2017 Volume 241 Issue 3 Pages 175-182
2017 Volume 241 Issue 3 Pages 175-182
Bach2 is a transcription factor which represses its target genes and plays important roles in the differentiation of B and T lymphoid cells. Bach2-deficient (KO) mice develop severe pulmonary alveolar proteinosis, which is associated with increased numbers of granulocytes and T cells. Bach2 is essential for the regulation of T cells, but its role in the regulation of granulocytes is not clear. Here, we observed increased numbers of eosinophils but not neutrophils in the bone marrow, spleen, peripheral blood, and bronchoalveolar lavage fluids of Bach2 KO mice compared with those of wild-type (WT) mice. Upon co-transplantation of the bone marrow cells from CD45.2 Bach2 KO and CD45.1/CD45.2 double-positive WT mice to irradiated WT CD45.1/CD45.2 mice, the reconstituted numbers of eosinophils were similar between Bach2 KO and WT cells. These results showed that the deficiency of Bach2 in eosinophils did not directly drive the differentiation of eosinophils. To investigate the effect of Bach2 KO CD4+ T cells upon eosinophils, we analyzed Rag2/Bach2-double deficient (dKO) mice which lack lymphocytes including CD4+ T cells. Rag2/Bach2 dKO mice did not show any increase in the numbers of eosinophils. Importantly, Bach2 KO mice showed an increase of interleukin-5 (Il-5) in the sera compared with WT mice. These results suggest that up-regulated functions of CD4+ T cells including secretion of Il-5 resulted in proliferation and/or migration to peripheral tissues of eosinophils in Bach2 KO mice. We propose that Bach2 controls homeostasis of eosinophils via restricting the production of Il-5 in CD4+ T cells.
Bach2 is a transcription repressor which belongs to the basic region-leucine zipper super-family and binds to Maf-recognition elements (MAREs) by forming heterodimers with some of the Maf family oncoproteins (Oyake et al. 1996). Bach2 plays important roles in B cell development from the progenitors (Itoh-Nakadai et al. 2014), the pre-B cell antigen receptor check point (Swaminathan et al. 2013), and immunoglobulin class-switch recombination and somatic hypermutation of immunoglobulin encoding genes in mature B cells upon activation (Muto et al. 2004; Muto et al. 2010). Bach2 is also essential for the development of effector T cells and regulatory T cells (Roychoudhuri et al. 2013; Tsukumo et al. 2013). In addition, Bach2 is essential for the functional maturation or maintenance of alveolar macrophages. Bach2 KO mice develop pulmonary alveolar proteinosis (PAP) due to a dysfunction of alveolar macrophages (Nakamura et al. 2013; Ebina-Shibuya et al. 2016). Interestingly, the diseased lungs of Bach2 KO mice contain increased numbers of granulocytes (Nakamura et al. 2013). However, the mechanism for this response has remained unclear.
Eosinophils play an important role in the immune response to the infection of helminth parasite. Eosinophils damage helminth parasite by releasing granules containing substances against these organisms (Glauert 1978). Interleukin-3 (Il-3), Il-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF) are essential for the expansion and activation of eosinophils (Warren and Moore 1988; Lopez et al. 1988). Il-5 strongly induces the proliferation and survival of eosinophils in mice (Yamaguchi et al. 1988; Dyer et al. 2008). Eosinophils differentiate from eosinophil lineage committed progenitors (EoPs) in both mouse and human (Iwasaki et al. 2005; Mori et al. 2009). EoPs differentiate from granulocyte macrophage progenitors (GMPs), which also branch into other granulocytes including neutrophils, monocytes and basophils. EoPs express the Il-5 receptor α (Il-5Rα), and therefore their terminal differentiation into eosinophils is strongly driven by Il-5 (Iwasaki et al. 2005).
In adaptive immune responses, the differentiation of CD4+ T cells into effector T helper (Th) cells is one of important regulatory steps. Th cells are distinguished based on their cytokine profiles. T helper cell type 1 (Th1) cells are characterized by the secretion of interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNFα), and play important roles in immune responses against tumor cells or cells infected by virus (Ohta et al. 2000). T helper cell type 2 (Th2) cells secret I1-4, Il-5 and interleukin-13 (Il-13), which are known to induce eosinophils in response to allergic conditions or helminth parasitic infection (Kurup et al. 1994). These cytokines are thus called Th2 cytokines. Eosinophils are regarded as one of the central effector cells in the Th2-type immune responses. Innate lymphoid cells (ILCs) have been described to represent the innate counterpart of the Th cells, and are classified by their cytokine production profile. Innate lymphoid cells 1 (ILC1s) produce IFN-γ (Fuchs et al. 2013). Innate lymphoid cells 2 (ILC2s) produce Il-4, Il-5 and Il-13 (Klein Wolterink et al. 2012). ILC2s are the early innate source of Il-5 and Il-13 after allergen exposure, leading to an induction of eosinophilia.
We have hypothesized that the Bach2 KO mice may suffer from altered regulation of eosinophils. We thus discovered that Bach2 KO mice showed increased numbers of eosinophils in multiple organs and that the increase of eosinophils in Bach2 KO mice was not cell autonomous using genetic depletion of T cells and bone marrow transplantation assays.
Bach2 KO mice on the C57BL/6 background were described previously (Muto et al., 2004). CD45.1 congenic mice were purchased from Sankyo Lab Service Corporation, Inc. (Tokyo, Japan). Recombination-activating gene 2 (Rag2) KO mice on the C57BL/6 background, lacking B and T cells, were previously described (Shinkai et al. 1992). All the mice were kept under specific pathogen-free conditions. All experiments involving mice were approved by the Institutional Animal Care and Use Committee of the Tohoku University Environmental and Safety Committee.
Spleen and bone marrow cellsMice were euthanized using isoflurane. Spleens and bone marrows were isolated and homogenized with slide glasses. Red blood cells were lysed using Red Blood Cell Lysing Buffer Hybri-MAX™ (Sigma Aldrich, Tokyo, Japan). The resulting suspensions of cells were counted and used for experiments.
Lung cellsMice were euthanized using isoflurane. After inserting the cannula into the trachea, the lungs were flushed with 1 ml phosphate buffered saline (PBS) containing 3% fetal bovine serum (FBS), 200 units/ml collagenase type 2 (Worthington, Lakewood, NJ, USA), 500 units/ml hyaluronidase type IV-S (Sigma Aldrich), and 50 units/ml DNase I (Roche, Basel Switzerland). The lungs were isolated and incubated for 1 hour at 37°C in 4 ml PBS containing the above enzymes, followed by homogenization using gentleMACS™ Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). The resulting suspensions of cells were counted and used for experiments.
Bronchoalveolar lavage (BAL) fluids and cellsMice were euthanized using isoflurane and cannula was inserted into the trachea. The lungs were washed three times with 1 ml of 3% FBS/PBS and this procedure was repeated twice. Eosinophils in BAL fluids were defined as F4/80+, CD11b+, SiglecF+ and CD11c− population.
Flow cytometryThe collected bone marrow cells, splenocytes and lung cells were suspended with staining buffer (PBS containing 3% FBS) and stained with fluorescent-conjugated antibodies specific for CCR3 (Biolegend, San Diego, CA, USA), SiglecF (BD Biosciences, Franklin Lakes, NJ, USA), Ly6G (Biolegend), CD11b (BD Biosciences), CD11c (BD Biosciences), Ly6C (BD Biosciences), CD3e (TONBO Biosciences, San Diego, CA, USA), CD4 (Biolegend), CD8 (TONBO Biosciences), CD14 (Biolegend), CD16/32 (BD Biosciences), CD19 (TONBO Biosciences), CD25 (TONBO Biosciences), CD44 (TONBO Biosciences), CD45.1 (Biolegend), CD45.2 (Biolegend), B220 (TONBO Biosciences), Gr-1 (Biolegend), Sca-1 (BD Biosciences), Il-5Rα (BD Biosciences), CD34 (BD Biosciences), and c-kit (Biolegend). The cells were sorted with a FACSAria II (BD Biosciences) and data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Identification of mouse EoPsEoPs were defined as lack of lineage markers (CD3e, CD4, CD8, B220, Gr-1, CD19) and Sca-1−, Il-5Rα+ CD34+ c-kitlo population (Iwasaki et al. 2005).
Identification of mouse ILC2sILC2s were defined as lack of lineage markers (CD3e, CD11b, CD14, CD16/CD32, B220) and CD25+ and CD44+ population (Bartemes et al. 2012).
Bone marrow transplantation experimentThe 8-week-old CD45.1/CD45.2 heterozygous mice were lethally irradiated with 10 Gy of gamma-ray (MDS Nordion, Ottawa, ON, Canada), and were injected on the same day via tail vein with bone marrow nucleated cells isolated from a CD45.1/CD45.2 WT mouse and a CD45.2 Bach2 KO mouse (1.0 × 106 cells each in 150 µl PBS). The mice were sacrificed after 16 weeks of the transplantation. Our pilot transplantation experiments revealed that more than 90% of eosinophils and neutrophils were reconstituted by the donor cells. According to these findings, most of CD45.1/CD45.2 eosinophils and neutrophils were regarded as cells derived from donor cells.
Enzyme-linked immunosorbent assay (ELISA)The level of Il-5 was measured with Quantikine® ELISA kit (R&D systems, Minneapolis, MN, USA). The ELISA measurements were conducted in strict accordance with the manual of the experimental kit.
Statistical analysesTwo tailed Student’s t-test and Welch’s t-test were used for statistical analysis of comparative data using Microsoft Excel software (Microsoft Corporation, Redmond, WA, USA). Values of p < 0.05 were considered as statistically significant.
To clarify how granulocytes of Bach2 KO mice are altered, we performed flow cytometric analysis, which revealed that FSCmid and SSChigh cells were increased in the spleens of Bach2 KO mice (Fig. 1A, left panels). Next we examined cells in peripheral organs such as the bone marrow and the peripheral blood. This analysis revealed that FSCmid and SSChigh cells were also increased in the bone marrow and the peripheral blood of Bach2 KO mice (Fig. 1A, right-middle and right panels). We then measured granulocytic cell surface markers in the FSCmid and SSChigh fraction of Bach2 KO splenocytes. The flow cytometric analysis revealed high expression of SiglecF and CCR3 in the cell fraction, which are known as specific cell surface markers of eosinophils (Fig. 1A, left-middle panels). Microscopically, the sorted FSCmid and SSChigh cells had segmented nuclei with multiple lobes or ring-shape and eosinophilic granules in the cytoplasm (Fig. 1B), which were consistent with known microscopic characters of eosinophils. Based on these observations, we concluded that the cells increased in Bach2 KO mice were mainly eosinophils.
We counted the numbers of eosinophils and neutrophils in the spleen with flow cytometry. Bach2 KO mice had increased numbers of eosinophils in the spleen compared with WT mice, whereas there was no significant difference in the numbers of neutrophils (Fig. 2A). Similar alterations were observed in the bone marrow and the BAL fluid (Fig. 2B, C). Therefore, Bach2 KO mice have increased numbers of eosinophils in multiple organs.
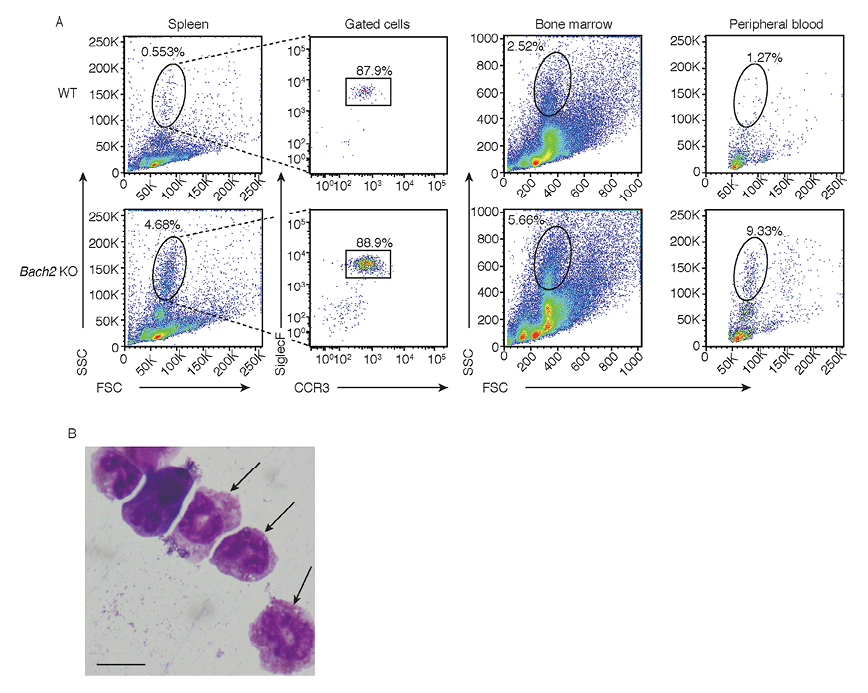
Increased FSCmid and SSChigh eosinophils in Bach2 KO mice.
A) The flow cytometric analysis of splenocytes (left panels), bone marrow cells (right-middle panels), and peripheral blood cells (right panels) from WT and Bach2 KO mice. The left-middle panels show cells expressing eosinophilic makers SiglecF and CCR3. The numbers are percentages of the cells in gated cells. B) May-Giemsa staining of the splenocytes in FSCmid and SSChigh fraction from a Bach2 KO mouse. The arrows indicate eosinophils. Scale bar = 10 µm.
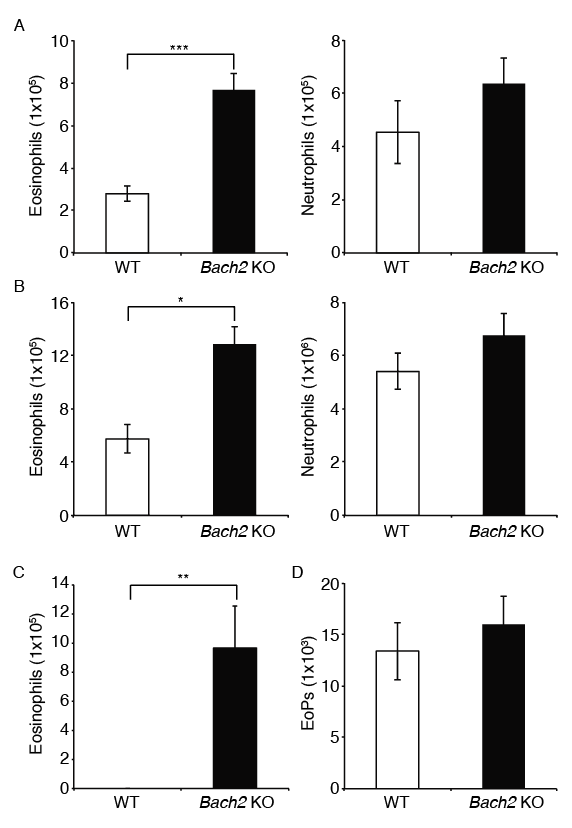
Bach2 KO mice exhibit increased numbers of eosinophils in multiple organs.
A) The cell numbers of eosinophils (left panel) and neutrophils (right panel) in the spleens from five WT mice (mean total cells; 6.7 × 107/mouse) and three Bach2 KO mice (mean total cells; 3.3 × 107/mouse). B) The cell numbers as in A in bone marrows from of three WT mice (mean total cells; 3.1 × 107/mouse) and three Bach2 KO mice (mean total cells; 3.1 × 107/mouse). C) The numbers of eosinophils in the BAL fluids from seven WT mice (mean total cells; 1.6 × 105/mouse) and seven Bach2 KO mice (mean total cells; 3.2 × 106/mouse). D) The cell numbers of EoPs in the bone marrows from four WT mice (mean total cells; 3.9 × 107/mouse) and three Bach2 KO mice (mean total cells; 3.0 × 107/mouse). Data are expressed as the means ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001.
According to the finding that Bach2 KO mice had increased numbers of eosinophils, not neutrophils, in multiple organs, we surmised that Bach2 might play a role in the differentiation of eosinophils after branching into EoPs from GMPs. To address this possibility, we compared the numbers of EoPs (Iwasaki et al. 2005). A flow cytometric analysis revealed that there was no significant difference in the numbers of EoPs between WT and Bach2 KO mice (Fig. 2D). This result suggests that deficiency of Bach2 may not affect the differentiation of EoPs from GMPs.
To investigate whether deficiency of Bach2 directly derives the differentiation and/or recruitment of eosinophils in the organs, we performed a bone marrow co-transplantation assay. The recipient CD45.1/CD45.2 WT mice were lethally irradiated and were injected via tail vein with bone marrow nucleated cells from a CD45.1/CD45.2 WT mouse and a CD45.2 Bach2 KO mouse. After 16 weeks, we assessed cells in the bone marrows and spleens. A flow cytometric analysis revealed that there was no significant difference in the numbers of reconstituted eosinophils and neutrophils derived from each of the donor cells (Fig. 3A, B). In the bone marrow, the numbers of hematopoietic progenitors derived from each donor was nearly equal (Itoh-Nakadai et al. 2017). These results suggest that deficiency of Bach2 in eosinophils does not directly drive the differentiation and/or recruitment of eosinophils. Rather, it appears that cells producing cytokines including Il-5 other than eosinophils affected the differentiation and/or recruitment of eosinophils in the organs of Bach2 KO mice.
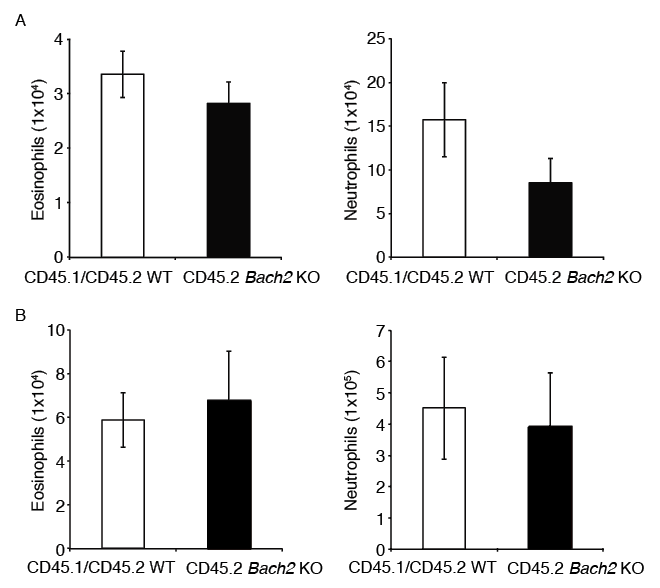
The increase of eosinophils in Bach2 KO mice is non-cell autonomous.
A, B) Co-transplantation experiment model. The recipient mice were 8-week-old CD45.1/CD45.2 WT mice irradiated with 10 Gy. They were injected with bone marrow nucleated cells from a CD45.1/CD45.2 WT mouse and a CD45.2 Bach2 KO mouse via tail vein. The cell numbers of eosinophils (left panels) and neutrophils (right panels) in the spleens (A) (mean total cells; 1.2 × 108/mouse) and bone marrows (B) (mean total cells; 7.6 × 107/mouse) from transplanted mice. The mean values and SEM from five mice are shown.
ILC2s induce terminal differentiation and proliferation of eosinophils by producing Il-5 in the lung (Klein Wolterink et al. 2012). Therefore, we hypothesized that ILC2s were associated with increase of eosinophils in Bach2 KO mice. A flow cytometric analysis revealed that the numbers of ILC2s in the Bach2 KO lung tended to increase, but it was not statistically significant (Fig. 4A).
Like ILC2s, Th2 cells promote eosinophil differentiation and/or proliferation by producing Il-5. Deficiency of Bach2 in CD4+ T cells results in elevated levels of Th2 cytokines such as Il-4, Il-5 and Il-13 (Kuwahara et al. 2014). To investigate the effect of Bach2 deficiency in lymphocytes including T cells on the differentiation and/or proliferation of eosinophils, we generated Rag2/Bach2 double KO (dKO) mice in which lymphocytes, including Th2 cells, are absent. A flow cytometric analysis of splenocytes and bone marrow cells revealed that Rag2/Bach2 dKO mice did not exhibit increased numbers of eosinophils in their spleens and bone marrows (Fig. 4B, C). These findings suggest that the increase of eosinophils in Bach2 KO mice depend on the lymphocytes. An ELISA revealed that the level of Il-5 in the peripheral blood serum was significantly higher in Bach2 KO mice than that of WT mice (Fig. 4D). Taken together, these results suggest that increased eosinophils in Bach2 KO mice are attributable to the altered functions of lymphocytes, particularly Th2 cells.

Depletion of lymphocytes in Bach2 KO mice normalizes numbers of eosinophils.
A) The cell numbers of ILC2s in the lung from WT mice (mean total cells; 2.5×107/mouse) and Bach2 KO mice (mean total cells; 3.4 × 107/mouse). The mean values from three mice for each group are shown. B) The cell numbers of eosinophils in the spleen from four WT mice (mean total cells; 9.6 × 107/mouse), one Bach2 KO mouse (total cells; 5.5 × 107/mouse) and four Rag2/Bach2 dKO mice (mean total cells; 2.1 × 107/mouse). See Fig. 2 for the comparison to Bach2 KO mice. The mean values from four WT and Rag2/Bach2 dKO mice are shown. C) The cell numbers of eosinophils in the bone marrow from one WT mouse (total cells; 3.1 × 107/mouse), one Bach2 KO mouse (total cells; 2.6 × 107/mouse) and four Rag2/Bach2 dKO mice (mean total cells; 0.9 × 107/mouse). See Fig. 2 for the comparison between WT and Bach2 KO mice. The mean values from four Rag2/Bach2 dKO mice are shown. D) The levels of Il-5 in peripheral blood from WT and Bach2 KO mice. The mean values from eight mice for each group are shown. Data are expressed as the mean ± SEM. **p < 0.01.
Our results show that Bach2 KO mice exhibit higher numbers of eosinophils than WT mice in the peripheral blood, spleen, bone marrow and BAL fluid. The bone marrow transplantation assay revealed that eosinophils were increased in Bach2 KO mice in non-cell-autonomous effect. Consistent with this interpretation, the flow cytometric analysis of Rag2/Bach2 dKO mice suggested that the increase of eosinophils in Bach2 KO mice was dependent on lymphocytes. Recent studies have revealed that Bach2 is essential for efficient formation of regulatory T cells by repressing differentiation of effector CD4+ T cells (Roychoudhuri et al. 2013; Tsukumo et al. 2013), and that Bach2 KO Th2 cells express elevated levels of Th2 cytokines (Kuwahara et al. 2014). Furthermore, we found here that the blood serum level of Il-5 was higher in Bach2 KO mice than WT mice. Since Il-5 strongly induces the proliferation and survival of eosinophils, the present results suggest that an increased secretion of Il-5 by Bach2 KO Th2 cells contribute to the increased numbers of eosinophils in various tissues of Bach2 KO mice. In this model, Bach2 controls differentiation and/or proliferation of eosinophils via regulating production of Th2 cytokines including Il-5 in CD4+ T cells. Since Bach2 KO eosinophils expressed the gene for Il-13 at a high level (data not shown), Il-13 secreted by Bach2 KO eosinophils may further promote the function of Th2 cells, generating a positive feedback between Th2 cells and eosinophils in Bach2 KO mice. Bach2 in T cells may restrict this feedback system to maintain the homeostasis of immune responses. Eosinophils play important roles in parasitic infection and allergic diseases such as asthma, allergic rhinitis and drug hypersensitivity. Importantly, genetic polymorphisms of BACH2 have been shown to associate with asthma and rheumatoid arthritis (Ferreira et al. 2011; McAllister et al. 2013; Igarashi and Watanabe-Matsui 2014). A reduction of BACH2 activity in T cells may unleash this positive feedback between eosinophils and Th2 cells, leading to these diseases.
LCs are the innate immune cells secreting various cytokine and without typical B or T cell markers (Crellin et al. 2010; Wilhelm et al. 2011; Xu et al. 2012; Bernink et al. 2013). Unlike the other innate immune cells such as natural killer (NK) cells and lymphoid tissue inducer (LTi) cells, ILCs can secrete some of the cytokines which are mainly secreted by adaptive immune cells like helper T cells, including Il-5 (Moro et al. 2010). However, we surmise that ILC2s are not regulated by Bach2. First, the alterations of eosinophils were rescued by the genetic ablation of T cells using Rag2 KO mice. Rag2 KO mice are known to retain ILC2s (Doherty et al. 2013). Second, there was no significant difference in numbers of ILC2s between WT and Bach2 KO mice. These results suggest that Bach2 is dispensable for proper differentiation and function of ILC2s.
Eosinophilia is a disease which is diagnosed by an increased numbers of eosinophils in peripheral blood (Brito-Babapulle 2003). The cause of eosinophilia is diverse but mainly due to allergic diseases and parasitic diseases. Eosinophilia is also caused by some types of tumors (Kato et al. 2010), auto-immune disease (Kane 1977) and drugs (Jordan and Cowan 1988). However, the etiology is unclear in a substantial part of patients. A dysfunction of Bach2 in lymphocytes might be one of the causes for eosinophilia
We thank Prof. N. Ishii, Tohoku University Graduate School of Medicine, for providing Rag2 KO mice, and members of the laboratories for technical advices, constructive criticism and discussion. The study was supported by Grants-in-aid from Japan Society for the Promotion of Science (15H02506, 24390066, 21249014) and AMED-CREST to K.I.
The authors declare no conflict of interest.