Abstract
Chronic hepatitis B virus (HBV) infection is associated with lower prevalence of hyperlipidemia (HLP). However, occult HBV infection (OBI) in HLP patients has not yet been explored. OBI is defined as the presence of detectable HBV DNA in serum or liver tissue but undetectable HBV surface antigen in serum. In this study, 1,036 HLP patients and 1,134 replacement blood donor controls were recruited. Among them, 252 HLP patients and 255 blood donors with antibody to HBV core positive were selected and analyzed. HBV DNA was confirmed by nucleic acid testing assays, and nucleotide mutations were analyzed. OBI was detected in 9.5% (24/252) of HLP patients and 2.4% (6/255) of blood donors, respectively (P < 0.001). In HLP population, 41.7% of OBI and 13.6% of non-OBI carriers were associated with daily alcohol consuming > 30 g/day (P < 0.01), while in control population those rates were not statistically different between OBI and non-OBI carriers (P > 0.05). Viral load of OBI in HLP patients was higher than that of OBI in blood donors (P < 0.05), which was a positive correlation between total cholesterol and HBV viral load levels (r = 0.474 P = 0.019). HBV vaccination rate was found significantly lower in OBI HLP patients than that in non-OBI HLP patients (P < 0.01). Importantly, mutations were found in basic core promoter region of HBV among OBI HLP patients. In conclusion, the frequency of OBI is significantly higher in HLP patients, especially those patients with heavy daily alcohol consumption.
Introduction
Hyperlipidemia (HLP) affects millions of people worldwide, which is a major risk factor for cardiovascular disease, fatty liver and diabetes (Bass et al. 1993; Bittner 2005; Milias et al. 2006), which is characterized by either elevated levels of serum total cholesterol, triglycerides or both. The known relevant factors associated with hyperlipidemia are high fat diet, diabetes, high blood pressure, smoking, age and gender (Primatesta and Poulter 2006; Li-Ng et al. 2007; Estoppey et al. 2011).
Previous studies showed that chronic hepatitis B virus (HBV) infection was associated with lower prevalence of hypertriglyceridemia (Jan et al. 2006; Chen et al. 2010; Wong et al. 2012; Chung et al. 2014; Huang et al. 2016) or hypercholesterolemia (Chen et al. 2010; Liu et al. 2013). HBV DNA load in chronic hepatitis B patients with normal range of total cholesterol was lower than those with high level of total cholesterol (Jarčuška et al. 2014). In contrast, the lower HBV viral load but higher triglyceride level were observed among patients with chronic HBV infection (Hsu et al. 2012).
In recent years, a special form of HBV infection called occult HBV infection (OBI) attracted attention towards blood transfusion and healthcare. OBI is defined as the presence of detectable HBV DNA in serum or liver tissue but undetectable HBV surface antigen (HBsAg) in serum (Raimondo et al. 2008), which is a risk factor for blood safety and development of hepatocellular carcinoma (HCC) (Allain and Cox 2011; Hassan et al. 2011). However, the association of occult HBV infection among certain groups of patients such as those with hyperlipidemia has not been reported.
In this study, we carried out a case-control survey for OBI among hyperlipidemic patients in comparison with a control population of replacement blood donors.
Subjects and Methods
Criteria for enrollment of patients and control populations
According to Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001), hyperlipidemia (HLP) is characterized as low-density lipoprotein (LDL)-cholesterol levels > 140 mg/dL or triglyceride (TG) levels > 150 mg/dL in patient’s serum. A total of 1,273 outpatients were diagnosed with hyperlipidemia between April 2010 and June 2014 in the affiliated hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, China. In this study, inclusive criteria for selecting participants were: (1) serum LDL level > 140 mg/dL or TG level > 150 mg/dL; (2) ages of 40-60 years; (3) treatment naive patients (especially who have medication for hypercholesterolemia such as taking statin); (4) HBsAg negative. All involved individuals were questioned for a history of medication, blood transfusion, HBV vaccination, smoking habit and daily alcohol drinking (Table 1). Patients with daily alcohol consumption > 30 g/day were examined separately (European Association for the Study of Liver 2012; Askgaard et al. 2015), patients with liver cirrhosis were recognized (Hashemi et al. 2015). According to these recruitment criteria, 237 patients were excluded (Demir et al. 2008), including 140 HBsAg+, 12 antibodies positive to hepatitis C virus (anti-HCV+), 1 antibody positive to human immunodeficiency virus (anti-HIV+), 3 liver fibrosis, 73 diabetes mellitus and 8 patients unavailable for follow-up examination. Based on detection of antibody to hepatitis B core antigen (anti-HBc), 252 hyperlipidemic patients (HPL group) with or without antibody to hepatitis B surface antigen (anti-HBs) were finally enrolled in this study (Fig. 1). In a control population, 1134 replacement blood donors with normal range of LDL or TG were recruited and tested in hospital, and among them 255 individuals with anti-HBc+ were randomly selected as controls between April 2012 and June 2014 (Fig. 1). All individuals involved in this study signed an informed consent. The study was approved by the Ethics Committee of Human Resources at the Jiangxi University of Traditional Chinese Medicine (No. [2010]-151).

Blood samples were tested for clinical biochemistry indicators, including alanine transaminase (ALT, normal range < 40 IU/L), aspartate transaminase (AST, normal range < 40 IU/L), γ-glutamyl transpeptidase (GGT, normal range < 40 IU/L). Low-density lipoprotein (LDL), triglyceride (TG) and total cholesterol (TC) levels were measured automatically with the Hitachi 7180 analyzer (Hitachi Ltd., Tokyo, Japan). Detection of HBsAg, anti-HBc, anti-HBs, anti-HCV and anti-HIV was performed with Abbott analyzer i2000SR (Abbott Laboratories, North Chicago, IL, USA).
HBV-DNA testing
HBV DNA was extracted from patient sera using Virus Acid Extraction Kits (Hoffmann-La Roche Ltd., Mannheim, Germany). RNase-Free water was extracted by the same way for the following negative control (Takara, Dalian, China). Detection of HBV DNA was performed in hospital by real-time PCR (qPCR) using the Premix Ex Taq™ kits according manufacturer’s instruction (Perfect Real Time, Takara, Dalian, China). The detection limit of HBV-DNA levels is 20 IU/mL (100 copies/mL). HBV DNA-negative serum samples were further detected in Guangzhou blood center by nucleic acid testing (NAT) with the multiplex Procleix Ultrio assay (individual donation [ID] NAT, Novartis Diagnostics, Emeryville, CA). HBV DNA positive samples were detected by confirmatory PCRs (Zheng et al. 2011). HBV DNA carriers with anti-HBc+/HBsAg− were defined as occult HBV infection (OBI) after exclusion of HBV window period infection in both patients and blood donors (Zheng et al. 2011).
Nested PCR technique and electrophoresis
HBV DNA was detected by nested PCR technique involving amplification of the HBV genome specific for the basic core promoter (BCP) region. The primers and cycling conditions described as a previous report (Allain et al. 2012). Nested PCR reactions were performed by using Takara’s rTaq and La-Taq enzyme (Takara, Dalian, China). 10 µl 2nd-round PCR products were analyzed by electrophoresis in 1.5% agarose gel stained with ethidium bromide.
Sequencing of HBV DNA from OBI HLP patients
BCP region amplification (295 bp) were directly sequenced commercially (Invitrogen, Guangzhou, China). The genotype and mutation were determined by nucleotide sequence alignments with the DNAMAN program (Lynnon Corporation, Quebec, Canada, version 6.0). Mutations in HBV BCP region were identified through comparison with reference sequences for genotype B or C (GenBank accession numbers of DQ463392 and AY167089), respectively.
Statistical analysis
Statistical analyses were performed by SPSS 19.0 (SPSS Inc., Chicago, Illinois, USA). Data was analyzed using parametric methods for normally distributed continuous data (t-test). Continuous variables were expressed as mean ± standard deviation. Chi-square test was performed to compare the variables of categories between two groups. For continuous variables, the non-parametric Mann-Whitney test was used to compare the median of viral load between two groups. P value < 0.05 was considered as significant. The Speraman correlation was used to access the association between two quantitative variables.
Results
Characteristics of population backgrounds
The information of clinical background was obtained individually from 252 HLP patients and 255 replacement blood donor controls carrying HBV serological markers (Table 1). All subjects were anti-HBc positive. No significant difference in the risk factors of age, gender, alcohol drinking, smoking, blood transfusion and HBV vaccination was observed between HLP patients and blood donors (P = 0.20-0.76), including the risk factor of daily alcohol consumption > 30 g/day (Table 1).
Clinical examination of hyperlipidemic patients and blood donor controls
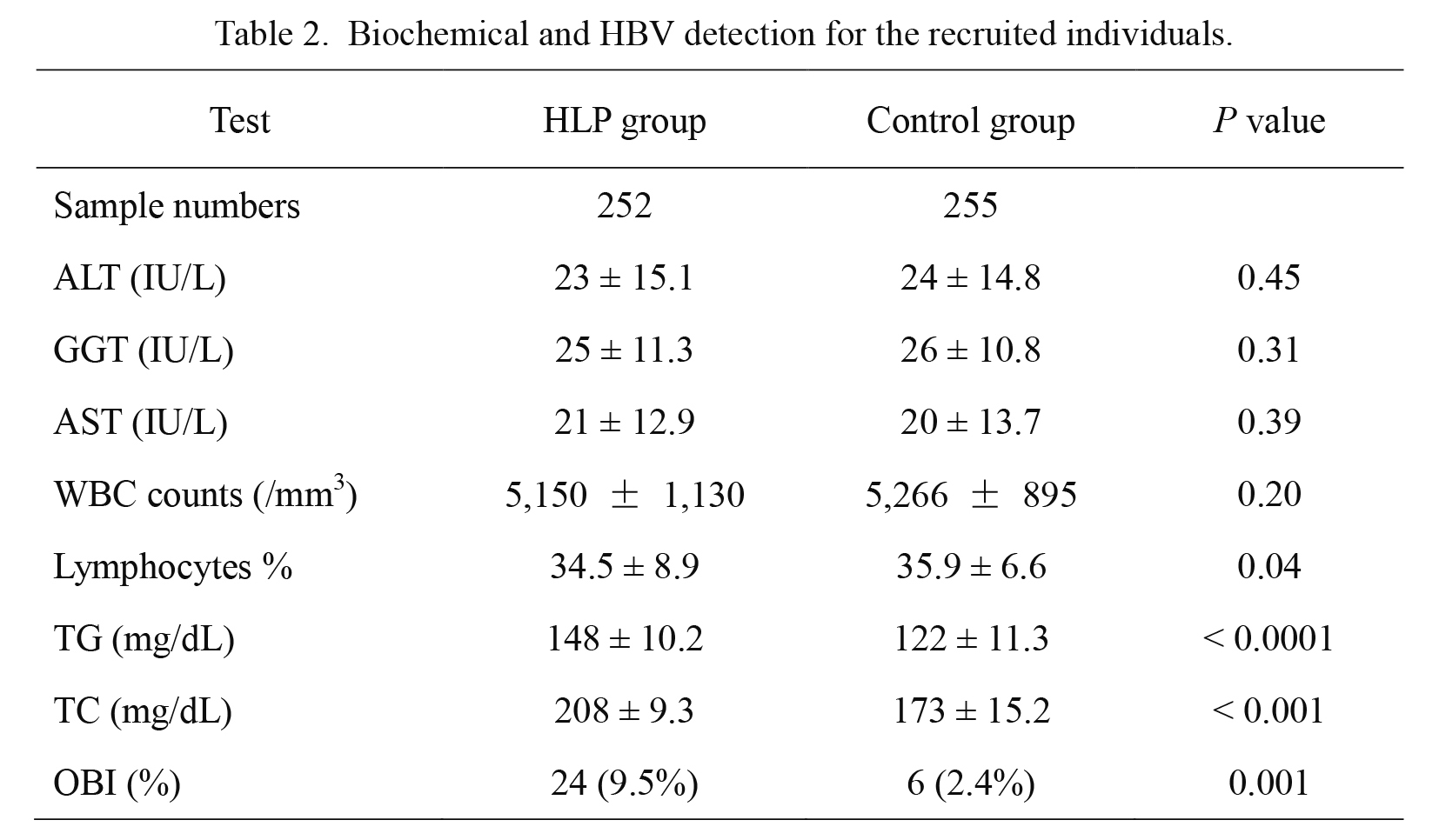
Individuals with anti-HBc, with/without anti-HBs and without HBsAg were selected from two groups of 1,036 HLP patients and 1,134 replacement blood donor controls, respectively, who were tested for serum biochemical activity of hepatic enzymes, total cholesterol (TC) and HBV DNA (Table 2). Levels of ALT, AST and GGT were measured in the normal ranges. For all these markers, no statistical differences were observed between HLP and control groups (P = 0.31-0.45). TC levels were significantly higher in the HLP group than those in the control group (P < 0.001). HBV DNA positive samples classified as OBI were detected in 9.5% (24/252) HLP patients and 2.4% (6/255) blood donor controls. The frequency of OBI appeared to be significantly higher in HLP patients than that in replacement blood donor population (P = 0.001).
Association of hyperlipidemic patients with occult HBV infection
Twenty-four HLP patients and six replacement blood donors, carrying OBI, were analyzed for association with the risk factors to HLP (Table 3). Level of LDL or TG was measured either > 140 mg/dL or > 150 mg/dL from HLP patients, respectively, but both factors levels were bellow hyperlipidemia criteria in replacement blood donors. TC level was significantly higher in OBI HLP patients than in OBI blood donors (P = 0.00012).
The rate of daily alcohol consumption > 30 g/day was 41.7% (10/24) in HLP patients with OBI, which was statistically higher than 13.6% (31/228) in HLP patients without OBI (P = 0.0016). The rates were not statistically different between OBI and non-OBI blood donors (16.7% [1/6] vs. 15.3% [38/249]) (P = 0.92). Among 24 OBI HLP patients, 8.3% (2/24) had received HBV vaccines, which was significantly lower than 52.2% (119/228) in non-OBI HLP patients (Table 3) (P = 0.0001).
Viral load (median 1,000 HBV DNA copies/ml) of OBI in HLP patients ranged between 700 and 1,200 copies/ml, which was significantly higher than that observed (median 800 copies/ml) in blood donor controls with OBI and normal range of LDL or TG level (P = 0.023). Among 24 OBI HLP patients, a positive correlation was observed between TC and HBV load levels (TC vs. HBV DNA load; r = 0.474, P = 0.019) (Fig. 2).
Genetic analysis in BCP region of OBIs from HLP patients
Ten out of 24 (42%) OBIs from HLP patients were analyzed for genotyping and mutations in BCP region of HBV (Table 4). Five OBIs were classified as genotype B and 5 were classified as genotype C. And among them 4 had a single nucleotide (nt) mutation, 4 had two mutations and 2 had three mutations. The typical mutations at nt 1762, 1764 and 1846 in BCP region of HBV were observed in OBIs from the HLP patients (Table 4).
Discussion
Chronic HBV infection is associated with lower prevalence of hypertriglyceridemia in men and women (Jan et al. 2006; Chen et al. 2010; Wong et al. 2012; Chung et al. 2014; Huang et al. 2016). In this study, however, OBI was detected at higher frequency in HLP patients than in replacement blood donor population controls (9.5% vs. 2.4%, P < 0.001), which suggested a positive association between OBI and HLP. Patients with hypercholesterolemia have higher total T cell counts, higher cluster of differentiation CD4+ cells and more interleukin-2 (IL-2) release in response to PHA (Muldoon et al. 1997). In addition, compared with the healthy controls, level of IL-2 increased significantly, while interleukin-10 (IL-10) decreased significantly among patients with hypercholesterolemia (P < 0.05) (Zhang et al. 2016). A similar situation was found with changes in immune system function such as the decrease of IL-10 in OBI patients co-infected with HIV infection compared to patients with overt HBV infection (Martin et al. 2009; Kwak and Kim 2014). In addition to inflammatory parameter, a previous study found that occult HBV infection lead to a significant increase in the levels of DNA damage, reactive oxygen species (ROS) and apoptosis in peripheral blood lymphocytes (Bhargava et al. 2010). In this study, whether high frequency of OBI in HLP patients is due to the increase of T-cell counts and decrease of IL-10 needs to be further investigated.
Higher frequency of OBI is found in HLP patients with heavy daily alcohol consumption than in HLP patients and blood donors without daily alcohol consumption > 30 g, suggesting that heavy alcohol consuming has a synergistic effect on the occurrence of OBI and HLP. In the case of the immune system, light or moderate alcohol consumption was associated with reduced inflammation and improved response to vaccination, while chronic heavy drinking was associated with decreased lymphocyte counts and increased risk of both bacterial and viral infections (Barr et al. 2016). Increased alcohol consumption has been related to increased prevalence of seroconversion from hepatitis B e antigen (HBeAg) to anti-HBe and to increasing prevalence of alcoholic liver disease and decreasing prevalence of chronic hepatitis (Itoh and Shimoji 1986), which might be a cause for OBI occurrence following HBV infection (Raimondo et al. 2013). Therefore, discontinuing heavy alcohol drinking should decrease the frequency of OBI occurrence in HLP patients and healthy individuals.
Viral load of OBI in HLP patients with LDL > 140 mg/dL or TG > 150 mg/dL was significantly higher than that of OBI in replacement blood donors with a normal range of LDL and TG levels (P < 0.05). In the meanwhile, a positive correlation between TC and HBV-load level in OBI HLP patients was observed (Fig. 2). Among chronic HBV infected individuals, a study showed that patients with metabolic syndrome (MS) or with hypercholesterolemia or hypocholesterolemia carried significantly higher HBV viral load than those without MS or with normal range of total cholesterol (Jarčuška et al. 2014). Another report indicated that HBV viral load was negatively proportional to serum TG (Jan et al. 2006). However, whether patient’s HLP benefits for maintaining a relatively high viral load of OBI still remains unclear. High frequency of OBI was found from HLP patients with low rate of HBV vaccination, suggesting the efficacy of HBV vaccination plays a critical role in preventing both occult and chronic HBV infections (Amponsah-Dacosta et al. 2015).
In our study, nucleotide mutations in the BCP region were detected from 10 of 24 OBI HLP patients, in which the meaningful mutations A1762T and G1764A were observed (Zheng et al. 2011; Chamni et al. 2014; Inuzuka et al. 2014; Yang et al. 2016). These could potentially be responsible for viral persistence and failure of detection of HBsAg in OBI HLP patients (Chandra et al. 2009; Fang et al. 2009; Pinarbasi et al. 2009; Chen et al. 2012). Some mutations in the BCP region are associated with not only immunoassay failure but also vaccine escape variants (Sa-Nguanmoo et al. 2012; Yimnoi et al. 2016). As all known, many researchers found that the disorders in lipid metabolism have contributed to the understanding of lipoprotein metabolism and the pathophysiological consequences of a particular mutation (Smit and Diamant 2004). However, it seems that there is no evidence of BCP mutation associated with the HLP patients. Further research requires to define whether the correlation between HLP and critical mutation of OBI exists.
In summary, we show the high frequency of OBI occurrence in HLP patients, in particular those patients with heavy daily alcohol consumption. Viral load of OBI in HLP patients was relatively higher than that of individuals without HLP. The positive correlation between TC and HBV DNA level was observed among OBI HLP patients. BCP mutations were detected in OBIs, which might contribute to occurrence of occult HBV infection in the HLP patients.
Acknowledgments
The authors thank Professor Junping Yang and Dr. Youjiang Min for blood or liver tissue sample collection at Department of Laboratory Medicine, the Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, China.
This work was supported by the grants from the National Natural Science Foundation of China (grant No. 81371801 and 81071348), the Guangzhou Key Laboratory for Blood Safety (grant No. 201509010009) and the Guangzhou Pearl River S&T Nova Program (grant No. 201506010075).
Conflict of Interest
The authors declare no conflict of interest.
References
-
Allain,
J.P. &
Candotti,
D.;
ISBT HBV Safety Collaborative Group
(2012) Hepatitis B virus in transfusion medicine: still a problem? Biologicals, 40, 180-186.
-
Allain,
J.P. &
Cox,
L.
(2011) Challenges in hepatitis B detection among blood donors. Curr. Opin. Hematol., 18, 461-466.
-
Amponsah-Dacosta,
E.,
Lebelo,
R.L.,
Rakgole,
J.N.,
Selabe,
S.G.,
Gededzha,
M.P.,
Mayaphi,
S.H.,
Powell,
E.A.,
Blackard,
J.T. &
Mphahlele,
M.J.
(2015) Hepatitis B virus infection in post-vaccination South Africa: occult HBV infection and circulating surface gene variants. J. Clin. Virol., 63, 12-17.
-
Askgaard,
G.,
Grønbæk,
M.,
Kjær,
M.S.,
Tjønneland,
A. &
Tolstrup,
J.S.
(2015) Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J. Hepatol., 62, 1061-1067.
-
Barr,
T.,
Helms,
C.,
Grant,
K. &
Messaoudi,
I.
(2016) Opposing effects of alcohol on the immune system. Prog. Neuropsychopharmacol. Biol. Psychiatry, 65, 242-251.
-
Bass,
K.M.,
Newschaffer,
C.J.,
Klag,
M.J. &
Bush,
T.L.
(1993) Plasma lipoprotein levels as predictors of cardiovascular death in women. Arch. Intern. Med., 153, 2209-2216.
-
Bhargava,
A.,
Khan,
S.,
Panwar,
H.,
Pathak,
N.,
Punde,
R.P.,
Varshney,
S. &
Mishra,
P.K.
(2010) Occult hepatitis B virus infection with low viremia induces DNA damage, apoptosis and oxidative stress in peripheral blood lymphocytes. Virus Res., 153, 143-150.
-
Bittner,
V.
(2005) Perspectives on dyslipidemia and coronary heart disease in women. J. Am. Coll. Cardiol., 46, 1628-1635.
-
Chamni,
N.,
Louisirirotchanakul,
S.,
Oota,
S.,
Sakuldamrongpanish,
T.,
Saldanha,
J.,
Chongkolwatana,
V. &
Phikulsod,
S.
(2014) Genetic characterization and genotyping of hepatitis B virus (HBV) isolates from donors with an occult HBV infection. Vox Sang., 107, 324-332.
-
Chandra,
P.K.,
Biswas,
A.,
Datta,
S.,
Banerjee,
A.,
Panigrahi,
R.,
Chakrabarti,
S.,
De,
B.K. &
Chakravarty,
R.
(2009) Subgenotypes of hepatitis B virus genotype D (D1, D2, D3 and D5) in India: differential pattern of mutations, liver injury and occult HBV infection. J. Viral Hepat., 16, 749-756.
-
Chen,
J.Y.,
Wang,
J.H.,
Lin,
C.Y.,
Chen,
P.F.,
Tseng,
P.L.,
Chen,
C.H.,
Chang,
K.C.,
Tsai,
L.S.,
Chen,
S.C. &
Lu,
S.N.
(2010) Lower prevalence of hypercholesterolemia and hyperglyceridemia found in subjects with seropositivity for both hepatitis B and C strains independently. J. Gastroenterol. Hepatol., 25, 1763-1768.
-
Chen,
S.J.,
Zhao,
Y.X.,
Fang,
Y.,
Xu,
W.Z.,
Ma,
Y.X.,
Song,
Z.W.,
Teng,
X. &
Gu,
H.X.
(2012) Viral deletions among healthy young Chinese adults with occult hepatitis B virus infection. Virus Res., 163, 197-201.
-
Chung,
T.H.,
Kim,
M.C. &
Kim,
C.S.
(2014) Association between Hepatitis B Surface Antigen Seropositivity and Metabolic Syndrome. Korean J. Fam. Med., 35, 81-89.
-
Demir,
M.,
Serin,
E.,
Göktürk,
S.,
Ozturk,
N.A.,
Kulaksizoglu,
S. &
Ylmaz,
U.
(2008) The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eur. J. Gastroenterol. Hepatol., 20, 668-673.
-
Estoppey,
D.,
Paccaud,
F.,
Vollenweider,
P. &
Marques-Vidal,
P.
(2011) Trends in self-reported prevalence and management of hypertension, hypercholesterolemia and diabetes in Swiss adults, 1997-2007. BMC Public Health, 11, 114.
-
European Association for the Study of Liver
(2012) EASL clinical practical guidelines: management of alcoholic liver disease. J. Hepatol., 57, 399-420.
-
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults
(2001) Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA, 285, 2486-2497.
-
Fang,
Y.,
Teng,
X.,
Xu,
W.Z.,
Li,
D.,
Zhao,
H.W.,
Fu,
L.J.,
Zhang,
F.M. &
Gu,
H.X.
(2009) Molecular characterization and functional analysis of occult hepatitis B virus infection in Chinese patients infected with genotype C. J. Med. Virol., 81, 826-835.
-
Hashemi,
S.J.,
Hajiani,
E.,
Masjedizadeh,
A.,
Makvandi,
M.,
Shayesteh,
A.A.,
Alavinejad,
S.P.,
Kadkhodaei,
A.,
Shahbazian,
H.,
Jasemi,
F. &
Karimi,
M.
(2015) Occult hepatitis B infection in patients with cryptogenic liver cirrhosis in southwest of Iran. Jundishapur J. Microbiol., 8, e16873.
-
Hassan,
Z.K.,
Hafez,
M.M.,
Mansor,
T.M. &
Zekri,
A.R.
(2011) Occult HBV infection among Egyptian hepatocellular carcinoma patients. Virol. J., 8, 90.
-
Hsu,
C.S.,
Liu,
C.H.,
Wang,
C.C.,
Tseng,
T.C.,
Liu,
C.J.,
Chen,
C.L.,
Chen,
P.J.,
Chen,
D.S. &
Kao,
J.H.
(2012) Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J. Viral Hepat., 19, e48-57.
-
Huang,
C.Y.,
Lu,
C.W.,
Liu,
Y.L.,
Chiang,
C.H.,
Lee,
L.T. &
Huang,
K.C.
(2016) Relationship between chronic hepatitis B and metabolic syndrome: a structural equation modeling approach. Obesity (Silver Spring), 24, 483-489.
-
Inuzuka,
T.,
Ueda,
Y.,
Morimura,
H.,
Fujii,
Y.,
Umeda,
M.,
Kou,
T.,
Osaki,
Y.,
Uemoto,
S.,
Chiba,
T. &
Marusawa,
H.
(2014) Reactivation from occult HBV carrier status is characterized by low genetic heterogeneity with the wild-type or G1896A variant prevalence. J. Hepatol., 61, 492-501.
-
Itoh,
S. &
Shimoji,
K.
(1986) Effects of alcohol on the liver in HBsAg carriers. Am. J. Gastroenterol., 81, 779-782.
-
Jan,
C.F.,
Chen,
C.J.,
Chiu,
Y.H.,
Chen,
L.S.,
Wu,
H.M.,
Huang,
C.C.,
Yen,
M.F. &
Chen,
T.H.
(2006) A population-based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung Community-based Integrated Screening study No. 10). Int. J. Obes. (Lond), 30, 794-799.
-
Jarčuška,
P.,
Janičko,
M.,
Kružliak,
P.,
Novák,
M.,
Veselíny,
E.,
Fedačko,
J.,
Senajová,
G.,
Dražilová,
S.,
Madarasová-Gecková,
A.,
Mareková,
M.,
Pella,
D.,
Siegfried,
L.,
Kristián,
P. &
Kolesárová,
E.;
HepaMeta Study Group
(2014) Hepatitis B virus infection in patients with metabolic syndrome: a complicated relationship. Results of a population based study. Eur. J. Intern. Med., 25, 286-291.
-
Kwak,
M.S. &
Kim,
Y.J.
(2014) Occult hepatitis B virus infection. World J. Hepatol., 6, 860-869.
-
Li-Ng,
M.,
Tropp,
S.,
Danoff,
A. &
Bini,
E.J.
(2007) Association between chronic hepatitis B virus infection and diabetes among Asian Americans and Pacific Islanders. Dig. Liver Dis., 39, 549-556.
-
Liu,
P.T.,
Hwang,
A.C. &
Chen,
J.D.
(2013) Combined effects of hepatitis B virus infection and elevated alanine aminotransferase levels on dyslipidemia. Metabolism, 62, 220-225.
-
Martin,
C.M.,
Welge,
J.A.,
Shire,
N.J.,
Shata,
M.T.,
Sherman,
K.E. &
Blackard,
J.T.
(2009) Cytokine expression during chronic versus occult hepatitis B virus infection in HIV co-infected individuals. Cytokine, 47, 194-198.
-
Milias,
G.A.,
Panagiotakos,
D.B.,
Pitsavos,
C.,
Xenaki,
D.,
Panagopoulos,
G. &
Stefanadis,
C.
(2006) Prevalence of self-reported hypercholesterolaemia and its relation to dietary habits in Greek adults; a national nutrition & health survey. Lipids Health Dis., 5, 5.
-
Muldoon,
M.F.,
Marsland,
A.,
Flory,
J.D.,
Rabin,
B.S.,
Whiteside,
T.L. &
Manuck,
S.B.
(1997) Immune system differences in men with hypo- or hypercholesterolemia. Clin. Immunol. Immunopathol., 84, 145-149.
-
Pinarbasi,
B.,
Onel,
D.,
Cosan,
F.,
Akyuz,
F.,
Dirlik,
N.,
Cakaloglu,
Y.,
Badur,
S.,
Besisik,
F.,
Demir,
K.,
Okten,
A. &
Kaymakoglu,
S.
(2009) Prevalence and virological features of occult hepatitis B virus infection in female sex workers who work uncontrolled in Turkey. Liver Int., 29, 227-230.
-
Primatesta,
P. &
Poulter,
N.R.
(2006) Levels of dyslipidaemia and improvement in its management in England: results from the Health Survey for England 2003. Clin. Endocrinol. (Oxf), 64, 292-298.
-
Raimondo,
G.,
Allain,
J.P.,
Brunetto,
M.R.,
Buendia,
M.A.,
Chen,
D.S.,
Colombo,
M.,
Craxì,
A.,
Donato,
F.,
Ferrari,
C.,
Gaeta,
G.B.,
Gerlich,
W.H.,
Levrero,
M.,
Locarnini,
S.,
Michalak,
T.,
Mondelli,
M.U., et al.
(2008) Statements from the Taormina expert meeting on occult hepatitis B virus infection. J. Hepatol., 49, 652-657.
-
Raimondo,
G.,
Caccamo,
G.,
Filomia,
R. &
Pollicino,
T.
(2013) Occult HBV infection. Semin. Immunopathol., 35, 39-52.
-
Sa-Nguanmoo,
P.,
Tangkijvanich,
P.,
Tharmaphornpilas,
P.,
Rasdjarmrearnsook,
A.O.,
Plianpanich,
S.,
Thawornsuk,
N.,
Theamboonlers,
A. &
Poovorawan,
Y.
(2012) Molecular analysis of hepatitis B virus associated with vaccine failure in infants and mothers: a case-control study in Thailand. J. Med. Virol., 84, 1177-1185.
-
Smit,
J.W. &
Diamant,
M.
(2004) Genetically defined hyperlipidemia. Pharmacogenomics, 5, 295-304.
-
Wong,
V.W.,
Wong,
G.L.,
Chu,
W.C.,
Chim,
A.M.,
Ong,
A.,
Yeung,
D.K.,
Yiu,
K.K.,
Chu,
S.H.,
Chan,
H.Y.,
Woo,
J.,
Chan,
F.K. &
Chan,
H.L.
(2012) Hepatitis B virus infection and fatty liver in the general population. J. Hepatol., 56, 533-540.
-
Yang,
Z.,
Zhuang,
L.,
Lu,
Y.,
Xu,
Q.,
Tang,
B. &
Chen,
X.
(2016) Naturally occurring basal core promoter A1762T/G1764A dual mutations increase the risk of HBV-related hepatocellular carcinoma: a meta-analysis. Oncotarget, 7, 12525-12536.
-
Yimnoi,
P.,
Posuwan,
N.,
Wanlapakorn,
N.,
Tangkijvanich,
P.,
Theamboonlers,
A.,
Vongpunsawad,
S. &
Poovorawan,
Y.
(2016) A molecular epidemiological study of the hepatitis B virus in Thailand after 22 years of universal immunization. J. Med. Virol., 88, 664-673.
-
Zhang,
M.,
Lu,
Y.,
Liu,
X.,
Zhang,
X.,
Zhang,
C.,
Gao,
W. &
Tie,
Y.
(2016) Relationship between XspI site polymorphisms of LDL-R gene and serum IL-2 and IL-10 in patients with hypercholesterolemia. J. Clin. Lab. Anal., 30, 1122-1127.
-
Zheng,
X.,
Ye,
X.,
Zhang,
L.,
Wang,
W.,
Shuai,
L.,
Wang,
A.,
Zeng,
J.,
Candotti,
D.,
Allain,
J.P. &
Li,
C.
(2011) Characterization of occult hepatitis B virus infection from blood donors in China. J. Clin. Microbiol., 49, 1730-1737.