2018 Volume 244 Issue 3 Pages 187-193
2018 Volume 244 Issue 3 Pages 187-193
The cytokine interleukin-21 (IL-21) is mainly produced from activated CD4+ T cells and natural killer T (NKT) cells. IL-21 enhances the proliferation and differentiation of T cells and B cells and also increases cytotoxicity of CD8+ T cells and NK cells through the IL-21 receptor and its downstream signaling molecules such as signal transducers and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase 1/2 (ERK1/2). SH2 domain-containing tyrosine phosphatase (SHP-2) is ubiquitously expressed, including hematopoietic cells. SHP-2 has been implicated in the regulation of IL-6 and IL-3 signaling, but its function in IL-21 signaling has not been investigated. Therefore, we studied the role of SHP-2 in IL-21 signaling by SHP-2 overexpression and knockdown experiments. For the SHP-2 overexpression, we used 293T human embryonic kidney cells, in which the IL-21 receptor system were easily reconstituted and high amounts of exogenous SHP-2 were expressed by vector transfection. In 293T cells, overexpressed SHP-2 caused the increase in the degree of the IL-21-induced ERK1/2 activation. Subsequently, SHP-2 knockdown experiments were performed in the mouse pro-B cell line, BAF21RWT-1, which constitutively expresses human IL-21 receptor and proliferates in an IL-21-dependent manner. SHP-2 knockdown reduced the degree of the IL-21-induced ERK1/2 activation and suppressed cell proliferation. These results suggest that SHP-2 may augment the ERK1/2 activity and cell proliferation activity in IL-21 signaling. We propose that SHP-2 is involved in the IL-21-mediated ERK1/2 activation and cell proliferation.
Interleukin-21 (IL-21) is a member of the common γ-chain receptor-sharing cytokine family (Asao et al. 2001; Habib et al. 2002). IL-21 is produced mainly from activated CD4+ T cells, including Th17 cells and follicular helper T cells, and NKT cells (Parrish-Novak et al. 2000). IL-21 profoundly regulates the growth, differentiation, and functional activation of T cells, B cells, NK cells, dendritic cells (DCs), and other immune cells (Habib et al. 2003; Coquet et al. 2007). IL-21 signals through its specific receptor, IL-21R and the common γ-chain subunit, which is shared by the IL-2, IL-4, IL-7, IL-9, and IL-15 receptor complexes (Kovanen and Leonard 2004). IL-21 activates Janus kinase (JAK) 1 and JAK3, STAT1 and STAT3, and to a lesser degree, STAT4, STAT5, and STAT6. Like other cytokines, IL-21 activates ERK1/2 and the phosphatidylinositol 3 (PI3) kinase-Akt pathway (Ozaki et al. 2000; Asao et al. 2001; Zeng et al. 2007).
Tyrosine-protein phosphatase non-receptor type 11 (PTPN11), also known as SHP-2, a protein tyrosine phosphatase (PTP) family member, contains a PTP domain and two Src homology 2 (SH2) domains, and elicits important biological functions in response to various growth factors, hormones, or cytokines (Neel et al. 2003). This phosphatase is ubiquitously expressed and also highly expressed in hematopoietic cells. SHP-2 is implicated in the negative regulation of IL-6 signaling (Atsumi et al. 2002), whereas SHP-2 was reported to play a positive role in IL-3-induced activation of the ERK pathway (Yu et al. 2006) and to bind Grb2-associated binding protein1 (Gab1) and positively regulate ERK1/2 and ERK5 after hepatocyte growth factor (HGF) stimulation in endothelial cells (Maroun et al. 2000; Shioyama et al. 2011). However, the function of SHP-2 in IL-21 signaling is not studied.
Here we examined the functional role of SHP-2 in IL-21-induced signaling, in particular through the ERK1/2 pathway.
Ba/F3 is an IL-3-dependent murine pro-B cell line (Palacios and Steinmetz 1985). A Ba/F3 subline, BAF21RWT-1, which expresses human IL-21 receptor, proliferates in an IL-21-dependent manner (Rahman et al. 2007). Ba/F3 sublines, including BAF21RWT-1, B21RSHP2KD-8, and B21RSHP2KD-12, were maintained in RPMI1640 medium supplemented with 10% heat-inactivated FBS, 50 μM 2-mercaptoethanol, 15% conditioned medium from WEHI3 mouse myelomonocytic leukemia cells as a source of IL-3, penicillin, and streptomycin. For transient transfection experiments, we used a human embryonic kidney cell line, 293T, which was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. All cells were cultured at 37˚C in a humidified incubator with 5% CO2.
Cytokines and antibodiesHuman recombinant IL-21 was purchased from PeproTech EC. The antibodies used were anti-phospho-ERK1/2 (Thr 202/Tyr 204), anti-ERK1/2, anti-phospho-Raf (Ser 259), anti-phospho-SHP-2 (Tyr 542), anti-phospho-STAT3 (Tyr 705), anti-phospho-JAK1 (Tyr 1022/1023), and anti-phospho-JAK3 (Tyr 980/981) (Cell Signaling), anti-SHP-2 (C18), anti-Raf-1 (E10), anti-JAK1 (HR785), and anti-STAT3 (C20) (Santa Cruz), and anti-JAK3 (Millipore), and anti-α-tubulin (B5-1-2) (Sigma).
Expression plasmids and transfectionThe human SHP-2 expression vector was a kind gift from Dr. N. Tanaka. Expression vectors for IL-21R, the common γ subunit, and JAK3 were described previously (Nara et al. 2010). Expression vectors were transferred into 293T cells by the calcium phosphate method, as described previously (Jordan and Wurn 2004).
Western blotting analysisTo analyze whole-cell proteins, cells were harvested and extracted with whole-cell extraction buffers as described elsewhere (Asao and Fu 2000). Whole-cell extracts (10 μg) were separated by SDS-PAGE and transferred to Immobilon-PTM polyvinylidene difluoride membranes (Millipore). Some membranes were soaked in 4% paraformaldehyde, 0.01% glutaraldehyde in PBS for 30 min at room temperature before the next step to enhance the immunoblot signals (Sasaki et al. 2015). The membranes were incubated in blocking buffer (5% nonfat milk in Tris-based saline containing 0.05% Tween20) and then with the indicated antibodies followed by horseradish peroxidase-coupled anti-rabbit IgG (Cell Signaling). The density of the bands in the immunoblot data was measured with ImageJ 1.48v software.
SHP-2 Knockdown using siRNA and shRNAA human SHP-2 siRNA and a mouse SHP-2-shRNA-containing plasmid, pLKO.1-puro-PTPN11 shRNA (NM_011202.3-1296s21c1), were purchased from Sigma-Aldrich. The SHP-2 siRNA was introduced into 293T cells by the calcium phosphate method. The ScaI-linearized pLKO.1-puro-PTPN11 shRNA plasmid was transferred into BAF21RWT-1 cells by electroporation as described previously (Asao and Fu 2000). Twenty-four hours later, 1 μg/ml puromycin was added to the cultures to select for puromycin-resistant clones (B21RSHP2KD clones).
Cell proliferation assayTo assess cell proliferation, a thymidine incorporation assay was performed as described previously (Asao and Fu 2000). Briefly, 1 × 105 cells/well were applied to 96-well plates in pentaplicate, methyl-[3H] thymidine was added at 1.0 μCi/well, and the cells were cultured for 24 or 48 hours. The incorporated [3H] thymidine was counted with a Tri-Carb 2910TR liquid scintillation counter (PerkinElmer).
Statistical analysisResult of the thymidine incorporation assay is presented as means ± standard deviation. The significance of differences among the groups was determined using one-way ANOVA with Bonferroni’s post-hoc test.
SHP-2 plays an important role in diverse signaling pathways, including those induced by several cytokines, growth factors, interferons, and insulin. Many cytokines induce the phosphorylation of SHP-2 on tyrosine 542 and tyrosine 580, which activates its phosphatase activity and induces downstream signaling. Therefore, to investigate whether SHP-2 plays a role in IL-21 signaling, we examined SHP-2’s phosphorylation. We first used an IL-21R signaling system reconstituted in 293T cells. However, IL-21 hardly induced SHP-2’s phosphorylation in these cells (Fig. 1A, upper panel, left half). We therefore overexpressed SHP-2 in addition to expressing the IL-21R signaling system in 293T cells, and found that IL-21 clearly upregulated SHP-2’s phosphorylation on tyrosine 542 in these cells (Fig. 1A, upper panel, right half). This finding suggested that SHP-2 is involved in the IL-21-induced signaling mechanism. Therefore, we next examined the ERK1/2 activation in the presence or absence of SHP-2 overexpression. While IL-21 slightly induced ERK1/2’s phosphorylation without SHP-2 overexpression, the IL-21-induced ERK1/2 phosphorylation was markedly upregulated in the SHP-2-overexpressing cells in a transfected IL-21R dose-dependent manner (Fig. 1B, upper panel). These data indicated that SHP-2 might positively control the ERK activity in IL-21 signaling.

Overexpression of the tyrosine phosphatase SHP-2 upregulates ERK activity.
(A) 293T cells were transfected with IL-21R, the common γ subunit, and JAK3 with or without an SHP-2 expression vector. The cells were stimulated with 1 nM hIL-21 for the indicated periods and then extracted with whole-cell extraction buffer. The cell extracts were analyzed by immunoblotting with an anti-phospho-SHP-2 antibody (Y542) (upper panel), and then the filter was re-probed with an anti-SHP-2 antibody (middle panel) or anti-α-tubulin antibody (lower panel). The position of the endogenous SHP-2 is indicated by an asterisk. (B) 293T cells were transfected with IL-21R, the common γ subunit, and JAK3 with or without an SHP-2 expression vector. The amount of IL-21R expression vector used for each transfection is indicated. The cells were stimulated with 1 nM hIL-21 and then extracted with whole-cell extraction buffer. The cell extracts were analyzed by immunoblotting with an anti-phospho-ERK1/2 antibody (upper panel), and then the filter was re-probed with an anti-ERK1/2 antibody (lower panel). The phospho-SHP-2 or phospho-ERK1/2 band intensity was divided by the internal control band intensity, α-tubulin (A) or ERK1/2 (B), respectively, and then indicated as relative changes. These experiments were repeated at least three times and the representative results are shown.
To confirm the function of SHP-2 in IL-21 signaling, we used SHP-2-siRNA knockdown to reduce the SHP-2 protein level in 293T cells. In these cells, 40 nM SHP-2-siRNA was sufficient to decrease the SHP-2 protein expression (Fig. 2A). In these SHP-2-knockdown cells, the IL-21-induced ERK1/2 activation was significantly hampered (Fig. 2B). To confirm these results, we used a different cell line, BAF21RWT-1, which is an IL-21- or IL-3-dependent mouse pro-B cell line that we previously established (Rahman et al. 2007). We introduced a SHP-2 shRNA expression vector into the BAF21RWT-1 cells, established some stable cell clones (named B21RSHP2KD), and examined the SHP-2 expression level of each clone by immunoblotting (Fig. 2C). We then examined the IL-21-induced ERK1/2 and Raf activation using B21RSHP2KD-8, the clone that exhibited the lowest SHP-2 expression (Fig. 2D). The results clearly showed that SHP-2 knockdown diminished the activation of ERK1/2 and its upstream signaling molecule Raf. Thus, SHP-2 may regulate IL-21 signaling at a point upstream of the Raf-ERK pathway.

SHP-2 knockdown reduces the IL-21-induced phosphorylation of ERK1/2 and Raf.
(A) SHP-2 was knocked down with SHP-2-siRNA in 293T cells. Whole-cell extracts were analyzed by immunoblotting with an anti-SHP-2 antibody. The amount of siRNA used for each transfection is indicated. (B) The IL-21R system was reconstituted in 293T cells as described in the legend for Figure 1 with or without SHP-2 siRNA. The cells were stimulated with 1 nM IL-21 for the indicated periods and then extracted with whole-cell extraction buffer. The whole-cell extracts were analyzed by immunoblotting with an anti-phospho-ERK1/2 antibody (upper panel). The filter was re-probed with an anti-ERK1/2 antibody (lower panel). (C) A SHP-2 shRNA expression vector was transferred into BAF21RWT-1 cells, and B21RSHP2KD clones were established. Whole-cell extracts of each clone were analyzed by immunoblotting with an anti-SHP-2 antibody. (D) BAF21RWT-1 and B21RSHP2KD-8 cells were starved of IL-3 for 12 hours and then stimulated with 1 nM IL-21 for the indicated periods. Whole-cell extracts were analyzed by immunoblotting with an anti-phospho-ERK1/2 antibody or an anti-phospho-Raf antibody (top and 3rd panels). The filters were re-probed with an anti-ERK1/2 antibody or an anti-Raf antibody, respectively (2nd and bottom panels). The SHP-2 band intensity was indicated as relative changes (A and C). The phospho-ERK1/2 or phospho-Raf band intensity was divided by the internal control band intensity, ERK1/2 (B and D upper half panels) or Raf (D lower half panels) respectively, and then indicated as relative changes. These experiments were repeated at least three times (B and C) or twice (A and C) and the representative results are shown.
We next examined the function of SHP-2 in IL-21-induced cell proliferation. A [3H]-thymidine incorporation assay demonstrated that B21RSHP2KD-8 and -12 cells showed remarkably impaired thymidine incorporation compared to the BAF21RWT-1 parent cells (Fig. 3), suggesting that SHP-2 has a critical role in the IL-21-induced cell proliferation.
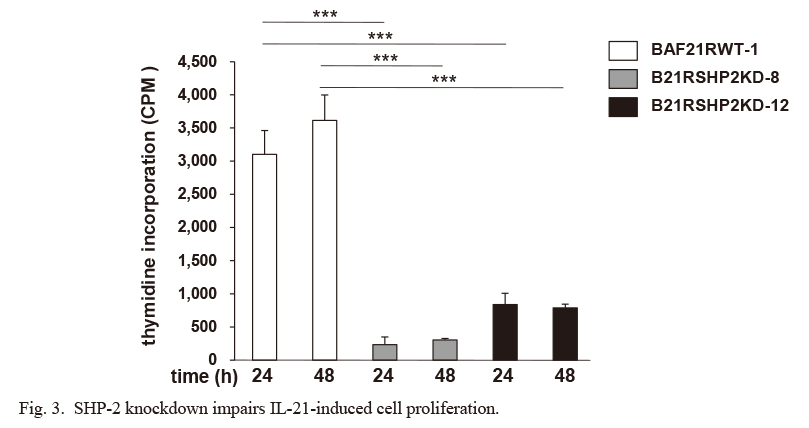
SHP-2 knockdown impairs IL-21-induced cell proliferation.
[3H] thymidine incorporation of BAF21RWT-1 and B21RSHP2KD cells in the presence of IL-21 for 24 or 48 hours was measured as described in Materials and methods.
***p < 0.001.
This experiment was repeated at least three times and the representative result is shown.
To identify the responsible molecules affecting the Raf-ERK1/2 pathway in the SHP-2-knockdown cells, we examined the JAKs, which are activated just downstream of the receptor. The JAK1 and JAK3 phosphorylation was not affected in the B21RSHP2KD cells (Fig. 4A, B). In addition, the phosphorylation of STAT3, which is the signal transducer directly downstream of the JAKs, was not affected in the B21RSHP2KD compared to BAF21RWT-1 cells (Fig. 4C). These data suggested that SHP-2 acts downstream of JAKs and upstream of Raf in IL-21-induced signaling.
Recently, in EGF-induced signaling, SHP-2 was reported to dephosphorylate Ras to increase its association with Raf and activate the downstream ERK pathway (Bunda et al. 2015). SHP-2 may have the same role in the IL-21-induced signaling system. Further studies are required to examine this point.

SHP-2 knockdown does not affect IL-21-induced JAK-STAT signaling.
BAF21RWT-1 and B21RSHP2KD-8 cells were starved of IL-3 for 10 hours and then stimulated with 1 nM IL-21 for the indicated periods. Whole-cell extracts were analyzed by immunoblotting with an anti-phospho-JAK1 (A, upper panel), anti-phospho-JAK3 (B, upper panel), or anti-phospho-STAT3 (C, upper panel) antibody. The filters were re-probed with an anti-JAK1, anti-JAK3, or anti-STAT3 antibody, respectively (A, B, and C, lower panels). The filter for the anti-phospho-JAK1 antibody immunoblot assay was pretreated with aldehyde solution after transferring the whole-cell extract to the filter as described in Materials and methods. The phospho-JAK1, phospho-JAK3 or phospho-STAT3 band intensity was divided by the internal control band intensity, JAK1 (A), JAK3 (B) or STAT3 (C) respectively, and then indicated as relative changes. These experiments were repeated at least three times and the representative results are shown.
We thank Dr. Nobuyuki Tanaka (Miyagi Cancer Center Research Institute) for providing the SHP-2 expression vector, and Dr. Naoto Ishii (Tohoku University) for generously providing us with critical reagents. This work was supported in part by a Grant-in-Aid for Scientific Research, 22590432 from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.