2017 Volume 243 Issue 2 Pages 95-100
2017 Volume 243 Issue 2 Pages 95-100
Polycystic ovary syndrome (PCOS) is a complex endocrine syndrome, resulting from the interaction of gene variants and environmental factors. PCOS is viewed as a proinflammatory state and is characterized by hyperandrogenism, hyperinsulinemia, and over-weight. In China, the incidence of PCOS is higher in the Uygur population than that in the Chinese Han population. The association of the tumor necrosis factor α (TNF-α) gene with PCOS remains to be clarified. Here, we investigated the association of TNF-α polymorphisms with PCOS in the Uygur population (393 patients with PCOS and 381 healthy subjects). Two single-nucleotide polymorphisms in TNF-α were analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method: rs1800629 (-308G/A polymorphism), a commonly tested variant and rs4645843 (6213C/T polymorphism) that causes a Pro-to-Leu substitution at position 84, the most damaging variant of TNF-α based on in silico analysis. We thus found that both the genotypic and allelic distributions of rs4645843 were significantly different between PCOS and control groups (p = 0.03 and 0.024, respectively), whereas those of rs1800629 were similar between the groups. Furthermore, rs4645843 was significantly associated with serum testosterone levels and the score of Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), but no such association was found with rs1800629. Importantly, both rs4645843 and rs1800629 were significantly associated with higher body mass index (p < 0.05). This is the first study that shows the association of TNF-α gene with PCOS in the Uygur population. The TNF-α gene may influence the pathogenesis of PCOS through regulating testosterone level, obesity and HOMA-IR.
Polycystic ovary syndrome (PCOS) is a common disease with various symptoms, which affects around 10% women of reproductive age worldwide (Franks 1995). In China, the incidence of PCOS is higher in the Uygur population than in the Chinese Han population (Palida and Tang 2011). The syndrome attracts the interests of many medical experts including gynecologists, endocrinologists, obstetrician, cardiologists and those who deal with reproductive medicine or metabolic disorders. The main clinical features of PCOS are hyperandrogenism, polycystic ovarian morphology, hyperinsulinemia, and over-weight (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004). Even through the etiology of PCOS has yet to be determined, it is believed to be hereditary in nature and caused by multiple factors, such as the lack of androgen’s hormonal balance, increased luteinizing hormone (LH), inflammation and oxidative stress (Ehrmann 2005; Szczuko et al. 2016). Thus, genetic factors interacting with biochemical, environmental, and immunological factors are implicated in the pathogenesis of this syndrome.
Tumor necrosis factor alpha (TNF-α) is an important multi-functional pro-inflammatory cytokine and is required for follicular development, ovulation and oocyte maturation (Gaafar et al. 2014; Cai et al. 2016a). Some studies have reported that the incidence of PCOS is closely related to chronic low-grade inflammation in human ovary, while TNF-α has found to exist in the follicular fluid of ovary and oocytes (Deepika et al. 2013; Kume et al. 2016). In addition, PCOS patients have been found to have higher TNF-α levels than healthy women (Deepika et al. 2013). The TNF-α gene has a size of approximately 3 kb and contains four exons, located on chromosome 6p21.3 within the spanning region of the major histocompatibility complex class III (MHC-III). The single nucleotide polymorphism (SNP) located in the promoter region of the TNF-α gene may influence its transcription. In fact, the -308G/A (rs1800629) polymorphism has been reported to be associated with elevated serum TNF-α concentrations (Kume et al. 2016). Various studies with small sample sizes have tested the association between PCOS and the rs1800629 polymorphism in TNF-α (Deepika et al. 2013; Kume et al. 2016). However, the results have been inconsistent. Moreover, a recent in silico analysis suggests that the SNP rs4645843, causing a Pro-to-Leu substitution at position 84 of TNF-α, has the greatest impact on the TNF-α protein structure stability (Dabhi and Mistry 2014). An association study of PCOS and rs4645843 of TNF-α has never been conducted.
Thus, to understand the effects of the TNF-α gene on the pathogenesis of PCOS, we used polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLP) method to genotype TNF-α polymorphisms, rs1800629 and rs4645843, in the Uygur ethnic patients with PCOS and healthy subjects. Furthermore, the relationship of the clinical feature parameters of PCOS patients and TNF-α polymorphisms was analyzed.
Totally 393 patients with PCOS were recruited from gynecology clinic of the first affiliated hospital of Xinjiang Medical University from October 2012 to March 2016. The diagnosis was based on the 2003 Rotterdam consensus criteria. All patients in the study had more than ten ovarian basal follicles and had a history of menstrual disorders, i.e., anovulatory infertility, amenorrhea/oligomenorrhea or frequent menstruation. Patients with other hyperandrogenism related diseases, such as hypothyroidism, androgen-secreting tumors, and congenital adrenal hyperplasia, were excluded from study. A total of 381 healthy women who had body examination at the same gynecology clinic during the same period were recruited as controls. Those healthy women had no hypertension or family history of disorders, such as diabetes. All participants were Uygur ethnic without thyroid abnormalities, cardiovascular system disorders, hepatic or renal dysfunction and other endocrine metabolic diseases. The medical ethical committee of the first affiliated hospital of Xinjiang medical university approved the study and all subjects participating in the project provided written informed consent.
Clinical testsA physical examination of every participant of this study was taken. Before anthropometric measurements were to be recorded, subjects refrained from exercise and fasted for at least 3 hours. Body weight was measured in minimal clothes and without shoes, and height was measured. Hip and wrist circumferences were measured according to standard procedures by using an anthropometric tape. Along with the subjects menstrual and fertility history, height, weight, waist and hip circumference were recorded. The waist-to-hip ratio (WHR) and body mass index (BMI) were calculated based on the following formula: WHR = Waist/Hip, and BMI = Weight/ (Height × Height).
About 5 mL of blood were extracted from the vein of each participant between 8:00 am-9:00 am after overnight fast during the 3rd-5th days of one menstrual cycle. After confirming no dominant follicle with the detection of B-ultrasound, about 5 mL of blood were also extracted from the amenorrhea women over the same period after overnight fast during any day of their menstrual cycle. The plasma estradiol (E2), total testosterone (T), follicle-stimulating hormone (FSH) and LH were measured by Axsym Automated Immunoassay Analyzer (Abbott, IL, USA). Plasma insulin was measured by an Insulin Human Direct enzyme-linked immunosorbent assay (ELISA) Kit (Invitrogen, UK), and glucose was detected by the hexokinase method. The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated based on the following formula: HOMA-IR = (fasting insulin × fasting glucose)/22.5.
Genotyping analysisA total of 2 ml anti-coagulated blood was drawn from each subject to extract genomic DNA using the previous methods (Cai et al. 2013; Jiang et al. 2016). The DNA was stored at −20℃ until it was used. The 25-μl PCR mixture contained 2.5 μg template DNA, 200 μmol/L for each deoxyribonucleotide triphosphate (dNTP), 100 pmol/L specific primer pairs (Table 1), 1.5 mmol/L magnesium chloride, and 2.5 U Taq DNA polymerase (Promega, WI). The PCR was performed under 94℃ for 5 min, 30 cycles of 95℃ for 1 min, 59.5℃ for 1 min and 72℃ for 1min, and then 72℃ for 10 min. The RFLP analysis was used to detect the known variants. For optimal RFLP analysis, NcoI endonuclease, which can recognize the sequence of 5′-CCATG|G-3′, was used for testing the rs1800629 polymorphism, and Ddel endonuclease, which can recognize the sequence of 5′-C|TNAG-3′, was used for testing the rs4645843 polymorphism (Thermo scientific, CA, USA). Each variant shown with underline and bold font is located at the cut site of an endonuclease, i.e the vertical line shown above. Based on the dbSNP (https://www.ncbi.nlm.nih.gov/projects/SNP/), the DNA sequence with a major allele, i.e., G allele for rs1800629, can be cut into two short fragments (20 and 119 base pairs) by the NcoI endonuclease, and the sequence with a minor allele, i.e., A allele for rs1800629 can keep the original length, while the DNA sequence with a minor allele, i.e., T allele for rs4645843, can be cut into two short fragments (29 and 154 base pairs) by the Ddel endonuclease, and the sequence without a cut is inferred to carry a major allele, i.e., C allele for rs4645843. The total endonuclease digestion reaction system (20 µL) included qualified PCR product (10 µL), 2-µL digestion buffer (10 ×), 1-µL endonuclease and 7-µL distilled water, and the mixture was incubated at 37°C for two-three hours. The digestion reaction products underwent 15% agarose gel electrophoresis. After electrophoresis, the gels were photographed under UV light after ethidium bromide (EB) staining. The results of the RFLP analysis were validated by the Sanger sequencing for all subjects.

PCR-RFLP primers for each detected SNP.
bp, base pair; Tm, annealing temperature.
A Mann-Whitney test or student T-test was used to detect the difference in the clinical characteristics of PCOS patients and healthy women. Hardy-Weinberg equilibrium (HWE) for each SNP in control subjects was evaluated using Fisher’s exact test. The genotypes of rs1800629 and rs4645843 in TNF-α were calculated by direct counting. Two allele frequencies were calculated using the following formula: P*A = (2N*AA + N*Aa)/2N, and P*a = (2N*aa + N*Aa)/2N.
•P* is the frequency of the allele (i.e., A or a); N* is sample number for each genotype; and N is the sample number.
A Chi-square test and Cochran-Armitage trend test were used to detect the difference of the genotypic and allelic distribution between PCOS patients and healthy women, and one way analysis of variance (ANOVA) and Kruskal-Wallis tests were performed to detect the differences among three groups (Cai et al. 2016b; Huang et al. 2016). The Power and Sample Size Program was used to calculate the power (Dupont and Plummer 1990). Odds ratios (OR), 95% confidence Interval (CI) and other statistical analyses were implemented using the R software version 3.1.2. The THESIAS program was used to perform the haplotype analysis (Tregouet et al. 2004; Cai et al. 2013). The global test for the association of the combined SNPs in TNF-α with PCOS was the (n−1) df test; here, n refers to the number of haplotypes.
The clinical characteristics of all 774 subjects are presented in Table 2. Among them, 393 PCOS patients and 381 healthy women had the age of 28.26 ± 4.63 years and 29 ± 5.11 years, respectively. There was no significant difference in age, menarche age, and basic estradiol (E2). However, the mean levels of BMI, WHR, basic FSH, basic LH, basic T, fasting plasma insulin, fasting glucose, and HOMA-IR were significantly decreased in healthy women than those in patients with PCOS.
The distributions of genotype/allele frequency in both PCOS patients and controls are shown in Table 3. The genotype frequencies for both rs1800629 and rs4645843 in TNF-α gene in the healthy control group showed no significant deviation from HWE. Both the genotypic and allelic frequency distributions of rs4645843 were significantly different between the PCOS patients and the controls (p = 0.03 and 0.024, respectively), while the genotypic and allelic frequency distributions of rs1800629 were similar between the groups (p = 0.14 and 0.13, respectively). Power analysis results demonstrated that the sample sizes of 393 patients and 381 controls in this study had about 77% power to detect associations in both the genotypic and allelic models under the minor allele with about 3% frequency, an OR of 2, and a threshold of significance at p = 0.05.
Haplotypes built up with these two SNPs with a frequency above 0.01 are listed in Table 4. Haplotype association analysis showed that the combination of these two variants was associated with PCOS (p-global = 0.003). With the GC haplotype as a reference, the GT haplotype had significant protective effects on PCOS (OR = 7.65, 95 % CI = 1.73-33.79, p = 0.007).
BMI and WHR are overweight indices suggested by World Health Organization (WHO). A criterion for obese is BMI ≥ 25 kg/m2 and WHR ≥ 0.8 is considered as obese in abdomen. In our study, nearly 53% of PCOS patients had BMI ≥ 25 kg/m2 and 18% had WHR < 0.80. Both rs1800629 and rs4645843 polymorphisms were significantly associated with higher BMI between patients and controls (p < 0.05). Only rs1800629 had a significant association with lower BMI (p = 0.022) and WHR (p = 0.042, Table 5).
Furthermore, we analyzed the association of these two TNF-α polymorphisms with clinical parameters in the PCOS patients (Fig. 1). The genotype of rs4645843 not rs1800629 was found to have significant different distribution of the levels of HOMA-IR and testosterone among the PCOS patients (p = 0.01 and 0.034, respectively). No further significant statistical differences for these two polymorphisms were found for the levels of FSH, LH and E2 (p > 0.05).

Clinical characteristics of patients with PCOS and healthy controls.
BMI, body mass index; WHR, waist/hip ratio; FSH, follicle-stimulating hormone; LH, luteinizing hormone; T, testosterone; E2, estradiol; HOMA-IR, homoeostasis model assessment.
aStudent’s t-test; bMann-Whtitney U test.

Distribution of TNF-α rs1800629 and rs4645843 polymorphisms in PCOS patients and controls.
*Armitage’s trend test which is used to compare three genotype frequencies between cases and controls.
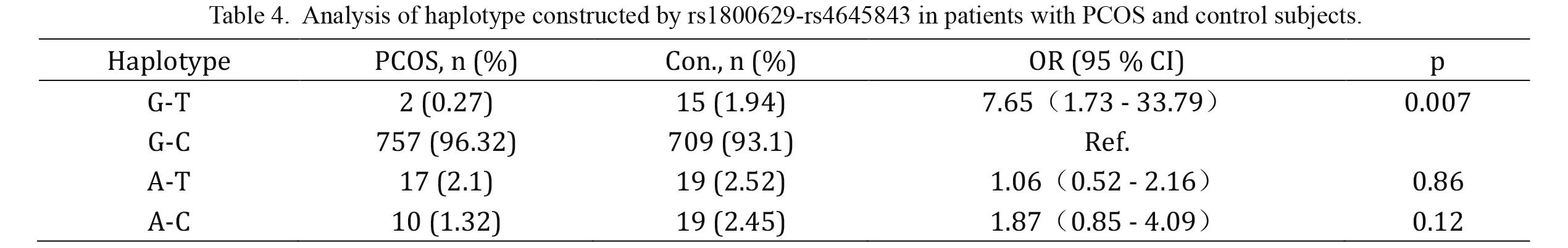
Analysis of haplotype constructed by rs1800629-rs4645843 in patients with PCOS and control subjects.
Con., control; Ref., reference; OR, odd ratio; CI, confidence interval.

Association of TNF-α rs1800629 and rs4645843 polymorphisms and obesity features.

Association of the TNF-α rs1800629 or rs4645843 and clinical parameters in PCOS patients.
The Kruskal-Wallis test was used to analyze the association of each parameter among three genotypes of rs1800629 or rs4645843. (A) FSH levels. p values for the FSH distribution among three genotypes are 0.074 (rs1800629) and 0.41(rs4645843). (B) LH levels. p values are 0.117 (rs1800629) and 0.077 (rs4645843). (C) Testosterone levels. p values are 0.091 (rs1800629) and 0.034 (rs4645843). (D) Estradiol (E2) levels. p values are 0.088 (rs1800629) and 0.052 (rs4645843). (E) HOMA-IR. p values are 0.064 (rs1800629) and 0.01 (rs4645843).
PCOS is a complex endocrine syndrome, resulting from the interaction of gene variants and environmental factors (Li et al. 2015). This disease is viewed as a proinflammatory state, and chronic low-grade inflammation was reported to promote the development of ovarian and metabolic dysfunction in PCOS. The analysis of the association of proinflammatory genes with PCOS may be helpful for better understanding of its etiology. TNF-α, an important pro-inflammatory cytokine, is present in follicular fluid of ovary and oocytes, and is involved in inflammation with dual roles (Cai et al. 2016a) and required for follicular development, ovulation and oocyte maturation (Deepika et al. 2016). There are several studies about association of the SNPs in TNF-α with PCOS, such as -1031 T>C (rs1799964) and rs1800629 in promoter region of TNF-α (Yun et al. 2011; Deepika et al. 2013, 2016). The SNP rs1799964 has been linked with altered promoter activity leading to varied plasma TNF-α levels in healthy subjects and repeatedly reported to be significantly associated with PCOS (Yun et al. 2011; Deepika et al. 2013, 2016). Unfortunately, the various association studies on rs1800629 have obtained conflicting results due to their relative small sample size (Deepika et al. 2013; Kume et al. 2016). Furthermore, rs4645843, giving rise to a Pro-to-Leu exchange at 84 position of TNF-α, has the greatest impact on the TNF-α protein structure stability based on the in silico analysis (Dabhi and Mistry 2014; Fang et al. 2016). However, this variant in TNF-α has never been investigated in the association study of PCOS.
The present study aims to investigate the genetic effects of TNF-α gene in the pathogenesis of PCOS within larger Uygur population samples. We thus found that the missense variant rs4645843, but not rs1800629, is significantly associated with PCOS. In addition, both rs1800629 and rs4645843 were significantly associated with BMI, which is consistent with the finding that TNF-α is linked to obesity (Li and Loos 2008; Bhagat et al. 2010). The rs1800629 has been demonstrated to have a significant effect on TNF-α transcriptional activity and regulate the expression level of TNF-α (Deepika et al. 2013; Kume et al. 2016). Increased TNF-α levels in women with PCOS have been verified by most studies (Deepika et al. 2016; Kume et al. 2016). Increased TNF-α can promote insulin resistance and cause hyperandrogenism. Obesity and insulin resistance are frequent events in hyperandrogenic women. In this study, rs4645843 is found to be significantly associated with the levels of HOMA-IR and testosterone among the PCOS patients. The HOMA score is a good indicator of insulin resistance, which is a crucial factor in the pathogenesis of PCOS. The effect of the TNF-α rs4645843 on PCOS may be related to its binding to other key molecules (Cai et al. 2011), resulting in a complex polygenic etiology of PCOS via its effects on testosterone levels. Usually, the high serum testosterone level may cause hyperandrogenism in women. Thus, current results support that TNF-α may play an important role in the pathogenesis of PCOS through regulating testosterone level, obesity and HOMA-IR in the Uygur population.
There are limitations in the present study. Only two SNPs in TNF-α were studied and a comprehensive analysis of other SNPs is not performed. Furthermore, serum TNF-α levels are not measured for the evaluation of clinical correlation in the Uygur population. Therefore, further extensive studies on the effects of TNF-α on PCOS involving a larger number of SNPs in different ethnic populations may help identify more potential variants as genetic markers of PCOS and also help the clinicians for treating the disorder based on the personal genetic background.
In conclusion, we suggest that the rs4645843 is involved in the predisposition to PCOS. Although TNF-α rs1800629 polymorphism is not associated with PCOS, it is significantly associated with obesity. Furthermore, the rs4645843 appears to affect PCOS via the significant association with obesity, testosterone and HOMA-IR in Uygur population. Further functional studies on this gene would be of help to gain more meaningful insights on the effects of TNF-α on PCOS phenotype.
Designed the experiments: X.H.W. Performed the experiments: L.Z, S.L. Analyzed the data: X.H.W. Wrote the paper: L.Z, S.L., X.H.W. All authors gave final approval for publication.
We would also like to say great thanks to all subjects participating in this study. The study was funded by departmental resources, First Affiliated Hospital of Xinjiang Medical University (201103).
The authors declare no conflict of interest.