Abstract
Prenatal glucocorticoid therapy is indicated in preterm delivery to prevent respiratory distress. This study was designed to evaluate the age-dependent effects of prenatal dexamethasone (DEX) therapy on the immune system using a rat model. Pregnant Sprague-Dawley rats received an intraperitoneal injection of DEX (0.1 mg/kg/day) or saline (VEH) over gestational days 14-20. Male offspring were sacrificed at postnatal day 7 (D7; infant stage), D120 (young adult stage), and D180 (adult stage) for evaluation of leukocyte subsets and isolation of splenocytes. The production of innate and adaptive immune cytokines was assessed from the culture supernatants of splenocytes, stimulated with lipopolysaccharide and concanavalin A, respectively. For innate cytokines, the levels of interferon gamma inducible protein 10 were significantly higher, but those of tumor necrosis factor-α were significantly lower, in the culture medium of splenocytes prepared from the DEX group at D120 than those in the VEH group. For adaptive cytokines, the levels of interleukin-4 (IL-4) were significantly higher at D7 and those of IL-10 were significantly higher at D120 after prenatal exposure to DEX. We also showed that the expression level of IL-4 mRNA was significantly higher in splenocytes prepared from the DEX group at D7, compared with the VEH group. Importantly, the mRNA expression level of T-bet, a key transcription factor for immune cells, was greatly decreased in the spleen of the DEX group at D7, compared with the VEH group. In conclusion, prenatal dexamethasone exposure shows the greater impact on immune responses of their male offspring in early life.
Introduction
Prenatal glucocorticoid treatment is used in preterm delivery between 24 and 34 weeks of gestation to stimulate lung maturation. This treatment effectively reduces mortality and morbidity rates after birth (Surbek et al. 2012; Brownfoot et al. 2013; Locatelli et al. 2015). However, animal studies have reported several adverse effects of prenatal steroid treatment on the development of central nervous system, endocrinal system, and immune system (Coe and Lubach 2005; Antonow-Schlorke et al. 2009). Our results agree with these observations (Tiao et al. 2014; Yu et al. 2014a; Lui et al. 2015; Sheen et al. 2015).
Several studies have been performed to investigate the modulatory effects of prenatal steroid exposure on the immune system development in humans. Kavelaars et al. (1999) reported that prenatal steroid treatment enhanced natural killer cell activity and decreased T cell proliferation in preterm cord blood. Other studies in infants whose mothers were administered immunosuppressants during pregnancy revealed that there was no difference in leukocyte population or cytokine and antibody production between infants who were exposed and those who were not exposed to prenatal steroid treatment (Cimaz et al. 2004; Biggioggero et al. 2007; Motta et al. 2009; Kumar et al. 2011). Thus, prenatal exposure to steroid appears to have a limited effect on the developing immune system in humans, and these effects were only observed in neonates to infants. In a large longitudinal population study over a span of 10 years, Pole and coworkers reported that the use of prenatal steroid was found to be an independent risk factor for the development of asthma between the age of 36 and 72 months and later between 3 and 5 years (Pole et al. 2009). Considering the difficulty in long-term observations in humans, animal models with a shorter life cycle may provide better insights and help determine mechanisms for human disease. In our previous study with pregnant rats, prenatal dexamethasone (DEX) exposure through intraperitoneal injection induced splenomegaly in the offspring. Data revealed that prenatal glucocorticoid reduced the capacity of tumor necrosis factor (TNF)-α to produce splenocytes in adolescent rats, making the offspring more vulnerable to pathogenic invasion even during adult life (Yu et al. 2014a). In this study, we investigated the effect of prenatal steroid on innate and adaptive immune function with rat spleen from infancy to adolescence and into adulthood [postnatal day 7 (D7), day 120 (D120), and day 180 (D180)]. The findings of this study will be helpful for the better understanding of the immune programming influenced by prenatal steroid exposure.
Materials and Methods
Animals
This study was conducted in strict accordance with the recommendations outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the Kaohsiung Chang Gung Memorial Hospital. Virgin Sprague-Dawley (SD) rats (12-16 weeks old; BioLASCO Taiwan Co., Ltd., Taipei, Taiwan) were housed and maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The study protocol was described in a previous study (Yu et al. 2014a). In brief, virgin SD female rats were allowed to mate with male rats for 24 hours and then were separated from the male rats and housed individually in a standard plastic home cage. After confirmation of pregnancy, the pregnant female rats were randomly assigned to the DEX exposure group or control group. In the DEX exposure group, DEX was administered intraperitoneally (0.1 mg/kg/day) over gestational days 14-20. The vehicle (VEH) group was intraperitoneally injected with normal saline daily over gestational days 14-20. The male offspring were divided into two groups (DEX and VEH) based on prenatal DEX exposure. The day of birth was designated as postnatal day 0 (D0). Male rat pups were weaned at postnatal day 21 (D21) and housed individually with food and sterile tap water available ad libitum.
Experimental procedures and specimen collection
Both DEX and VEH group rats were sacrificed at D7, D120, and D180 to assess the age-related effects of prenatal DEX treatment. Body, thymus, and spleen weights were recorded after sacrifice. The spleens were used for further studies, and blood specimens were collected for analysis.
Peripheral blood analysis and plasma immunoglobulin detection
Blood samples were collected in heparin tubes. Total blood cell counts and white blood cell (WBC) differential counts were obtained using Sysmex XT-1800i (Sysmex, Hyogo, Japan). For lymphocyte subset analysis, leukocytes were stained with the following antibodies: PE-conjugated anti-rat CD3, APC-conjugated anti-rat CD45RA, PE-conjugated anti-rat CD4, and FITC-conjugated anti-rat CD8a. All these antibodies were purchased from BD Biosciences. Data were acquired using a FACSAria I cytometer (Becton Dickinson, Franklin, NJ, USA) and analyzed using Flow Jo software. The levels of plasma immunoglobulins (Ig), including IgG, IgA, and IgM, were analyzed by ELISA (eBioscience, San Diego, CA, USA).
Splenocyte culture and drug treatment
Splenocytes were isolated from the whole spleen as previously described (Yu et al. 2014a). In brief, the spleen was washed with phosphate-buffered saline (PBS) and pressed with a syringe plunger through a 30-µm nylon mesh. After erythrocytes were lysed, the remaining splenocytes were washed with PBS. All spleen cells were counted and 2 × 106 cells/ml were plated in 24-well plates containing enriched medium (RPMI 1640 medium supplemented with 1% non-essential amino acids, 1% pyruvate, 10% heat-inactivated fetal bovine serum (FBS), and antibiotics). Cultured splenocytes were stimulated with or without 100 ng/ml of lipopolysaccharide (LPS) or 5 µg/ml of concanavalin A (Con A; Sigma). The cell pellets and culture supernatants were collected at the indicated time for further studies.
5-Bromo-2ʹ-deoxyuridine cell proliferation assay
The proliferation of splenocytes was assessed by the BrdU assay, as described previously (Yu et al. 2014b). Splenocytes (5 × 105 cells/ml) were plated in a 96-well plate and maintained in enriched RPMI-1640 medium. Then, the splenocytes were stimulated with 5 µg/ml of Con A or PBS. After 48 hours of ConA or PBS stimulation, BrdU was added to the proliferating splenocytes and labeled in the following 24 hours. Proliferation was measured by the BrdU assay (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. The results were presented as the ratio of optical density (OD) of ConA stimulation to OD of PBS stimulation for every group.
Cytokine analysis
The isolated splenocytes (2 × 106 cells/ml) were plated in 24-well plates containing enriched medium and treated with or without 100 ng/ml of LPS for 24 hours or 5 µg/ml of Con A for 72 hours. The cell culture supernatants were collected for analysis of cytokines related to innate and adaptive immunity using the Luminex 200 system (Luminex, Austin, Tex, USA). Supernatant concentrations of interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, transforming growth factor (TGF)-β1, TGF-β2, TGF-β3, monocyte chemotactic protein-1 (MCP-1), and interferon gamma inducible protein 10 (IP-10) were measured using the Multiplex Assay (Millipore) system. Antibody conjugated beads were incubated first with diluted standards or supernatants for 2 hours and then with detector antibodies for 1 hour at room temperature. Fluorescent detection was performed after the sample was incubated for 1.5 hours with biotin as a reporter and for 30 min with fluorescent dye-conjugated streptavidin-phycoerythrin. Cytokine levels were measured using a flow cytometer and analyzed with the FlowMetrix software. The TNF-α/IFN-γ secretion was detected by ELISA (Biolegend, San Diego, CA, USA).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
RNA was prepared from Con A-stimulated splenocytes of 7-day-old rats and from spleens of 7-day-old rats. qRT-PCR was performed as previously described (Yu et al. 2014a). In brief, 5 µg of extracted RNA sample was reversed transcribed with Moloney murine leukemia virus reverse transcriptase. PCR was performed in 20 µl of total reaction volume containing 2 µl of 1:10 diluted cDNA obtained from reverse transcribed RNA, specific primers, 2.5 mM MgCl, and Maxima SYBR Green/Fluorescein qPCR Master Mix (2X) (#K0242, Thermo Scientific, CA, USA). The cycling protocol comprised one cycle of 10 min at 95°C followed by 45 cycles of denaturation for 10 s at 95°C, annealing for 20 s at 55°C, and extension for 20 s at 72°C. The primers were as follows: IL-4: 5′-AGACGTCCTTACGGCAACAAG-3ʹ (sense) and 5ʹ-AGCACCCTGGAAGCCCTGC-3ʹ (antisense); T-bet: 5ʹ-TCC ACC CAG ACT CCC CAA CCA-3ʹ (sense) and 5ʹ-GGC TCA CCG TCA TTC ACC TCC A-3ʹ (antisense); GATA-3: 5ʹ- CAC CCA GAC ACG CAC CAC CC-3ʹ (sense) and 5ʹ- CGG CAT ACC TGG CTC CCG TG-3ʹ (antisense); Foxp3: 5ʹ-CCC AGG AAA GAC AGC AAC CTT-3ʹ (sense) and 5ʹ- CTG CTT GGC AGT GCT TGA GAA-3ʹ (antisense); peptidylprolyl isomerase B (PPIB): 5ʹ-CTG TCG ATT CCC TCA CAG GT-3ʹ (sense) and 5ʹ-AAA ATC AGG CCT GTG GAA TG-3ʹ (antisense). Each PCR primer set used for qRT-PCR was located in the different exons. Serial dilutions of the standard cDNA were also used for parallel amplifications. The threshold cycles (Ct) were calculated using the LightCycler software (ver. 1.5.0). Standard curves were plotted with Ct-versus-log cDNA quantities, and the quantities of the samples were determined from the standard curves. For the relative quantification of mRNA expression, the comparative Ct method was employed. The averaged Ct was subtracted from the corresponding averaged PPIB value for each sample, resulting in ΔCt. ΔΔCt was obtained by subtracting the average control ΔCt value from the average experimental ΔCt value. The fold increase was established by calculating 2-ΔΔCt for the experimental versus control samples.
Chromatin immunoprecipitation assay
The chromatin immunoprecipitation (ChIP) assay was performed as previously described (Yu et al. 2014a). In brief, after cutting and fixing with warmed 1% formaldehyde (37°C), the spleen tissue samples of 7-day-old rats were centrifuged and washed with ice-cold PBS. The ChIP assay was performed using an EZ-Magna ChIPTMA kit (Cat# 17-408, Millipore) according to the manufacturer’s instructions. DNA was sheared by sonication to an average length of 200-1,000 bp, and 5 μl of the supernatant was removed as the “Input”. The supernatant was incubated overnight at 4°C with specific antibodies or nonspecific IgG anti-body. The antibodies used in the immunoprecipitation were specific for acetyl histone H3 (Millipore, #06-599), acetyl histone H3 lysine 4 (Cell Signaling, #9649), acetyl histone H3 lysine 9 (Cell Signaling, #7627), monomethyl histone H3 lysine 4 (Abcam, Cambridge, UK, ab8895), trimethyl histone H3 lysine 4 (Abcam, ab8580), and trimethyl histone H3 lysine 36 (Abcam, ab9050). The immunoprecipitated DNA was eluted in a total volume of 50 μl and amplified and quantitated by Q-PCR at an annealing temperature of 57°C for a total of 45 cycles. The primers for the rat IL-4 promoter 1 were 5ʹ-CAC TTC CAG AAA GAT GGC AGA A-3ʹ (forward) and 5ʹ-TTG CGC CCA TTG TCT TAA TCT A-3ʹ (reverse); for the IL-4 promoter 2 were 5ʹ-ACA CAC ATC CTC CCA TGA AAT AAA GTA-3ʹ (forward) and 5ʹ-GGT TGA CGA TTG TTC CTT CCA-3ʹ (reverse); and for the IL-4 promoter 3 were 5ʹ-AGA GAT ACA CAC ATC CTC CCA TGA-3ʹ (forward) and 5ʹ-GGA CCA TGA AAT GAG GCC TTT-3ʹ (reverse). The Ct values of the diluted input were adjusted to 100% of the input by subtracting 3.322 cycles (log 2 of 10) from the Ct value of the diluted input. One percent of starting chromatin was used as input. The amount of DNA precipitated by the indicated antibodies was calculated as percentage of the input using the following formula: % of input = 2ΔCt × 100, where ΔCt = Ct input − Ct IP (Allan et al. 2012). The results were expressed as fold over the control.
Statistics
The data are expressed as mean ± standard error of the mean. The Mann-Whitney U test was used when two groups were analyzed. Results with a p value of < 0.05 were considered statistically significant. All statistical tests were performed using SPSS 15.0 for Windows XP (SPSS, Inc., Chicago, IL, USA).
Results
Prenatal DEX exposure increases lymphoid organ-to-body weight ratio and adulthood body weight
The DEX group rats at D7 had a lower birth body weight than the VEH group rats did, but the birth weight was similar to the VEH group at D120 (Table 1). Prenatal DEX exposure resulted in a higher body weight (BW) at D180. The DEX group rats had a higher spleen weight and spleen weight-to-BW ratio than the VEH group rats did at D7 and D120; however, these parameters decreased thereafter (Table 1).
Prenatal DEX exposure modifies leukocyte subsets before adulthood
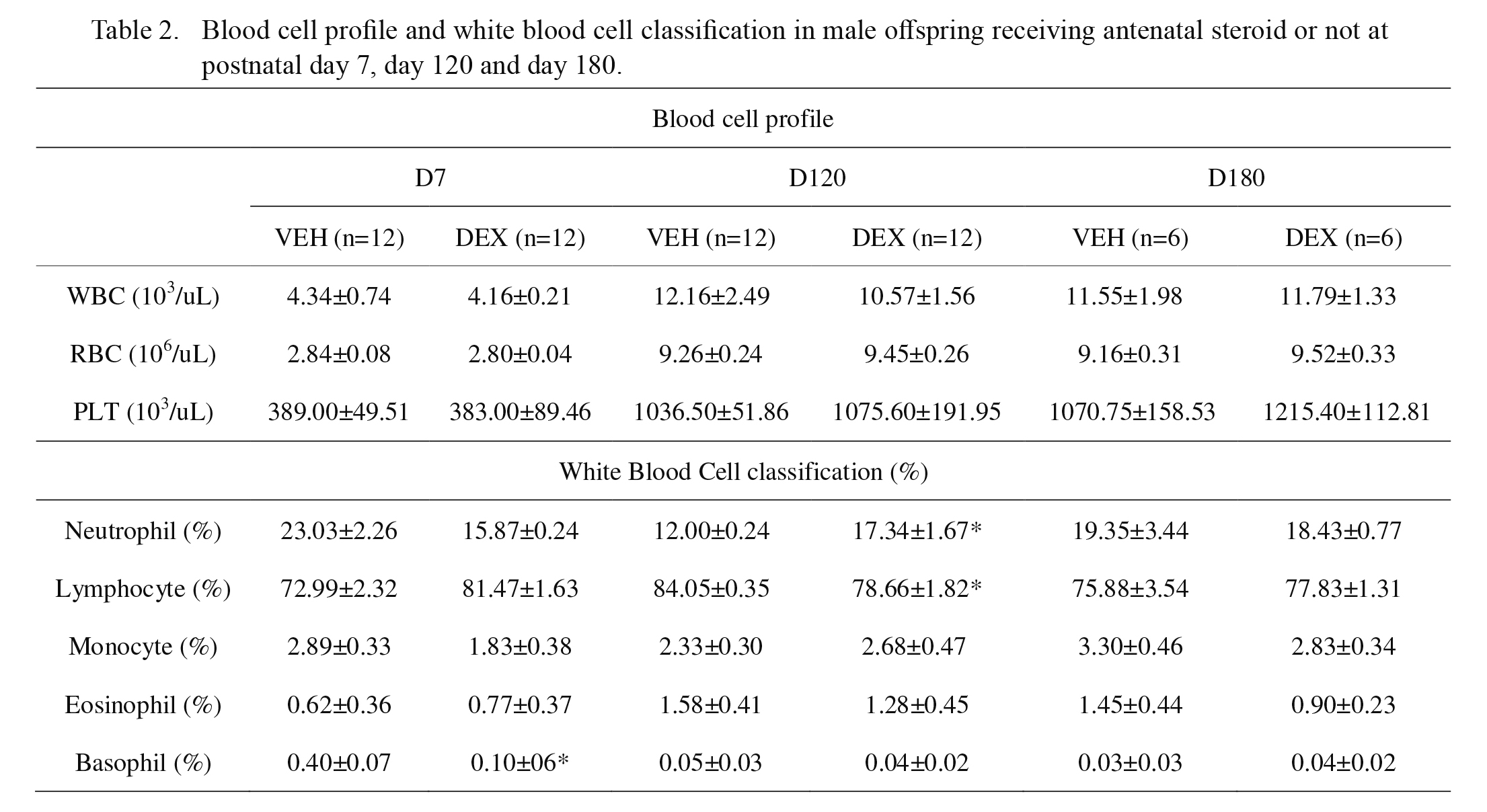
There was no significant difference between the DEX group and VEH group in terms of total leukocyte counts, red blood cell counts, and platelet counts at D7, D120, and D180 (Table 2). Regarding the leukocyte subsets, the DEX group had a higher neutrophil-to-lymphocyte ratio than that of the VEH group at D120 (0.143 vs. 0.218, p = 0.014). The DEX group had a lower percentage of basophils than that of the VEH group at D7 (Table 2).
Dietert et al. (2003) found no change in splenic cell population of rats after prenatal DEX exposure when evaluated at adult stage (13 weeks old). Therefore, we performed flow cytometry analysis of leukocytes from offspring of different ages using antibodies directed against the indicated cell surface markers (Table 3). Samples were analyzed and compared for CD3, CD4, CD8a, the CD4/8a ratio, and CD45RA (Ox-33 antibody). The DEX group had a lower CD4-to-CD8a ratio at infancy (D7). The percentage of CD45RA+ cells in the DEX group was significantly lower than that in the VEH group at infancy (D7). At young adult stage (D120) and adulthood (D180), there were no significant differences between the DEX group and VEH groups in the percentage of CD4+, CD8a+, and CD45RA+ cells (Table 3).
Prenatal DEX exposure reduces plasma IgA and IgM levels during infancy
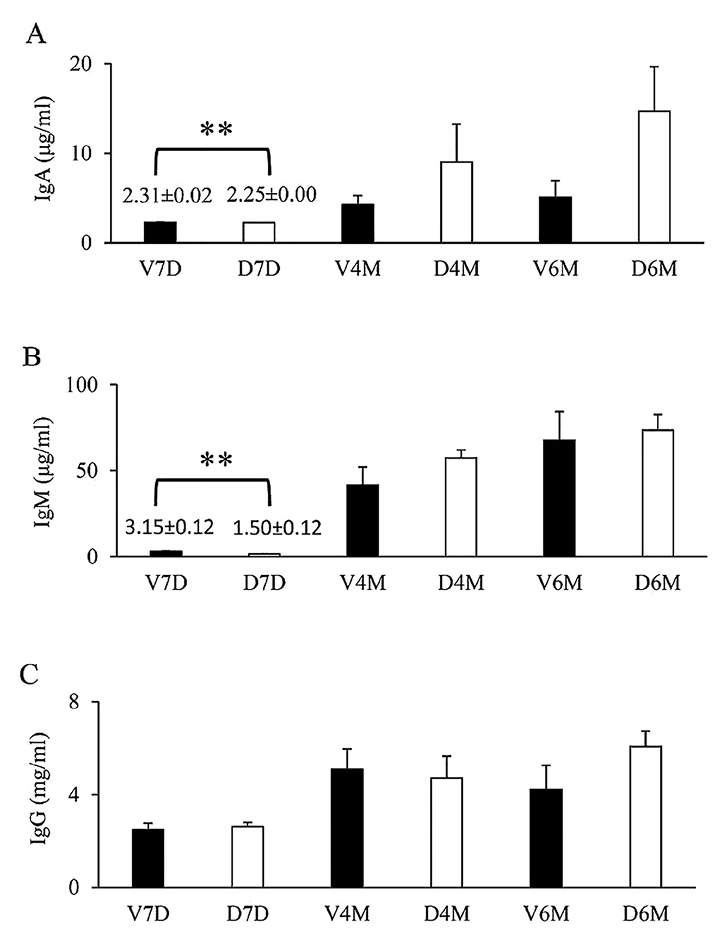
Total plasma IgA, IgM, and IgG were measured by ELISA (Fig. 1). Overall, IgA, IgM, and IgG levels were the lowest at D7, and the highest at D180 (V6M and D6M). The DEX group had significantly lower plasma IgA and IgM levels than the VEH group did at D7. A trend of higher IgA levels was observed at D180 (V6M and D6M) with prenatal DEX exposure; however, the levels were not statistically significant. At D7, D120, and D180, IgG levels in the DEX group were not different from those in the control group (Fig. 1).
Splenocyte proliferation rate is transiently higher at infancy and lower at adolescence
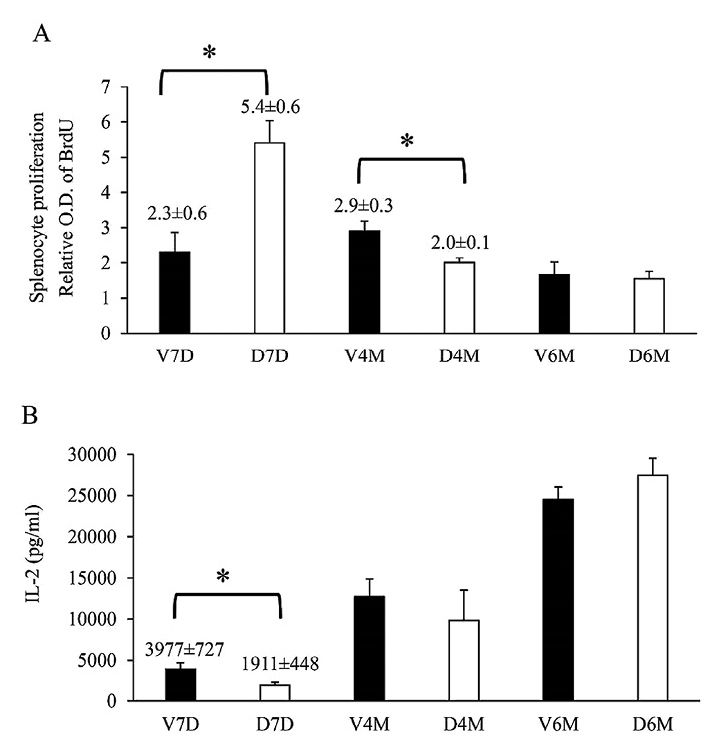
With ConA stimulation, splenocyte proliferation in the DEX group increased transiently at D7 and then decreased at D120 compared to that reported for the VEH group (Fig. 2). However, IL-2 level in the DEX group was lower than that in the VEH group at D7, which may be due to consumption by leukocytes during proliferation. Overall, IL-2 levels were the highest during adulthood (D180).
Prenatal DEX exposure alters innate immunity cytokines at adolescence
In a previous study, we had reported that prenatal dexamethasone exposure in rats resulted in a decrease in TNF-α production at D120 (Yu et al. 2014a). In this study, we investigated additional innate immunity-related cytokines over a longer period of time. For innate immunity-related cytokines, splenocytes were cultured with LPS (100 ng/ml) for one day, and the supernatants were collected to measure the cytokine concentrations. In general, the levels of IP-10, IL-1β, and TNF-α were the lowest in both groups at D7 compared to that at the more mature stages (Fig. 3). The IP-10 level was the highest at D120, and TNF-α and IL-1β levels were the highest at D180. By contrast, the MCP-1 level was the highest at D7 (Fig. 3C). Importantly, splenocytes in the DEX group produced higher IP-10 levels than those in the VEH group did at D120 (Fig. 3A), whereas the TNF-α levels were lower in the DEX group at D120 (Fig. 3D). However, the differences in the IP-10 and TNF-α levels were not detected at D180.

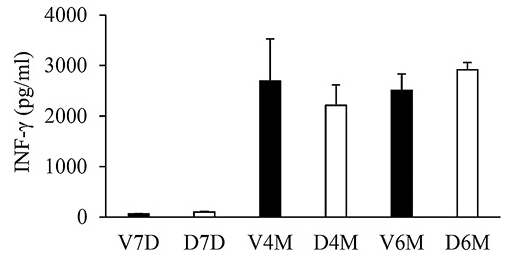
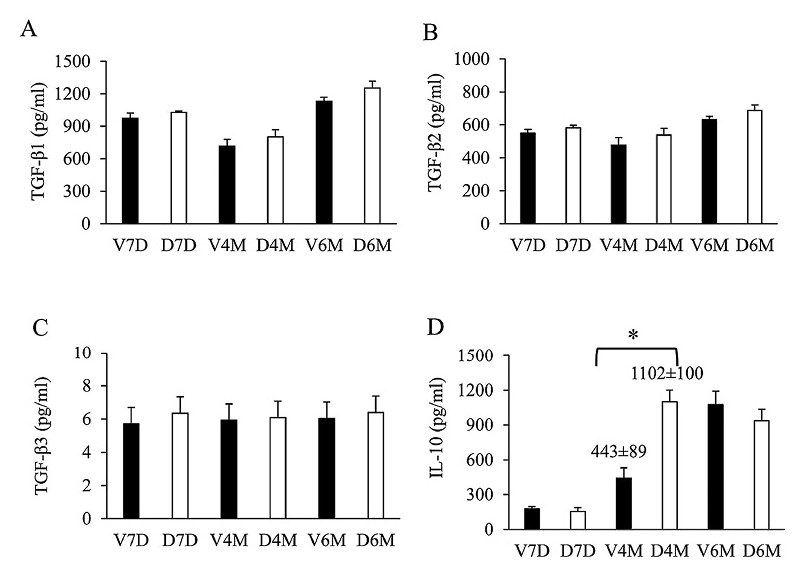
We investigated the effects of prenatal DEX exposure on the adaptive immunity-related cytokines. To produce adaptive immunity-related cytokines, splenocytes were stimulated with ConA stimulation for 3 days, and the supernatants were collected to measure the cytokine concentrations. IFN-γ production was the lowest at D7 compared to that at the more mature stages (Fig. 4). This phenomenon is compatible with Th1 immune development in humans (Yu et al. 2003). However, prenatal DEX exposure did not alter IFN-γ production at D7, D120, or D180 (Fig. 4). It was interesting to note that prenatal dexamethasone exposure resulted in higher IL-4 production by splenocytes at D7, but not at D120 or D180 (Fig. 5A). This suggests that the impact of prenatal dexamethasone exposure on IL-4 production can be compensated and reversed at a later age. Prenatal DEX exposure did not influence the production of other Th2-related cytokine (IL-5 and IL-13) (Fig. 5B, C). IL-13 levels were the highest in the adult stage (D180).
Among the TGF-β cytokines, TGF-β1 levels were the highest, followed by TGF-β2 and TGF-β3; however, there was no significant difference in TGF-β1, TGF-β2, and TGF-β3 levels between the DEX group and VEH group (Fig. 6A-C). IL-10 levels were higher in the DEX group than in the VEH group at D120 (Fig. 6D), but the levels were similar at D180.
Prenatal DEX exposure decreases expression of T-bet mRNA and increases expression of IL-4 mRNA
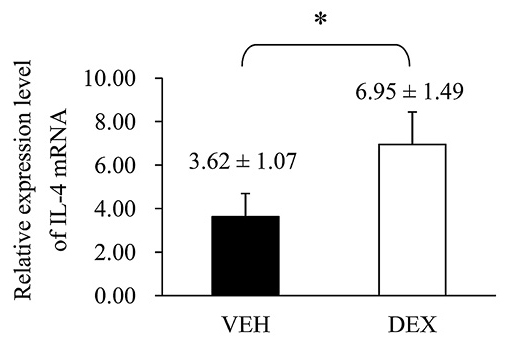
Since the IL-4 is an important Th2 cytokine that related to allergic reaction, we tried to explore the mechanism for its increase in prenatal DEX exposure group. Next, we investigated the expression of IL-4 mRNA of splenocytes treated with ConA at D7. As shown in Fig. 7, the expression level of IL-4 mRNA was significantly higher in the DEX group than in the VEH group. Then, we attempted to determine the mRNA expression of Th1-, Th2- and CD4+CD25+ regulatory T cells (the mostly well-understood regulatory T cells) related transcription factors in rat spleen. T-bet, Gata-3, and FoxP3 correspond to Th1-, Th2- and CD4+CD25+ regulatory T-related transcription factors, respectively. These molecules are master regulators in the polarization of helper T cells (Yu et al. 2003; Sundrud and Nolan 2010). The DEX group showed a significantly lower T-bet mRNA expression level than the VEH group did (Fig. 8A), although prenatal dexamethasone exposure did not alter GATA-3 or Foxp3 mRNA expression (Fig. 8B, C).
Previously, we had demonstrated that prenatal DEX treatment modified TNF-α production through histone modifications (Yu et al. 2014a). In this study, we investigated the effects of prenatal DEX exposure on histone modifications of IL-4 promoter in the spleen. ChIP assays were performed on the spleen nuclear extracts of 7-day-old rats using antibodies directed against acetyl-histone H3 (total Lys sites), acetyl-histone H3K9 (Lys 9), and acetyl-histone H3K14 (Lys 14). RT-PCR analysis was used to determine the percentage of IL-4 promoter input DNA bound to these proteins. We designed 3 pairs of IL-4 primer for RT-PCR (Fig. 9A). As shown in Fig. 9B, there were no significant differences in the total acetyl-histone H3, acetyl-histone H3K9, and acetyl-histone H3K14 expression on the IL-4 promoters between the VEH and DEX groups. We also investigated the effects of prenatal DEX exposure on promoter histone H3 lysine methylation in rat spleens. ChIP assay was performed on the nuclear extracts using antibodies directed against H3K4me1, H3K4me3, and H3K36me3. As seen in Fig. 9C, there were no significant differences in H3K4me1, H3K4me3, and H3K36me3 expression on the IL-4 promoters between the VEH and DEX groups.

Discussion
In humans, glucocorticoid treatment during pregnancy appears to have a limited effect on the immune system of neonates and infants. However, few studies have been conducted to evaluate the potential effects of prenatal glucocorticoid treatment after the childhood stage and beyond. Animal models with a shorter life cycle are ideal for such studies. Therefore, we conducted our study using an animal cohort model to evaluate the short- and long-term effects of prenatal DEX treatment on the immune system. However, owing to differences in development and physiology, special care needs to be taken when correlating the age of rats and humans (Quinn 2005; Sengupta 2013). In SD rats, the weaning period is over postnatal days 14-21, and the average weaning age in humans is until about 6 months. The period of sexual maturation in male SD rats is during postnatal days 40-76, which denotes the beginning of the adolescent period. The average age of tapering growth of musculoskeletal and social developmental maturity in SD rats is postnatal day 210 onward, which corresponds to an age of 20 years in humans (Quinn 2005; Andreollo et al. 2012; Sengupta 2013). In this study, rats were investigated on day 7, 120, and 180 after birth, which corresponds to the newborn, young adult, and middle age adult stages in humans, respectively (Andreollo et al. 2012; Sengupta 2013). We found that prenatal DEX exposure had a greater impact during the earlier stages. Prenatal DEX exposure increased the lymphoid organ-to-body weight ratios and altered the leukocyte subsets before the adulthood stage. Moreover, plasma IgA and IgM levels decreased during infancy. Prenatal DEX exposure also altered the innate and adaptive immunity-related cytokine production, including increased IL-4 at the infancy stage and increased IL-10 production at the adolescent stage. Rats recover from most of the effects of prenatal DEX exposure on their immunity during the mature adolescent stage. Reversibility following DEX exposure is important because this may lead to completely different considerations for the strategies implemented to prevent the adverse programming of prenatal DEX treatment. However, any significant impact of prenatal DEX exposure on the older stages requires further investigation.
In previous animal studies, prenatal steroid treatment resulted in decreased fetal body weight, particularly at an earlier gestational age, which suggests that prenatal steroid exposure affects fetal body weight (Bunton and Plopper 1984). Prenatal steroid exposure was also observed to transiently reduce body weight in rats (Noorlander et al. 2014). Our results partly agree with these reports: prenatal DEX exposure led to an evident decrease in body weight at D7, and the between-group difference in body weight disappeared at D120. These findings suggest the transient effect of prenatal steroid exposure on body weight. In previous reports, no significant difference in body weight was found between prenatal DEX exposure group and control group, even in premature infants (Liggins and Howie 1972; Schutte et al. 1980; Schmand et al. 1990; Dessens et al. 2000; Roberts and Dalziel 2006). However, these studies evaluated the body weights of offspring at 4-6 years of age or around 10-12 years (Liggins and Howie 1972; Schutte et al. 1980). However, at these check points, the possible effects of prenatal steroid exposure on body weight may be diminished. In our results, prenatal DEX exposure led to a higher body weight even at D180 (adult stage). Similar results of prenatal corticosteroid treatment have been more recently recorded in a sheep model (Berry et al. 2013). A long-term follow up is required to observe whether prenatal corticosteroid exposure causes obesity.
In our prenatal DEX exposure group, relative splenomegaly was noted at D7 and D120 and an enlarged thymus at D120. However, previous studies have reported that prenatal steroid exposure resulted in growth retardation of the thymus (Wu et al. 1993; Michie et al. 1998; Quinlivan et al. 1998; Kuypers et al. 2012) and spleen (Quinlivan et al. 1998) at birth. These differences in results may be explained by the differences in animal model and prenatal DEX regimens. Currently, the data on lymphocyte subtype modifications following prenatal DEX exposure are limited. A study on premature subjects who received prenatal steroid revealed a decrease in CD4+CD8−/CD4−CD8+ ratios in the cord blood of premature infants (Chabra et al. 1998). In our study, the CD4+CD8a−/CD4−CD8a+ ratios decreased in the prenatal DEX exposure group at D7 (infancy stage). CD4-positive lymphocytes play a crucial role in the effective immune response to pathogens; (Luckheeram et al. 2012) therefore, a decreased number of CD4 T cells may be related to weakness in initiating an appropriate adaptive immunity response. However, whether a clinical immune response is influenced by prenatal steroid exposure remains unclear and further studies are required to determine the possible relationships.
Previous studies have shown a controversial correlation between prenatal steroid exposure and the development of asthma in childhood (Palta et al. 2001; Hung et al. 2010; Byrjalsen et al. 2014). This controversial presentation could be explained by the age difference among the individual study groups. Prenatal steroid exposure was associated with an increased risk of asthma in children aged 3-5 years, but not in older children (Palta et al. 2001; Hung et al. 2010; Byrjalsen et al. 2014). The risk appears to be age-dependent and the highest during early childhood and appears to diminish with age. The mechanism underlying the association between prenatal corticosteroid therapy and the onset of asthma during childhood, which is related to the dominant presentation of Th2 immunity (Lilja and Wickman 2000; Ownby 2001; Busse and Rosenwasser 2003; Macaubas et al. 2003), has not yet been fully established. In this study, we have provided evidence of prenatal corticosteroid exposure influencing the Th1/Th2 ratio in favor of Th2 in early life. We observed elevated IL-4 production in the prenatal steroid exposure group at D7, which was attenuated at D120 and D180. This finding may explain the age-dependent risk of asthma demonstrated in previous studies. In a study of 5-week-old female rat offspring, increased IL-4 production was not noted after prenatal DEX exposure (Dietert et al. 2003). However, a sex-specific difference has been well established, with male predominantly developing childhood asthma (van Merode et al. 2007). The influence of age and sex can thus explain these controversial results. In a previous report, no difference was found in plasma Th1/Th2 cytokine levels between the control group and prenatal dexamethasone exposure group (Kuo et al. 2014) which suggests that extra-stimulation is required to trigger the potential effects of prenatal dexamethasone exposure. In this study, we found that the increase in IL-4 production and IL-4 mRNA expression in the prenatal dexamethasone exposure group was related to a decrease in the levels of Th1-determined mRNA. This T-bet mRNA expression modulated by prenatal DEX exposure is contradictory to a previous report that showed higher T-bet mRNA levels with prenatal DEX exposure (Yu et al. 2003). The discrepancy in findings may be due to a difference in the reference gene used for qRT-PCR. We used PPIB mRNA, instead of 18S rRNA, as the reference gene to normalize the target genes, because the PPIB mRNA shows a smaller degree of variation in the rat spleen tissue compared with GAPDH (Cai et al. 2007).
This study has several limitations. First, in addition to histone modifications, there are other epigenetic regulation mechanisms such as CpG methylation, non-coding RNAs, and structure inheritance (Margueron and Reinberg 2010; Zheng et al. 2013). Whether these mechanisms are involved in IL-4 regulation needs to be investigated. Second, although acetylation is the most studied type of histone modification, there are several other types of histone modification involved in transcript regulation, such as lysine or arginine methylation, lysine ubiquitylation, serine and threonine phosphorylation, lysine sumoylation, lysine ribosylation, and arginine citrullination (Kim et al. 2009; Zhang and Pradhan 2014). Although we investigated several popular histone acetylation and methylation active markers, we cannot exclude the possibility that other histone modifications were involved in the prenatal DEX-regulated IL-4 production. Third, we measured mRNA levels of T-bet, GATA-3 and Foxp3 of unactivated spleen tissue in this study rather than activated splenocytes. If spleen cells from the DEX group at day 7 were stimulated with Con A, upregulation of GATA-3 transcript may be observed. Thus further more compressive studies are needed regarding the Th1/Th2 balance.
We found that innate immunity was affected by prenatal DEX exposure and that its effects persisted at least until young adult stage. In contrast to the data on female offspring reported by Dietert et al. (2003), in our male offspring, TNF-α was suppressed but IP-10 and IL-10 levels were increased at D120 by prenatal DEX treatment. Sex should be considered an important parameter for these conflicting results. On follow-up until D180, the altered cytokine levels returned to normal; however, Dietert et al. (2003) did not investigate the mature adult period. Age-dependent complications associated with prenatal corticosteroid therapy have been noted previously (Lanteri et al. 1994), and our data suggest that the effects of prenatal DEX exposure on immunity were age-dependent, although the exact mechanism is unclear. We studied the effects of prenatal DEX on immunity of newborn to middle age adult rats. Further research related to immune programming in older stages is required to fully understand the long-term effects of prenatal corticosteroid exposure on the immune system.
Acknowledgments
This study was supported by grants CMRPG8D0761, CMRPG8D0181, CMRPG8D0182 (Yu, H.R.), CMRPG8D0261 (Chou, M.Y.) from Chang Gung Medical Foundation and NSC 102-2314-B-182-066-MY3 (Yu, H.R.) from the National Science Council, Taiwan.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Allan,
R.S.,
Zueva,
E.,
Cammas,
F.,
Schreiber,
H.A.,
Masson,
V.,
Belz,
G.T.,
Roche,
D.,
Maison,
C.,
Quivy,
J.P.,
Almouzni,
G. &
Amigorena,
S.
(2012) An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature, 487, 249-253.
-
Andreollo,
N.A.,
Santos,
E.F.,
Araujo,
M.R. &
Lopes,
L.R.
(2012) Rat’s age versus human’s age: what is the relationship? Arq. Bras. Cir. Dig., 25, 49-51.
-
Antonow-Schlorke,
I.,
Helgert,
A.,
Gey,
C.,
Coksaygan,
T.,
Schubert,
H.,
Nathanielsz,
P.W.,
Witte,
O.W. &
Schwab,
M.
(2009) Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet. Gynecol., 113, 142-151.
-
Berry,
M.J.,
Jaquiery,
A.L.,
Oliver,
M.H.,
Harding,
J.E. &
Bloomfield,
F.H.
(2013) Antenatal corticosteroid exposure at term increases adult adiposity: an experimental study in sheep. Acta Obstet. Gynecol. Scand., 92, 862-865.
-
Biggioggero,
M.,
Borghi,
M.O.,
Gerosa,
M.,
Trespidi,
L.,
Cimaz,
R. &
Meroni,
P.I.
(2007) Immune function in children born to mothers with autoimmune diseases and exposed in utero to immunosuppressants. Lupus, 16, 651-656.
-
Brownfoot,
F.C.,
Gagliardi,
D.I.,
Bain,
E.,
Middleton,
P. &
Crowther,
C.A.
(2013) Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev., 8, CD006764.
-
Bunton,
T.E. &
Plopper,
C.G.
(1984) Triamcinolone-induced structural alterations in the development of the lung of the fetal rhesus macaque. Am. J. Obstet. Gynecol., 148, 203-215.
-
Busse,
W.W. &
Rosenwasser,
L.J.
(2003) Mechanisms of asthma. J. Allergy Clin. Immunol., 111, S799-804.
-
Byrjalsen,
A.,
Froslev,
T.,
Telen Andersen,
A.B.,
Olsen,
M. &
Sorensen,
H.T.
(2014) Use of corticosteroids during pregnancy and risk of asthma in offspring: a nationwide Danish cohort study. BMJ Open, 4, e005053.
-
Cai,
J.H.,
Deng,
S.,
Kumpf,
S.W.,
Lee,
P.A.,
Zagouras,
P.,
Ryan,
A. &
Gallagher,
D.S.
(2007) Validation of rat reference genes for improved quantitative gene expression analysis using low density arrays. BioTechniques, 42, 503-512.
-
Chabra,
S.,
Cottrill,
C.,
Rayens,
M.K.,
Cross,
R.,
Lipke,
D. &
Bruce,
M.
(1998) Lymphocyte subsets in cord blood of preterm infants: effect of antenatal steroids. Biol. Neonate, 74, 200-207.
-
Cimaz,
R.,
Meregalli,
E.,
Biggioggero,
M.,
Borghi,
O.,
Tincani,
A.,
Motta,
M.,
Airo,
P. &
Meroni,
P.L.
(2004) Alterations in the immune system of children from mothers treated with immunosuppressive agents during pregnancy. Toxicol. Lett., 149, 155-162.
-
Coe,
C.L. &
Lubach,
G.R.
(2005) Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci. Biobehav. Rev., 29, 227-235.
-
Dessens,
A.B.,
Haas,
H.S. &
Koppe,
J.G.
(2000) Twenty-year follow-up of antenatal corticosteroid treatment. Pediatrics, 105, E77.
-
Dietert,
R.R.,
Lee,
J.E.,
Olsen,
J.,
Fitch,
K. &
Marsh,
J.A.
(2003) Developmental immunotoxicity of dexamethasone: comparison of fetal versus adult exposures. Toxicology, 194, 163-176.
-
Hung,
Y.L.,
Hsieh,
W.S.,
Chou,
H.C.,
Yang,
Y.H.,
Chen,
C.Y. &
Tsao,
P.N.
(2010) Antenatal steroid treatment reduces childhood asthma risk in very low birth weight infants without bronchopulmonary dysplasia. J. Perinat. Med., 38, 95-102.
-
Kavelaars,
A.,
van der Pompe,
G.,
Bakker,
J.M.,
van Hasselt,
P.M.,
Cats,
B.,
Visser,
G.H. &
Heijnen,
C.J.
(1999) Altered immune function in human newborns after prenatal administration of betamethasone: enhanced natural killer cell activity and decreased T cell proliferation in cord blood. Pediatr. Res., 45, 306-312.
-
Kim,
J.K.,
Samaranayake,
M. &
Pradhan,
S.
(2009) Epigenetic mechanisms in mammals. Cell. Mol. Life Sci., 66, 596-612.
-
Kumar,
P.,
Venners,
S.A.,
Fu,
L.,
Pearson,
C.,
Ortiz,
K. &
Wang,
X.
(2011) Association of antenatal steroid use with cord blood immune biomarkers in preterm births. Early Hum. Dev., 87, 559-564.
-
Kuo,
H.C.,
Guo,
M.M.,
Liu,
S.F.,
Chen,
C.C.,
Sheen,
J.M.,
Yu,
H.R.,
Tiao,
M.M.,
Tain,
Y.L. &
Huang,
L.T.
(2014) Cross-fostering increases TH1/TH2 expression in a prenatal dexamethasone exposure rat model. PLoS One, 9, e115554.
-
Kuypers,
E.,
Collins,
J.J.,
Jellema,
R.K.,
Wolfs,
T.G.,
Kemp,
M.W.,
Nitsos,
I.,
Pillow,
J.J.,
Polglase,
G.R.,
Newnham,
J.P.,
Germeraad,
W.T.,
Kallapur,
S.G.,
Jobe,
A.H. &
Kramer,
B.W.
(2012) Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PLoS One, 7, e38257.
-
Lanteri,
C.J.,
Willet,
K.E.,
Kano,
S.,
Jobe,
A.H.,
Ikegami,
M.,
Polk,
D.H.,
Newnham,
J.P.,
Kohan,
R.,
Kelly,
R. &
Sly,
P.D.
(1994) Time course of changes in lung mechanics following fetal steroid treatment. Am. J. Respir. Crit. Care Med., 150, 759-765.
-
Liggins,
G.C. &
Howie,
R.N.
(1972) A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics, 50, 515-525.
-
Lilja,
G. &
Wickman,
M.
(2000) The immunology of fetuses and infants. Allergy, 55, 589-590.
-
Locatelli,
A.,
Consonni,
S. &
Ghidini,
A.
(2015) Preterm Labor: approach to Decreasing Complications of Prematurity. Obstet. Gynecol. Clin. North Am., 42, 255-274.
-
Luckheeram,
R.V.,
Zhou,
R.,
Verma,
A.D. &
Xia,
B.
(2012) CD4+T cells: differentiation and functions. Clin. Dev. Immunol., 2012, 925135.
-
Lui,
C.C.,
Hsu,
M.H.,
Kuo,
H.C.,
Chen,
C.C.,
Sheen,
J.M.,
Yu,
H.R.,
Tiao,
M.M.,
Tain,
Y.L.,
Chang,
K.A. &
Huang,
L.T.
(2015) Effects of melatonin on prenatal dexamethasone-induced epigenetic alterations in hippocampal morphology and reelin and glutamic acid decarboxylase 67 levels. Dev. Neurosci., 37, 105-114.
-
Macaubas.,
C,
de Klerk,
N.H.,
Holt,
B.J.,
Wee,
C.,
Kendall,
G.,
Firth,
M.,
Sly,
P.D. &
Holt,
P.G.
(2003) Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet, 362, 1192-1197.
-
Margueron,
R. &
Reinberg,
D.
(2010) Chromatin structure and the inheritance of epigenetic information. Nat. Rev. Genet., 11, 285-296.
-
Michie,
C.A.,
Hasson,
N. &
Tulloh,
R.
(1998) The neonatal thymus and antenatal steroids. Arch. Dis. Child. Fetal Neonatal Ed., 79, F159.
-
Motta,
M.,
Rodriguez-Perez,
C.,
Tincani,
A.,
Lojacono,
A.,
Nacinovich,
R. &
Chirico,
G.
(2009) Neonates born from mothers with autoimmune disorders. Early Hum. Dev., 85, S67-70.
-
Noorlander,
C.W.,
Tijsseling,
D.,
Hessel,
E.V.,
de Vries,
W.B.,
Derks,
J.B.,
Visser,
G.H. &
de Graan,
P.N.
(2014) Antenatal glucocorticoid treatment affects hippocampal development in mice. PLoS One, 9, e85671.
-
Ownby,
D.R.
(2001) Pediatric asthma and development of atopy. Curr. Opin. Allergy Clin. Immunol., 1, 125-126.
-
Palta,
M.,
Sadek-Badawi,
M.,
Sheehy,
M.,
Albanese,
A.,
Weinstein,
M.,
McGuinness,
G. &
Peters,
M.E.
(2001) Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am. J. Epidemiol., 154, 521-529.
-
Pole,
J.D.,
Mustard,
C.A.,
To,
T.,
Beyene,
J. &
Allen,
A.C.
(2009) Antenatal steroid therapy for fetal lung maturation: is there an association with childhood asthma? J. Asthma, 46, 47-52.
-
Quinlivan,
J.A.,
Archer,
M.A.,
Dunlop,
S.A.,
Evans,
S.F.,
Beazley,
L.D. &
Newnham,
J.P.
(1998) Fetal growth retardation, particularly within lymphoid organs, following repeated maternal injections of betamethasone in sheep. J. Obstet. Gynaecol. Res., 24, 173-182.
-
Quinn,
R.
(2005) Comparing rat’s to human’s age: how old is my rat in people years? Nutrition, 21, 775-777.
-
Roberts,
D. &
Dalziel,
S.
(2006) Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev., CD004454.
-
Schmand,
B.,
Neuvel,
J.,
Smolders-de Haas,
H.,
Hoeks,
J.,
Treffers,
P.E. &
Koppe,
J.G.
(1990) Psychological development of children who were treated antenatally with corticosteroids to prevent respiratory distress syndrome. Pediatrics, 86, 58-64.
-
Schutte,
M.F.,
Treffers,
P.E.,
Koppe,
J.G. &
Breur,
W.
(1980) The influence of betamethasone and orciprenaline on the incidence of respiratory distress syndrome in the newborn after preterm labour. Br. J. Obstet. Gynaecol., 87, 127-131.
-
Sengupta,
P.
(2013) The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med., 4, 624-630.
-
Sheen,
J.M.,
Yu,
H.R.,
Tiao,
M.M.,
Chen,
C.C.,
Huang,
L.T.,
Chang,
H.Y. &
Tain,
Y.L.
(2015) Prenatal dexamethasone-induced programmed hypertension and renal programming. Life Sci., 132, 41-48.
-
Sundrud,
M.S. &
Nolan,
M.A.
(2010) Synergistic and combinatorial control of T cell activation and differentiation by transcription factors. Curr. Opin. Immunol., 22, 286-292.
-
Surbek,
D.,
Drack,
G.,
Irion,
O.,
Nelle,
M.,
Huang,
D. &
Hoesli,
I.
(2012) Antenatal corticosteroids for fetal lung maturation in threatened preterm delivery: indications and administration. Arch. Gynecol. Obstet., 286, 277-281.
-
Tiao,
M.M.,
Huang,
L.T.,
Chen,
C.J.,
Sheen,
J.M.,
Tain,
Y.L.,
Chen,
C.C.,
Kuo,
H.C.,
Huang,
Y.H.,
Tang,
K.S.,
Chu,
E.W. &
Yu,
H.R.
(2014) Melatonin in the regulation of liver steatosis following prenatal glucocorticoid exposure. Biomed. Res. Int., 2014, 942172.
-
van Merode,
T.,
Maas,
T.,
Twellaar,
M.,
Kester,
A. &
van Schayck,
C.P.
(2007) Gender-specific differences in the prevention of asthma-like symptoms in high-risk infants. Pediatr. Allergy Immunol., 18, 196-200.
-
Wu,
F.F.,
Momma,
K. &
Takao,
A.
(1993) Cardiovascular and pulmonary effects of betamethasone during midtrimester on fetal rats. Fetal Diagn. Ther., 8, 89-94.
-
Yu,
H.R.,
Chang,
J.C.,
Chen,
R.F.,
Chuang,
H.,
Hong,
K.C.,
Wang,
L. &
Yang,
K.D.
(2003) Different antigens trigger different Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to T-bet/GATA-3 expression. J. Leukoc. Biol., 74, 952-958.
-
Yu,
H.R.,
Kuo,
H.C.,
Chen,
C.C.,
Sheen,
J.M.,
Tiao,
M.M.,
Chen,
Y.C.,
Chang,
K.A.,
Tain,
Y.L. &
Huang,
L.T.
(2014a) Prenatal dexamethasone exposure in rats results in long-term epigenetic histone modifications and tumour necrosis factor-alpha production decrease. Immunology, 143, 651-660.
-
Yu,
H.R.,
Kuo,
H.C.,
Huang,
L.T.,
Chen,
C.C.,
Tain,
Y.L.,
Sheen,
J.M.,
Tiao,
M.M.,
Huang,
H.C.,
Yang,
K.D.,
Ou,
C.Y. &
Hsu,
T.Y.
(2014b) L-Arginine modulates neonatal lymphocyte proliferation through an interleukin-2 independent pathway. Immunology, 143, 184-192.
-
Zhang,
G. &
Pradhan,
S.
(2014) Mammalian epigenetic mechanisms. IUBMB Life, 66, 240-256.
-
Zheng,
G.,
Dahl,
J.A.,
Niu,
Y.,
Fu,
Y.,
Klungland,
A.,
Yang,
Y.G. &
He,
C.
(2013) Sprouts of RNA epigenetics: the discovery of mammalian RNA demethylases. RNA Biol., 10, 915-918.