2018 Volume 244 Issue 2 Pages 145-149
2018 Volume 244 Issue 2 Pages 145-149
Leukemoid reaction (LR) is a reactive disease that exhibits abnormal blood values similar to leukemia, but not due to leukemia. One report showed that neonatal LR (NLR) was associated with elevated serum granulocyte colony stimulating factor (G-CSF) in only 30% of the study neonates. NLR is not always associated with the elevation of serum G-CSF. NLR was defined as a white blood cell count of ≥ 40 × 103/μL and/or blast cell concentration of > 2%. We have focused on NLR with fetal inflammatory response syndrome (FIRS), defined as a fetal systemic inflammatory reaction triggered by intrauterine infection. FIRS was diagnosed based on a cord serum interleukin-6 (IL-6) concentration ≥ 17.5 pg/mL and histopathological chorioamnionitis. Because NLR is highly associated with FIRS, we have hypothesized that NLR is associated with the elevation of both G-CSF and IL-6. This is the first report to measure multiple cytokines in NLR at the same time. The study comprised 19 preterm infants with FIRS: 8 with NLR (study group) and 11 without NLR (control group). Serum G-CSF and IL-6 concentrations were significantly higher in the study group than the control group. There was a positive correlation between G-CSF and IL-6 levels in the study group but not in the control group. These results suggest that elevated serum G-CSF and IL-6 may underlie NLR. Thus, G-CSF and IL-6 concentrations may be predictive of the onset of NLR. Measuring these cytokines is useful for judging the prognosis of preterm infants and for their post-natal clinical management.
The concept of fetal inflammatory response syndrome (FIRS) advocated by Gomez et al. (1998) has become widely accepted today. When an intrauterine infection occurs and the fetus is exposed to amniotic fluid contaminated by bacteria, microorganisms, or inflammatory cells that have migrated from the uteroplacental circulation, the fetus reacts with a systemic inflammatory response. Thus, systemic inflammatory response syndrome (SIRS) has been proposed, and concepts that involve cytokines are considered equally in the uterus. In this condition, a series of reactions mainly driven by the fetus is called FIRS. As a result of intrauterine infection, FIRS is a pathology in which the fetus responds with its own systemic inflammatory response. The importance of FIRS has drawn attention today through investigations of the relationship between interleukin-6 (IL-6) concentrations in amniotic fluid and serum by cordocentesis and the prognosis of the neonates. Romero and coworkers (2011) reported that about two-thirds of the preterm infants with FIRS develop leukocytosis and that granulocyte colony stimulating factor (G-CSF) is involved in the leukocytosis (Chaiworapongsa et al. 2011). We investigated characteristic neonatal leukemoid reactions (NLR) in infants with hyperleukocytosis and reported that they complicated FIRS, and the infants developed a high rate of chronic lung disease (Nakamura et al. 2002). It was also reported that G-CSF is involved in the mechanism of NLR onset and that NLR was associated with elevated serum G-CSF in only 30% of the study neonates (Calhoun et al. 1996). Although leukocytosis may develop with the involvement of G-CSF alone, an excessive increase in leukocytes does not always lead to LR. In the current study, we examined the possibility that in addition to G-CSF, IL-6 is also involved in the development of NLR.
This retrospective cross-sectional study included infants who were admitted between January 2003 and December 2011 to the National Organization Hospital Nishi-Saitama Chuo National Hospital and between January 2012 and December 2016 to the Japanese Red Cross Musashino Hospital. The inclusion criteria for this study were (1) a premature baby who was hospitalized at the relevant NICU and who had undergone blood sampling on a daily examination, (2) inclusion of the infant in this study was approved in writing by the parents, and (3) serum separated from the blood specimens was stored frozen and was available for cytokine measurement.
Clinical definitionComplete blood cell counts were routinely obtained on the 1st day after birth and were repeated once/day until the white blood cell (WBC) count peaked. The WBC count was corrected according to the count of nucleated erythrocytes. NLR was defined as a WBC count of ≥ 40 × 103/µL (Zanardo et al. 2006) and/or the presence of a > 2% concentration of blast cells in the peripheral blood (Hill and Duncan 1941).
Infants with FIRS who developed features of NLR formed the study group, and the remainder without NLR, matched for gestational age, formed the control group. Newborns with congenital and/or chromosomal abnormalities were excluded from the study.
Because our hospitals do not perform cordocentesis, FIRS was defined based on a cord serum IL-6 concentration at birth of ≥ 17.5 pg/mL (Yoon et al. 2000) and the presence of histopathological chorioamnionitis (Pacora et al. 2002).
Clinical assaysSerum was separated by centrifugation and stored in aliquots at –80°C until analysis. Many of these samples were used in previous studies (Nakamura et al. 2007). Serum IL-6 and G-CSF concentrations were determined with either commercially available enzyme-linked immunoassays (ELISA) (R&D Systems, Inc., Minneapolis, MN, USA) or the cytometric bead array method, which combines the principles of the sandwich-based immunoassay with cytometry using the BioPlex protein array (Bio-Rad, Hercules, CA, USA) and Luminex 100 (Mirai Bio, Alameda, CA, USA), as previously described (Takahashi et al. 2009). Assay time constraints and the relatively large volume of neonatal samples required to measure multiple cytokines made the ELISA technique expensive. Therefore, we switched to the faster and more inexpensive and sensitive cytometric bead array method during the study period. The average inter-assay percent coefficient of variation was ≤ 10% (G-CSF: 9.5%, IL-6: 6.0%). Therefore, we considered the data measured by the two methods to be satisfactory when examining them collectively.
Statistical analysisResults are presented as mean ± standard deviation (SD). Groups were compared using the Student t-test for normally distributed data and the Mann-Whitney U-test for non-normally distributed data. Correlations between G-CSF and other continuous variables were determined using the Spearman’s rank correlation test. Proportions were compared by contingency tables or Fisher’s exact test. A p value of < 0.05 was considered statistically significant. StatMate IV (Tokyo, Japan) statistical software was used for the statistical analysis.
Ethical approvalThis study was approved by the ethics committees of the Japanese Red Cross Musashino Hospital, Nishisaitama-Chuo National Hospital, and University of Tokyo Hospital. Parents of the infants were informed of the study design, and their written informed consent was obtained.
The study comprised 19 preterm infants with FIRS: 8 infants with NLR (study group) and 11 without NLR (control group). The mean gestational ages and birth weights of the study and control groups were 30.6 ± 3.5 weeks and 1,641 ± 620 g and 31.2 ± 2.2 weeks and 1,479 ± 625 g, respectively. There was no significant difference in these values between the study and control groups (p = 0.673 and 0.582, respectively). As well, no statistically significant differences were observed in sex, fetal growth retardation, premature membrane rupture, delivery mode, antenatal steroids, maternal depression, including therapeutic drugs such as selective serotonin re-uptake inhibitors (SSRIs), histological chorioamnionitis, and funisitis between the study group and the control group (Table 1).
Both the median cord serum concentration of G-CSF and the median concentration of IL-6 were higher in the study group than the control group (both, p < 0.05) (Fig. 1). The cord serum concentration of G-CSF in the study group significantly correlated with that of IL-6 (r = 0.999, [G-CSF] = 2.974 × [IL-6] − 725.867, p < 0.001), but there was no relationship between these two cytokines in the control group (p = 0.4167) (Fig. 2). There was also no relationship between the cord serum concentration of G-CSF and initial and maximum WBC counts in either the study or control group. The WBC counts of the study group normalized within two weeks.

Maternal and fetal variables.
LR, leukemoid reaction; SSRIs, selective serotonin re-uptake inhibitors.
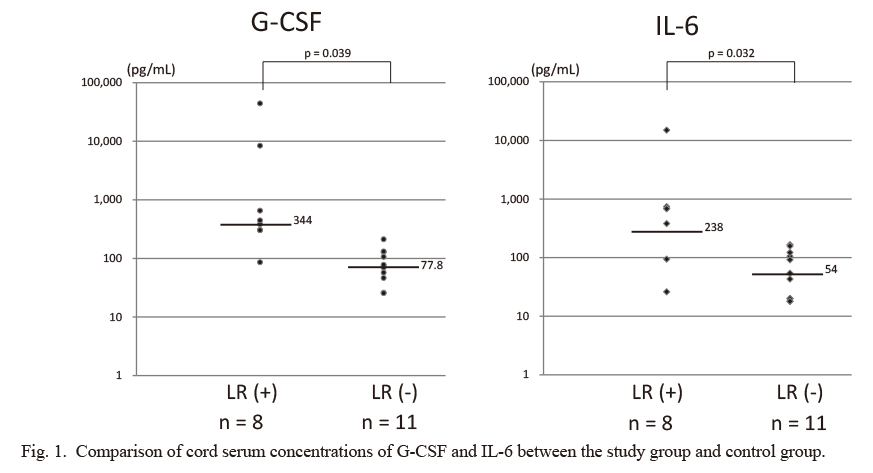
Comparison of cord serum concentrations of G-CSF and IL-6 between the study group and control group.
The median G-CSF concentration was higher in the study group (n = 8) than in the control group (n = 11): median 344 pg/mL, interquartile range [IQR] 302.6-650 pg/mL vs. median 77.8 pg/mL, IQR 63.5-129.7 pg/mL; p < 0.05. The median IL-6 concentration was also higher in the study group than in the control group: median 238 pg/mL, IQR 74-681 pg/mL vs. median 54 pg/mL, IQR 20-113 pg/mL; p < 0.05. The y-axis is depicted in log scale.

Relation between cord serum concentrations of G-CSF and IL-6.
(1) The concentrations of G-CSF in the study group (n = 8) correlated significantly with those of IL-6 (r = 0.999, [G-CSF] = 2.974 × [IL-6] – 725.867; p < 0.001). (2) In contrast, there was no significant relation between these two cytokines in the control group (n = 11) (r = 0.272; p = 0.4167). Both x and y axes are depicted in log scale.
Gomez et al. (1998) discovered that fetal umbilical cord serum IL-6 concentration is an independent risk factor for the occurrence of severe neonatal complications, and they initially defined FIRS as a cord serum IL-6 concentration > 11 pg/mL. However, in addition to intrauterine infection, an increase in IL-6 occurring during the fetal period can also be due to intrauterine growth retardation (Amarilyo et al. 2011; Lausten-Thomsen et al. 2014) and maternal depression (although there are reports that maternal administration of SSRIs decreases the IL-6 level) (Latendresse et al. 2013). Therefore, in the present study, the required and sufficient conditions for a diagnosis of FIRS were both an increase in the level of cord serum IL-6 and the presence of histological chorioamnionitis.
We already reported the frequent occurrence of NLR in premature infants following intrauterine inflammation and their high rates of bronchopulmonary dysplasia (Nakamura et al. 2002). Hsiao and Omar (2005) reported the association of NLR with the prolonged need for ventilatory and oxygen support, a higher incidence of bronchopulmonary dysplasia, and a tendency for lower mortality. We also previously measured concentrations of cord serum IL-6 and G-CSF at the same time in infants who developed NLR and reported that the values of these two cytokines were high and, in some infants, might be related to a mechanism causing NLR (Nakamura et al. 2007). Therefore, in the present study, we increased the number of FIRS-associated cases and examined the association of these two cytokines with the presence or absence of NLR. Interestingly, there was a very high positive correlation between IL-6 and G-CSF in the infants in the study group (r = 0.999), whereas there was no such correlation in those in the control group.
Four mechanisms control the increase in the peripheral blood leukocyte count: (1) stimulation and promotion of leukocyte production in the bone marrow, (2) mobilization from the bone marrow storage pool, (3) change from the marginal pool to the circulation pool, and (4) suppression of leukocyte apoptosis. G-CSF is mainly responsible for (1), (2), and (4) as mechanisms causing the increase in leukocyte number, whereas IL-6 mainly has effects on (2) and (3). Thus, some of the leukocytotic actions of IL-6 and G-CSF are independent of each other, and when there is a positive correlation between the two, we suggest that NLR may develop.
Corticosteroids have effects on (2) and (4), and several reports of corticosteroid-induced NLR in premature infants have been published (Anday and Harris 1982; Cohen et al. 1993). We investigated the relationship between the administration of maternal corticosteroids and the occurrence of NLR in infants and found no causal relationship (Nakamura et al. 2002). Once again, the data in the present study did not show any association between NLR and the use of antenatal steroids.
In case of adults, the synergistic effect of IL-6 and G-CSF was also reported to cause the transient leukocytosis that occurs during an exercise burden (Yamada et al. 1985), and this effect was later confirmed in experimental pulmonary injury models by Yokota et al. (2008).
Although G-CSF may be the primary factor behind the increase in the WBC count, it is extremely rare for NLR to develop based on the concentration of G-CSF alone. Furthermore, although the concentration of cord serum cytokines reflects the condition of the infant immediately before birth, the time from birth to the onset of FIRS varies among individuals. However, there is a high possibility that these two cytokines are related not only to leukocytosis but are also the main factors causing NLR. The measurement of cord serum concentrations of G-CSF and IL-6 may predict the occurrence of NLR. Furthermore, these values may be useful in judging the prognosis of preterm infants and in their clinical management after birth. We plan to accumulate additional cases and perform additional studies to elucidate the pathology of NLR.
The authors declare no conflict of interest.