamponade. Their patient had 61% blast cells in his peripheral blood but no eosinophils. His condition was also improved by oral administration of prednisolone. All 4 cases were controlled by prednisolone and the patients survived. These 4 cases are very similar to our case, but they did not investigate the mechanism of pericardial effusion. We think that their pericardial effusion might have been related to TAM, eosinophilia and cytokines, as was the case in our patient.
In other medical fields not related to the perinatal period, there have been some reports on cytokines related to pericardial effusion (Shikama et al. 2000; Kazama et al. 2003). Shikama et al. (2000) reported a case of rheumatoid pericarditis with cardiac tamponade. IL-6 and IL-8 were elevated at 2,950 pg/mL and 233 pg/mL, respectively, in pericardial fluid. But these cytokines were at normal levels in peripheral blood. Kazama et al. (2003) reported a 25-year-old male with acute necrotizing eosinophilic myocarditis with cardiac tamponade. The patient recovered without administration of a corticosteroid. The patient did not show inflammation of the pericardium, and they did not investigate the pathology of the myocardium. The authors found 4,320 pg/mL of IL-5 and 220 pg/mL of IL-13 in pericardial effusion fluid, and IL-5 was elevated at 94 pg/mL in peripheral blood.
In our patient, TNF-α and IL-6 might have played a role in retention of pericardial effusion fluid, because they are known to increase microvascular permeability. IL-13, a Th2 cytokine, may play a major role in increasing eosinophils, while IL-5, the most potent cytokine to induce accumulation of eosinophils may not be effective in this patient. We think that cytokines related to TAM or eosinophilia can induce pericardial effusion. However, it is still not clear what mechanism induced the hyper-cytokine state at the pericardial site in our patient. Further studies are needed to clarify the immunological mechanism of TAM and pericardial effusion in patients with DS. In this study, a limitation exists. Our control group is non-DS population. We should compared cytokine levels of our patient to DS infants without TAM. But we did not have non-TAM cytokine profile in serum.
Clinically, corticosteroids can be effective in reducing the cytokine level in pericardial effusion fluid and the amount of pericardial effusion in patients with DS. We presume that administration of a corticosteroid would be highly effective to improve effusion in DS patients with TAM.
This research was supported by Japan Society for the Promotion of Sciences (JSPS), Grant-in-Aid for Scientific Research [KAKENHI] Grant Number 22390215.
The authors declare no conflict of interest.