2017 Volume 241 Issue 3 Pages 183-188
2017 Volume 241 Issue 3 Pages 183-188
Atherosclerotic cardiovascular diseases, such as coronary heart disease, have become a major public health problem all over the world. MicroRNA-29a (miR-29a) modulates expression levels of collagen, inflammatory reaction and other extracellular matrix mRNAs, while adiponectin (APN), a circulating protein secreted by adipocytes, has anti-inflammatory properties. Both play multifaceted roles in angiogenesis or vascular remodelling. However, little is known about plasma miR-29a and APN levels in patients with atherosclerosis. We therefore investigated the relationship between the plasma levels of miR-29a or APN and carotid intima-media thickness (cIMT) in atherosclerosis patients (n = 85, cIMT ≥ 1.2 mm) and the controls (n = 85, cIMT < 1.2 mm). We found that the atherosclerosis group showed higher miR-29a levels (31.15 ± 3.99 vs. 26.39 ± 1.05 Ct, P < 0.001) and lower APN levels (15.93 ± 4.61 vs. 21.80 ± 7.74 ng/ml, P < 0.001), compared with control group. Thus, increased cIMT was associated with higher plasma miR-29a levels (r = 0.688, P < 0.001) and with lower plasma APN levels (r = −0.494, P < 0.001). Furthermore, multiple logistic regression analysis indicated that higher miR-29a levels (OR: 1.136, 95% CI: 1.042-1.240, P = 0.004) increased the risk for atherosclerosis, whereas higher APN levels appeared to be protective (OR: 0.122, 95% CI: 0.055-0.271, P < 0.001). The present study indicates that elevated miR-29a levels and reduced APN levels are associated with atherosclerosis.
Atherosclerotic cardiovascular diseases such as coronary heart disease and stroke have become a major public health problem all over the world (Murray et al. 2012). It is necessary to detect atherosclerotic cardiovascular diseases at earlier stages. In recent decades, microRNAs (miRs) are short non-coding RNA able to bind specific sequences on target mRNAs, thereby modulating gene expression (Gaudet and Brisson 2015). They are involved in almost every aspect of cellular or biological processes, and dysregulation of miRs correlated with cardiovascular diseases, such as atherosclerosis (Condorelli et al. 2014). MiR-29a was one of the members of the miR-29 family (Hysolli et al. 2016). The miR-29 family, modulating mRNA level of collagen, inflammatory reaction and other extracellular matrix genes, play a multifaceted role in tissue remodelling and vessels injury (He et al. 2013; Bretschneider et al. 2016). It was reported that miR-29 were up-regulated in the region of the heart adjacent to a myocardial infarction (MI) (van Rooij et al. 2008). Among other miRs, circulating miR-29a levels may represent one of the new biomarkers which significantly correlate with cardiovascular diseases (Roncarati et al. 2014). In addition, a previous study showed that miR-29b was involved in vascular smooth muscle cell migration and proliferation by mediating an epigenetic mechanism (Chen et al. 2011), which was related to atherosclerosis. With highly similar sequence and nature to miR-29b, miR-29a may contribute to vascular atherosclerosis.
Adiponectin (APN), a circulating protein secreted by adipocytes, has insulin-sensitizing and anti-inflammatory properties (Ouchi et al. 2000; Berg et al. 2001) and may represent a link between obesity and atherosclerosis. It was shown that lower APN levels were related to diabetes mellitus (Lindsay et al. 2002; Spranger et al. 2003), dyslipidaemia (Matsushita et al. 2006) and hypertension (Adamczak et al. 2003), all of which increased the risk of developing atherosclerosis. Although the miR-29 family and APN appear to play a role in the development of angiogenesis, little is known about plasma miR-29a and APN profile in patients with atherosclerosis. Therefore, the aim of the present study was to examine the plasma levels of miR-29a and APN, and investigate their relationship to cIMT in both subjects with atherosclerosis and the controls.
This was a cross-sectional study and all participants were selected continuously from the Guangdong General Hospital, China, from January 2015 to July 2015. All participants underwent physical examination, office blood pressure monitoring, and carotid artery Doppler ultrasonography (CDUS). A total of 170 participants were enrolled in this study: 85 patients (41 males and 44 females) with atherosclerosis (cIMT ≥ 1.2 mm) as our research group and 85 healthy volunteers (48 males and 37 females) with cIMT < 1.2 mm as control group. The participants in research group had atherosclerosis based on the value of cIMT. The cIMT of the common carotid artery was measured by ATL HDI 3000 ultrasound system (Advanced Technology Laboratories, Bothell, WA) equipped with a 5-MHz linear array transducer as previously described (Cardellini et al. 2007; Polak et al. 2010). A cIMT ≥ 1.2 mm was defined as atherosclerosis according to the 2007 Guidelines for the management of arterial hypertension released by the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) (Mancia et al. 2007). We measured the cIMT in both carotid arteries and the higher value of cIMT was used for analyses. A trained sonographer with registered diagnostic medical sonography certification performed the measurements.
Participants with a history of coronary heart disease, diabetes mellitus, neck surgery, thyroid diseases, heavy smokers, hypertension, tumour, and relevant medications (lipid-lowering drugs, antiplatelet or antihypertensive drugs) were excluded from the study. Among those exclusion criteria, cerebrovascular diseases were defined as a documented history of ischemic cerebrovascular event with or without sequelae, transient ischemic attack, or amaurosis fugax in the last 6 months.
All samples were collected from all the participants after obtaining informed consent in accordance with a protocol approved by the Ethics Committee of Guangdong General Hospital (Guangzhou, China). The study was conducted in accordance with the Declaration of Helsinki.
Sample collectionAll fasting blood sample were collected in the morning from each patient before any treatment. Plasma was collected by centrifuging whole blood sample at 3,000 rpm for 10 min at room temperature. Plasma was divided into 2 aliquots, and then frozen as separate aliquots at −80°C for storage until it was used for analysis. Fasting blood glucose, blood lipid, routine laboratory tests, and renal function were tested by routine methods.
The measurement of plasma miR-29a and APNTotal RNA containing small RNAs was extracted from plasma using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and purified with mirVana miR Isolation Kit (Ambion, Austin, TX, USA) according to the manufacture’s protocols (Mitchell et al. 2008; Huang et al. 2016). We evaluated the expression of plasma miRs using the S-Poly (T) reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) method as previously described (Kang et al. 2012), using miR-54 as a control (Baraniskin et al. 2013; Huang et al. 2016). The comparative cycle threshold (Ct) (ΔCt) was used to calculate the relative expression level of miRs. Mean Ct values and deviations between the duplicates were calculated for all samples. ΔCt = Ct (miR) − Ct (miR-54). Primers used were 5′-uagcaccaucugaaaucgguua-3′ for miR-29a and 5′-uacccguaaucuucauaauccg-3′ for miR-54. Plasma APN levels were measured using a commercially available enzyme linked immunosorbent assay (ELISA) kit (Cusabio Biotech, Newark, DE, USA) as previously described (Jin et al. 2016). The detectable concentration ranges for APN were 1.56-100 ng/ml.
StatisticsAll the continuous variables were expressed as mean ± standard deviation. The Student t-test was used for continuous variables between patients and controls. Pearson’s correlation was used for numerical data. Spearman’s correlation was used for nominal data. Multiple logistic regression analysis was used to assess factors contributing to atherosclerosis. An odds ratio (OR) and 95% confidence interval (CI) were calculated. Results are presented as odd ratios (ORs; 95% CI). Statistical significance was defined as two-sided P < 0.05. Statistical analyses were performed using the statistical Package for the Social Sciences (SPSS) version 17.0 software (SPSS Inc, Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
We recruited 170 participants and they were divided into two groups as control group (n = 85, aged 50.36 ± 5.49 years) and atherosclerosis group (n = 85, aged 52.34 ± 5.68 years). The control group comprised 48 males and 37 females, while the atherosclerosis group comprised 41 males and 44 females. The demographic data, laboratory parameters, and plasma APN and miR-29a levels are summarized in Table 1. There were no significant differences in sex, age, body mass index, heart rate, blood pressure, fasting blood-glucose and glomerular filtration rate (GFR) between atherosclerosis group and control group. The atherosclerosis group had lower APN (15.93 ± 4.61 vs. 21.80 ± 7.74 ng/ml, P < 0.001) levels and higher miR-29a levels (31.15 ± 3.99 vs. 26.39 ± 1.05 Ct, P < 0.001) than control group (Fig. 1A, B).

Demographic, clinic and laboratory characteristics between groups.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; GFR, glomerular filtration rate; DBP, diastolic blood pressure; SBP, systolic blood pressure. Data are presented as the mean ± standard deviation. P < 0.05 was considered statistically significant.

Plasma adiponectin levels and miR-29a levels between groups.
A. Adiponectin levels between groups; B. miR-29a levels between groups; carotid intima-media thickness. Data are presented as the mean ± standard deviation. The Student t - test was used for analysis between groups. Note that a total of 170 samples were analyzed. P < 0.05 was considered statistically significant.
As shown in Table 2, we found the levels of APN were negatively related with triglyceride (r = −0.280, P = 0.004), cIMT (r = −0.494, P < 0.001), and miR-29a (r = −0.259, P = 0.006). The levels of miR-29a were positively related with cIMT (r = 0.688, P < 0.001), and triglyceride levels were positively associated with cIMT (r = 0.390, P = 0.001). As shown in Table 3, plasma APN and miR-29a were found to contribute to atherosclerosis. Higher plasma miR-29a levels may increase the risk of atherosclerosis (OR, 1.136; 95% CI, 1.042-1.240, P = 0.004), while APN appeared a protective factor (OR, 0.122; 95% CI, 0.055-0.271, P < 0.001).

Relationship of miR-29a levels with adiponectin and cIMT.
cIMT, carotid intima-media thickness. The Pearson’s correlation coefficient was used for statistical analysis. P < 0.05 was considered statistically significant.
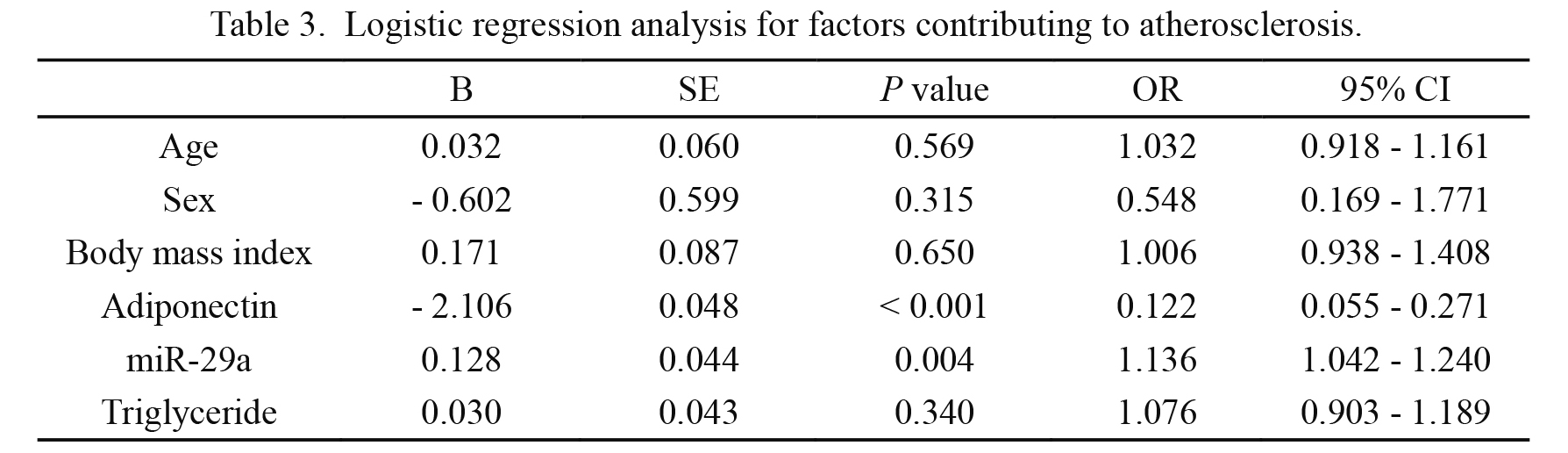
Logistic regression analysis for factors contributing to atherosclerosis.
OR, odds ratio; CI, confidence interval; SE, standard error. Multivariable logistic regression was used for statistical analysis. P < 0.05 was considered statistically significant.
As far as we know, this is the first study to document the relationship between miR-29a or APN and atherosclerosis. We found that plasma miR-29a and APN levels were associated with cIMT, which were related to atherosclerosis. Our study found higher miR-29a levels and lower APN levels in patients with atherosclerosis than in the controls. We also showed the levels of miR-29a were positively related with cIMT. Moreover, this study demonstrated that plasma APN levels were negatively correlated with cIMT, further indicating that lower APN levels might expose people to the higher risk of atherosclerosis.
Atherosclerosis is a complex process. In recent years, more new biomarkers were found to play vital roles in atherosclerosis via different mechanisms. Plasma miR-29a was a new biomarker that could play a role in the pathophysiology of atherosclerosis (Hulsmans and Holvoet 2013). Previous studies (Liu et al. 2010; Maurer et al. 2010; Kriegel et al. 2012) have revealed that target genes of miR-29, such as type I and type III collagens, were important in the development of atherosclerosis (Ulrich et al. 2016). The usage of miR-29 antagonists may prevent plaque remodelling (Ulrich et al. 2016). Furthermore, a basic research demonstrated that the expression of Quaking protein was modulated by miR-29a and that inhibiting the expression of Quaking protein in turn resulted in downregulation of scavenger receptor A and lipid uptake, an important process involved in atherosclerosis (Wang et al. 2015). In addition, miR-29a could promote angiogenesis by targeting HMG box-containing protein-1 (Yang et al. 2013) or phosphatase gene (Wang et al. 2013). These studies suggest that miR-29a may play a role in atherogenesis.
Our data showed a negative correlation between APN and cIMT. It was reported that APN played an important role in metabolic and cardiovascular homeostasis (Lim et al. 2014). A meta-analysis showed that the higher APN levels, the lower cardiovascular events (Gasbarrino et al. 2016). Dyslipidaemia was an important risk factor for atherosclerosis (Gasbarrino et al. 2016). Probably due to the small sample size, APN was found only significantly associated with triglyceride levels. A previous study showed that APN could lower serum triglycerides through enhancement of the catabolism of triglyceride-rich lipoproteins (Christou and Kiortsis 2013). Furthermore, statins were found to raise APN levels as well as decrease the cIMT mainly by improving the lipid profile, and suppression of miR29 will decrease hepatic cholesterol synthesis (Phillips and Kung 2010; Chrusciel et al. 2016). In addition, APN exerts its anti-inflammatory effects in multiple ways. Previous researches demonstrated that plasma APN could protect vascular injury, alleviate endothelial dysfunction, and suggested it could be a potential therapeutic target to reduce cardiovascular risk (Matsuda and Shimomura 2014; Ebrahimi-Mamaeghani et al. 2015). All these results suggested that high levels of APN protect against atherosclerosis. We found a negative correlation of APN with atherosclerosis, suggesting that insufficient expression of APN may aggravate atherosclerosis.
Of course, this study has several limitations. First, as a cross-sectional study, we only show a relationship but cannot identify causality. Second, the sample size was small; multicentre studies with larger sample sizes are needed. Third, the underlying mechanisms responsible for the correlation of miR-29a and APN with cIMT were not elucidated. The potential role of miR-29a and APN in atherosclerosis needs to be further investigated.
In conclusion, patients with atherosclerosis had lower APN levels and higher miR-29a levels than the controls. There was a positive correlation between miR-29a levels and cIMT, whereas a negative correlation between APN levels and cIMT. Higher plasma miR-29a levels may increase the risk for atherosclerosis, while higher APN level appeared to be protective. In the future, miR-29a and APN may be the potential biomarkers for atherosclerosis.
We thank all the participating volunteers. This work was supported by grants from the Guangdong Natural Science Foundation (No. 2015A030313660), Technology Project Foundation of Guangzhou (No. 2014y2-00140, No. 1563000381), Technology Project Foundation of Guangdong Province (No. 2014B020212008) and the National Natural Science Foundation of China (No. 81300230), Science and Technology Innovation Project of Hunan Province Development and Reform Commission (2014658).
The authors declare no conflict of interest.