2017 Volume 243 Issue 4 Pages 289-295
2017 Volume 243 Issue 4 Pages 289-295
Histological chorioamnionitis (CAM) is one form of intrauterine inflammation that is often seen in cases of preterm birth and are usually confirmed based on pathological examination after delivery. Histological CAM is related to significant neonatal morbidity and mortality; however, its etiology is unknown. The objective of this study was to determine the risk factors for histological CAM, using medical background, including fetal heart rate (FHR) patterns in preterm birth cases. The preterm birth cases delivered between 28 and 36 weeks were categorized into two groups according to the presence of histological CAM. Ninety-five preterm infants were included: 48 infants without histological CAM and 47 cases with histological CAM. The odds ratio for histological CAM was adjusted for FHR patterns, gestational age, and delivery mode (vaginal delivery or Caesarean section). Logistic regression analysis showed that vaginal delivery and gestational age were associated with histological CAM (odds Ratio [OR]: 3.1, 95% confidence interval [CI]: 1.0-9.4, p < 0.05, and OR: 0.8, 95% CI: 0.6-0.9, p < 0.05, respectively). However, there were no specific FHR patterns associated with histological CAM. Our study indicates that in preterm birth cases, histological CAM is not related to any specific FHR pattern. However, labor uterine contraction and immature gestational age at the delivery are related to histological CAM. These results may provide better delivery management methods for preterm birth cases.
Chorioamnionitis (CAM) is strongly related to preterm birth, leading to significant neonatal morbidity and mortality, including periventricular leukomalacia, bronchopulmonary dysplasia, pneumonia, and cerebral palsy (Wu and Colford 2000; Willoughby and Nelson 2002; Jobe 2003). It has been reported that histological CAM is one form of histological intrauterine inflammation that is usually confirmed based on pathological examination after delivery in cases of preterm birth. Even in mature neonates, there is an association between histological CAM and brain injury (Redline and O’Riordan 2000). It has also been reported that inflammation with intrapartum hypoxic-ischemic stress can easily lead to brain injury (Nelson and Grether 1998; Peebles and Wyatt 2002). Recently, a concern for intrauterine inflammation includes increases in neonatal neurological morbidity and whether this form of brain injury can be detected by fetal assessment or prevented by therapy such as mode of delivery (Holcroft et al. 2004).
Electronic fetal heart rate (FHR) monitoring has been clinically used since the 1960s and is now a standard management technique to assess fetal well-being during the intrapartum period (American College of Obstetricians and Gynecologists 2010) and provide the obstetric management techniques for the healthcare provided (Clark et al. 2013). For example, minimal or undetectable FHR variability in the presence of recurrent late decelerations or variable decelerations is a specific FHR pattern that is associated with the presence of fetal academia (Williams and Galerneau 2003; Parer et al. 2006); medical intervention is then required depending on the progress of labor (Clark et al. 2013). However, the relationship between a specific FHR pattern considering the patient’s clinical background and histological CAM is yet to be established.
Therefore, the purpose of the present study was to compare the FHR patterns and clinical backgrounds between cases with and without histological CAM at 28 to 36 weeks gestation in order to determine the risk factors for histological CAM and to provide the appropriate management for preterm delivery.
This was a multicenter case-control study conducted in two tertiary fetal medicine units in Fukushima Prefecture, Japan. Histological CAM was determined by histologic placenta and cord examination. The study population comprised pregnant women who delivered at 28-36 weeks gestation and underwent histological placenta and cord examination between January 1, 2011, and December 31, 2015.
We excluded cases with multiple pregnancies, fetal growth restriction, fetal and cord anomaly, obstetric complications including preeclampsia, abruption, and placenta previa, elective delivery due to maternal complications, intrauterine fetal death, Rhesus (Rh) isoimmunization, and insufficient data. The present study was approved by the institutional review board of Fukushima Medical University (No. 2218) and informed consent was waived for this study. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Maternal and neonate characteristicsInformation on maternal and neonatal outcomes was extracted from the medical records of each unit. Maternal background information included maternal age at delivery, parity, gestational age, height, weight before pregnancy, weight at delivery, whether labor was induced, and mode of delivery. Neonatal outcomes included birth weight, Z-score of birth weight, birth height, umbilical arterial pH, 1- and 5-minute Apgar scores, placenta weight, cord length, maximum cord diameter, and sex. Gestational age was determined in the early stages of pregnancy based on the last menstrual period and/or ultrasound examination. Induction of delivery refers to the use of an augmenting agent for labor induction. The indications of labor induction were membrane rupture or abnormality in labor progress. The modes of delivery were categorized as vaginal delivery and elective Caesarean section. Caesarean section was performed only for breech presentation of previous caesarean section. The Z-scores of birth weight were calculated using the ‘New Japanese neonatal anthropometric charts’ (Itabashi et al. 2014). Placenta weight, cord length, and maximum cord diameter were measured by a midwife soon after delivery.
In the two units, histologic examination of the placenta and cord in cases of preterm birth was routinely conducted to examine the severity of inflammation by pathologists based on Blanc’s classification (Blanc 1981). According to these examinations, the participants were divided into the following two groups. The histological CAM group consisted of patients with pathological intervillositis, chorionitis, chorioamnionitis, and funisitis; all other patients were categorized into the control group. Before multivariable logistic regression, maternal and neonatal background information was compared between the two groups.
FHR patternIn this analysis, we referred to the previous study that examined the correlation between FHR patterns 2 hours before delivery and the presence of cerebral palsy during pregnancy with intrauterine bacterial infection (Sameshima et al. 2005). The FHR patterns taken 1 to 2 hours before delivery were retrospectively evaluated by a single obstetric specialist certified by the Japan Society of Perinatal and Neonatal Medicine. The physician was blinded to neonatal outcomes and the results of histologic examination except for gestational age.
The FHR patterns were interpreted according to the National Institute of Child Health and Human Development guidelines (Macones et al. 2008). Tachycardia was defined as a baseline FHR > 160 beats/min. The baseline variability was calculated based on the amplitude of peak to trough in beats/minute and categorized as decreased variability when interpreted as absent (undetectable) or minimal (≤ 5 beats/minute). Before gestational week 32, FHR acceleration was defined as an increased baseline of ≥ 10 beats/minute with a duration of ≥ 10 seconds and < 2 minutes. At gestational week 32 and beyond, acceleration was defined as an increased baseline of ≥ 15 beats/minute with a duration of ≥ 15 seconds and < 2 minutes. In management during labor, acceleration was evaluated every 20 minutes. Reactive pattern was defined as any episodes of acceleration, and non-reactive pattern was defined as the absence of a reactive pattern. FHR deceleration patterns were classified into variable deceleration (VD), prolonged deceleration (PD), and late deceleration (LD). VD was further classified as mild, moderate, or severe (Mild: all variable decelerations that did not meet criteria for moderate or severe, Moderate: < 70 bpm nadir and 30-60 seconds duration or 70-80 bpm at nadir and > 60 seconds duration, Severe: > 60 seconds in duration and < 70 bpm at nadir) (Kubil et al. 1969). If multiple VD patterns were present, the most severe pattern was used for this classification. PD was defined as a visually apparent decrease to ≥ 15 beats/min from the baseline lasting for ≥ 2 minutes, but < 10 minutes. Recurrent LD was defined as LD lasting for more than 50% of the uterine contractions. All FHR parameters were treated as categorical variables.
Statistical methodsThe maternal background, neonatal outcomes, and FHR patterns between the two groups were compared. Then, multivariable logistic regression models were used to determine whether FHR patterns or maternal background were associated with histological CAM. The inclusion criteria of variables for this model were determined by clinical importance and univariate analysis. SPSS version 21 (IBM Corp., Armonk, NY, USA) was used for the statistical analyses. T-tests and Mann-Whitney U tests were conducted depending on whether the data were normally or non-normally distributed, respectively, and Chi-square tests were used to compare categorical variables. The level of statistical significance was set at p < 0.05.
During the study period, there were 4,350 pregnancy records, and 485 cases were delivered between 28 and 36 weeks at two tertiary fetal medicine units. Ninety-five infants were selected for this analysis according to the inclusion criteria (Fig. 1). Among the 485 cases delivered between 28 and 36 weeks of gestational age, 44 cases presented with fetal anomalies (9.1%). Of the selected 95 cases, 47 infants had histological CAM and 48 cases did not have histological CAM. In this study, there were no cases of emergency cesarean deliveries due to abnormal FHR patterns.

Study enrollment flowchart.
IUFD, intrauterine fetal death; Rh, Rhesus.
Fetal anomaly (44 cases) included 2 cases of chromosomal abnormality, 3 cases of spina bifida, 11 cases of congenital heart defect, 10 cases of gastrointestinal tract abnormality, 4 cases of hydrocephalus, and 14 cases of others.
There was a significant difference in weight before pregnancy (control, 52 ± 9 kg vs. histological CAM, 57 ± 13 kg, p < 0.05 (Table 1). No significant differences were observed regarding maternal age, ratio of primipara, gestational age, and weight at delivery (p = 0.34, p = 0.38, p = 0.14, and p = 0.07, respectively). Vaginal delivery was more common in the histological CAM group (p < 0.05).

Maternal backgrounds of the two groups.
There was no significant difference in birth weight between groups (p = 0.32), but the Z score of birth weight was higher in the histological CAM group (p < 0.05) (Table 2). No differences were seen in the umbilical arterial pH and the proportion of 5-minute Apgar Scores less than 7 (p = 0.30 and p = 0.96, respectively). Although cord length and placental weight were not significantly different (p = 0.38 and p = 0.09, respectively), cord diameter was higher in the histological CAM group (control, 1.2 ± 0.3 cm vs. histological CAM, 1.5 ± 0.5 cm, p < 0.05)
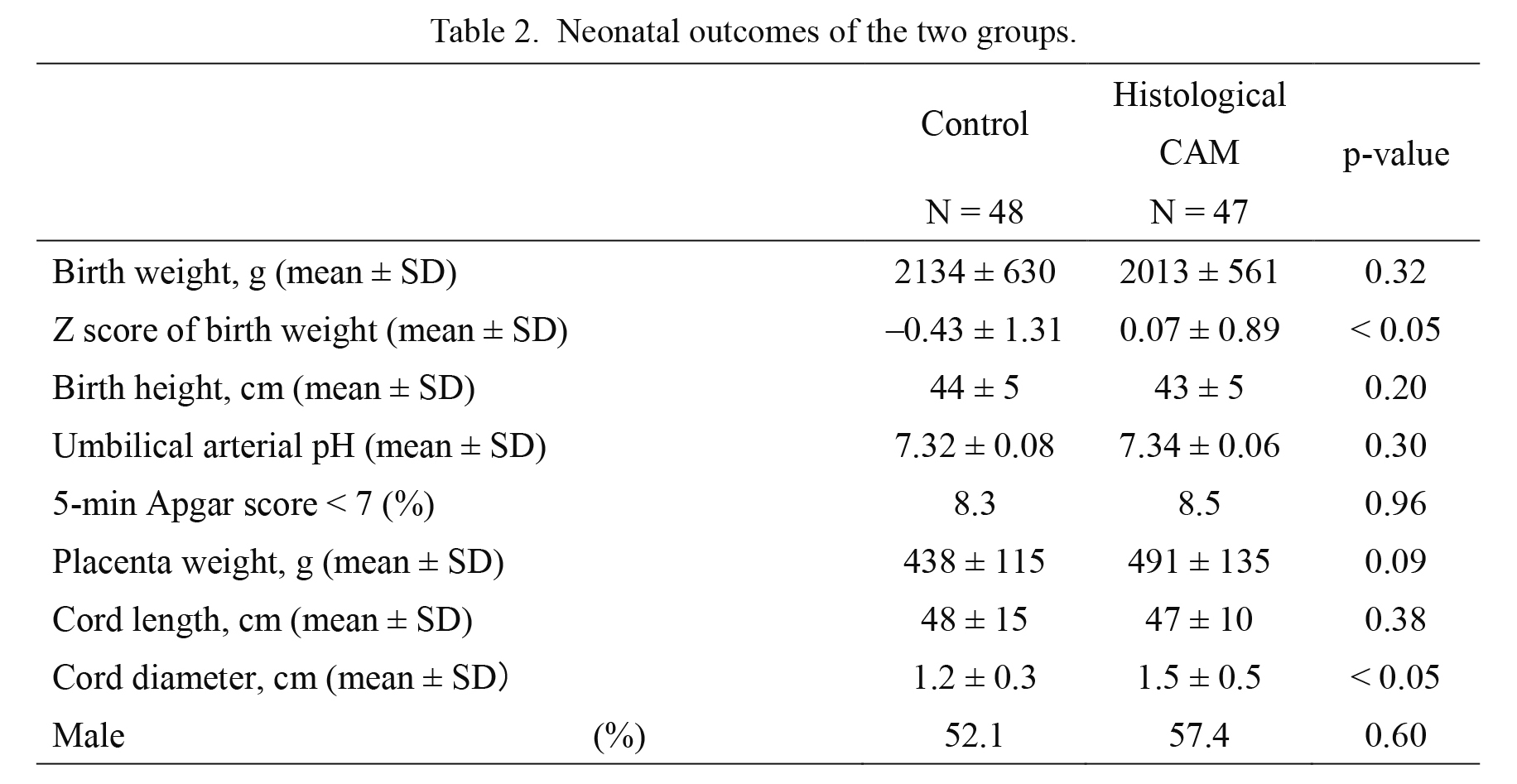
Neonatal outcomes of the two groups.
No significant differences were observed between the two groups in terms of proportion of tachycardia (control 6.3% vs. histological CAM 17.0%, p = 0.10), decreased variability (control 4.2% vs. histological CAM 4.3%, p = 0.98), or non-reactive FHR pattern (control 14.6% vs. histological CAM 10.6%, p = 0.56) (Table 3). Although mild VD was more common in the histological CAM group (control 41.7% vs. histological CAM 62.2%, p < 0.05), the presence of deceleration was not significantly different between the two groups (control 52.1% vs. histological CAM 68.1%, p = 0.11).

Differences in FHR patterns 1-2 hours before delivery in the two groups.
FHR, fetal heart rate; VD, variable deceleration; PD, prolonged deceleration; LD, late deceleration.
Multiple logistic regression analysis (Table 4) showed that vaginal delivery was significantly and independently associated with histological CAM (OR: 3.1, 95% CI: 1.0-9.4, p < 0.05). Conversely, recurrent LD and higher gestational age were significant independent factors associated with lower risk of histological CAM (OR: 0.1, 95% CI: 0.02-0.96, p < 0.05 and OR: 0.8, 95% CI: 0.6-0.9, p < 0.05, respectively). Mild VD was not significantly associated with histological CAM in multivariable analysis.
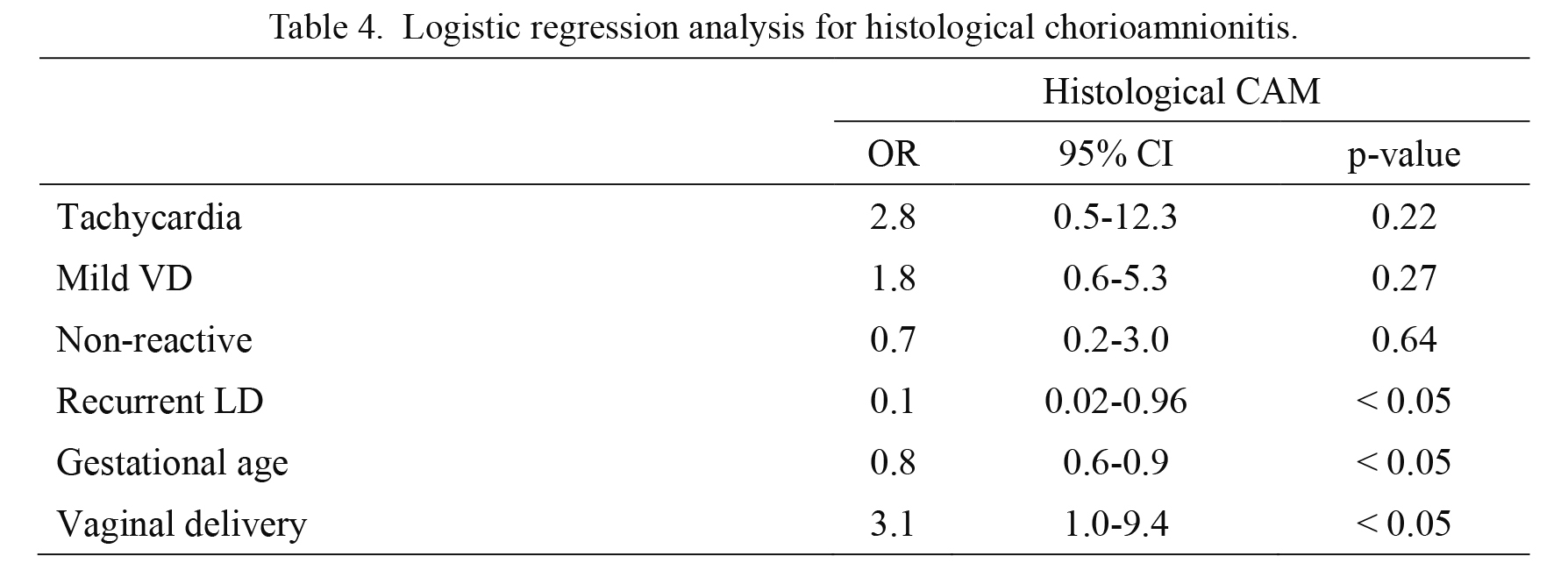
Logistic regression analysis for histological chorioamnionitis.
Outcome: histological chorioamnionitis.
Independent variables: Tachycardia (1, no; 2, yes), Mild VD (1, no; 2, yes), Non-reactive (1, reactive; 2, non-reactive), Recurrent LD (1, no; 2, yes), Gestational age, Vaginal delivery (1, Caesarian section; 2, Vaginal delivery)
OR, odds ratio; CI, confidence interval.
In this study, we found that, among women in preterm labor between 28 and 36 weeks of gestation, obstetric backgrounds such as gestational age and mode of delivery were associated with histological CAM. With regard to FHR pattern, contrary to our expectations, recurrent LD, which is a decrease in blood flow during uterine contraction that is beyond the capacity of the fetus to extract sufficient oxygen, (Murata et al. 1982), was independently associated with reduced risk of histological CAM.
The diagnostic definition of CAM has not been clearly determined, which leads to confusion among obstetricians and complicates clinical decision-making (Higgins et al. 2016). A diagnosis of CAM is usually determined by clinical symptoms before delivery (clinical CAM), evidence of microbial organisms in the amniotic fluid, and histopathologic findings after delivery (histological CAM). Methods of CAM diagnosis and management of preterm labor using FHR monitoring vary depending on the institution. Therefore, in the present study, we selected histological CAM cases treated in two tertiary fetal medicine units led by obstetricians with equivalent training, and using a standardized method of pathological examination of the placenta and cord. Our study also showed a higher prevalence of fetal anomalies (9.1%) than that in the entire Fukushima Prefecture (2.7%) during the same period (Fujimori et al. 2014). The difference in prevalence may be a result of the study design and selection bias from tertiary fetal medicine units.
In a previous study, Salafia et al. (1989) reported some abnormal FHR patterns were associated with histological CAM cases, but not related to the severity of inflammation. They also reported that sensitization of the umbilical vessel smooth muscle by surrounding inflammatory cytokines may cause uterine contractions or other minor trauma, as well as potentially more severe VD (Salafia et al. 1998). However, the present study did not show any association between histological CAM and abnormal FHR patterns indicative of a hypoxic state or acidemia. A previous study reported that FHR deceleration was not associated with intra-amniotic infection in patients who subsequently developed cerebral palsy (Sameshima et al. 2005). An observational study also reported that intrauterine histological CAM is not associated with fetal acidosis (Holcroft et al. 2004). Generally, antenatal stress such as inflammation leads to sensitization of the fetal brain, and the fetus exposed to intrauterine inflammation may be vulnerable to perinatal secondary insults such as hypoxic-ischemia, which themselves are not enough to induce significant brain damage (Fleiss et al. 2015). Thus, the conventional method of using the FHR pattern to determine the presence of a fetal hypoxic or acidosis state has not been shown to be useful for detecting intrauterine inflammation to reduce neurologic morbidity.
Our study finds that early gestational age and vaginal delivery are related to histological CAM. A previous study reported that CAM is seen in as many as 40-70% of preterm birth cases (Yoon et al. 2001), and lower gestational age was associated with a higher prevalence of histological CAM (Hillier et al. 1988). The latter results were similar to our own. With regard to mode of delivery and histological CAM, one previous study (involving sonohysterography with contrast media) reported that uterine contractions act as a peristaltic pump by which vaginal fluid can ascend into the uterine cavity (Zervomanolakis et al. 2007). It is, therefore, possible that the frequency of microbial invasion into the amniotic cavity and histological CAM could increase in pregnancies with vaginal deliveries (Seong et al. 2008). These factors associated with histological CAM by logistic regression indicate the possibilities of two different etiologies for preterm histological CAM. One scenario is that histological CAM has already established regardless of uterine contraction in earlier gestational age due to immaturity of infection defense mechanism, resulting in preterm labor. The other is that histological CAM is only one form of inflammation as a result of labor uterine contraction at the time of labor and delivery.
This study has some limitations. First, the FHR pattern of preterm birth cases of less than 28 weeks was not assessed in this study. Because previous studies reported that fetal maturity affects FHR patterns via the fetal autonomic nerve system (Schifferli and Caldeyro-Barcia 1973; Wakatsuki et al. 1992), we think that FHR patterns of early preterm birth cases of less than 28 weeks are difficult to compare with those of late near-term cases.
Second, the present study compared the FHR patterns recorded 1 to 2 hours before delivery, and therefore is a cross-sectional rather than a longitudinal study. Additionally, the current study included vaginal delivery cases, and did not consider the longitudinal effect of uterine contractions on the FHR pattern. Third, all evaluation items of the FHR pattern were categorical variables. The absence or decrease of variability is thought to represent a state of fetal acidosis (Siira et al. 2013), and we focused on decreased variability as reported in other studies (Salafia et al. 1998; Sameshima et al. 2005). However, in animal experiments of preterm fetal sheep, fetal inflammation induced by lipopolysaccharides (LPS) was associated with a transient increase in FHR variability and loss of FHR variability by repeated exposure to LPS (Lear et al. 2014), so rigorous FHR evaluation including increased variability might be required in future studies focused on the evaluation of intrauterine infection. Furthermore, in certain cases, variability is not determined quantitatively but judged subjectively based on the recorded data, and, thus, quantitative analysis in observational study might be also required.
In the current study, it is difficult to detect the histological CAM based on specific FHR pattern prior to delivery. However, labor uterine contraction and earlier gestational age at delivery were related to the presence of histological CAM. These findings suggest that histological CAM is not only the cause of preterm birth, but also the result of labor uterine contraction in preterm birth. In order to reduce the incidence of histological CAM, the mode of delivery of women in near-term labor needs to be taken into consideration.
In summary, we demonstrated that vaginal delivery and younger gestational age at delivery are associated with increased risk of histological CAM in preterm birth cases. Since the study of human FHR patterns is restricted to retrospective and cross-sectional research, further prospective and longitudinal studies are needed to evaluate the association between FHR patterns and intra-amniotic infection in animal models.
H. Kyozuka: Data analysis, Manuscript writing, S. Yasuda: Data analysis, T. Hiraiwa: Data collection, M. Ishibshi: Data management, K. Kato: Data collection, K. Fujimori: Project development.
The authors declare no conflict of interest.