2018 Volume 244 Issue 3 Pages 201-207
2018 Volume 244 Issue 3 Pages 201-207
Ankle and foot injuries are common among athletes and physically active individuals. The most common residual disability, ankle sprain, is characterized by instability along with postural sway. If the supporting structures around a joint become lax, posture stability and balance are also affected. Previous studies have examined muscle stiffness and elasticity and postural sway separately; however, the relationship between these factors is yet unknown. It is well known that the levels of sex hormones, especially estrogen, change in women over the phase of the menstrual cycle. Therefore, this study examined the relationship between the mechanical properties of tissue and balance activity using a non-invasive digital palpation device to determine if they undergo any changes over the menstrual cycle in young women. Sixteen young women with regular menstrual cycles completed the study. Tone, stiffness, and elasticity of the ankle muscles (lateral gastrocnemius, peroneus longus, and tibialis anterior) were measured using a non-invasive digital palpation device. Postural sway was recorded while the participants performed balance tasks during ovulation and menstruation. Significantly greater posture sway characteristics and ankle muscle elasticity were found during ovulation than during menstruation; lower tone and stiffness of the ankle muscles were observed at ovulation (p < 0.05). Additionally, weak-to-strong relationships between ankle muscle mechanical properties and postural sway characteristics were found (p < 0.05). These results suggest the effect of estrogen on human connective tissues. We therefore postulate that estrogen increases joint and muscle laxity and affects posture stability according to the phase of the menstrual cycle.
Ankle and foot injuries are common among athletes and physically active individuals (Chinn and Hertel 2010). The most common residual disability, ankle sprain, is characterized by instability along with postural sway (Denegar and Miller 2002). An increasing number of women incur knee injuries during sports activities (Beynnon et al. 2005; Eiling et al. 2007; Kjaer and Hansen 2008; Hansen et al. 2009b; Lee et al. 2013a, 2015). The incidence, especially of non-contact anterior cruciate ligament injuries, is 2 to 8 times greater in women compared to men when participating in the same sports activities (Park et al. 2009a, b; Hansen et al. 2009b; Boden et al. 2010; Lee et al. 2013a; Stijak et al. 2015).
The ankle plays a major role in posture and locomotion (Baumhauer et al. 1995; Vieira et al. 2013; Cattagni et al. 2014; Duclos et al. 2014; Pozzi et al. 2015). Small changes in the center of gravity are corrected by the position of the knee, hip and ankle to maintain the center of gravity in the basal plane (Page et al. 2010). Combined with the tibialis muscles, the peroneus muscles support and stabilize the ankle joint (De Ridder et al. 2014, 2015). The peroneus longus (PL) is well known to provide lateral stability, and the tibialis anterior (TA) muscle is increasingly activated when the lateral displacement of the tibia is being controlled (Page et al. 2010; De Ridder et al. 2014; Duclos et al. 2014; Pozzi et al. 2015). Calf muscles also support anterior/posterior stability of the ankle joint, especially when postural stability is challenged (Vieira et al. 2013).
Balance is maintained when the center of pressure (CoP) stays within the support base (Choi and Shin 2016). The vertical projection of the center of mass within their base of support should be kept when standing to maintain balance, resulting in little medial-lateral or anterior-posterior sway (Inanir et al. 2014). Conventionally, a series of balance tasks are presented that will cause an increased demand on postural stability and hence increase sway.
Previous research has shown that PL plays a major role in maintaining balance and that TA supports some aspects of balance (Louwerens et al. 1995). TA activity increases to stabilize the ankle joint when balance is disturbed (Baumhauer et al. 1995; Louwerens et al. 1995). The CoP displacement has been used to identify definite postural instability (Kalron and Achiron 2013) and has a negative correlation with the maximal isometric torque of ankle muscles (Cattagni et al. 2014). Weakness of ankle-stabilizing muscles is a major cause of postural instability (Cattagni et al. 2014, 2016).
Almost 40% of patients with ankle sprains also have ankle instability and increased body sway, resulting from poor proprioception and impaired postural control (McCriskin et al. 2015). Previous evidence has also shown that postural stability is more impaired if there is fatigue of the muscles controlling the hip, knee and ankle. Similarly, reductions in muscle tone also can increase instability in balance. Laxity in tendons and ligaments has also been shown to affect stability (Petrofsky and Lee 2015; Lee and Yim 2016).
Many researches have studied the relation between sex hormones and structure and mechanical properties found in human connective tissue (Heitz et al. 1999; Slauterbeck and Hardy 2001; Shultz et al. 2005; Lee et al. 2013a, 2014). An obvious sex-related difference was observed in the role of estrogen in regulating muscle mass and ligament laxity (Lee et al. 2013b), and, specifically, its relation to ligament laxity has been investigated (Hansen et al. 2009a; Lee et al. 2013a). Human connective tissue, such as muscle and ligament, consists of collagen fibers closely packed together. Estrogen has also been shown to control protein synthesis in human connective tissue (Liu et al. 1996; Hansen et al. 2011). Decreased collagen synthesis causes decreased muscle tone and stiffness and increased elasticity when estradiol increases with decreases in collagen formation and fibroblast proliferation (Park et al. 2009b; Lee et al. 2013a).
Therefore, it can be predicted that if the menstrual cycle alters both muscle tone and tendon laxity, there would be a difference in balance during the menstrual cycle. The purpose of this study was to determine the effect of the menstrual cycle on muscle tone and mechanical properties including stiffness and elasticity of ankle stabilizing muscles in relation to balance, by comparing the two phases of the menstrual cycle (follicular vs. ovulation) and how it correlates to postural sway. We hypothesized that muscle tone and stiffness would be higher and elasticity lower during menstruation than during ovulation in women and that these would be positively related to postural stability.
A convenience sample of 20 physically active women with a mean age of 20.7 ± 1.6 years and body mass index (BMI) of 22.4 ± 2.7 kg/m2 volunteered to participate in this investigation; all participants were recruited from the G University, Incheon. All volunteers were screened for eligibility criteria as follows; 1) performed between 60 and 150 minutes of moderate or lighter physical activity per week; 2) no history of lower extremity injuries and neuromuscular disorders; 3) no use of any medication that would affect sex hormones; 4) nonsmokers; 5) dysmenorrhea (menstrual pain) no more than 5 out of 10. In addition, female subjects had to have a regular menstrual cycle (cycle length 26-35 d) for at least one year, with no history of pregnancy. This study was approved by the Gachon University Institutional Review Board (IRB). All participants provided prior written informed consent.
Self-reported menstrual cycleAll female subjects were asked to report their last 3 months of their menstrual cycles. According to their average menstrual cycle (days), their expected ovulation date was calculated by the research coordinator. For the ovulation phase, the participants were instructed to start using a digital ovulation predictor kit (Clearblue, SPD GmbH, Geneva, Switzerland) (Ellis et al. 2011), with 99% accuracy from day 10 to 12 of the menstrual cycle, based on their cycle length. When a positive result was detected, the participants were asked to immediately contact the research coordinator to schedule data collection. For the follicular phase, the participants contacted the research coordinator once menstruation began, and then were scheduled between day 1 and day 3 of their cycles.
Measurement of muscle tone and mechanical propertiesA non-invasive digital palpation device MyotonPRO (Myoton AS, Tallinn, ESTONIA) was used to measure muscle tone, dynamic stiffness and elasticity of the subjects’ dominant side lower limb muscles including lateral gastrocnemius (LG), tibialis anterior (TA), and peroneus longus (PL). The subjects were asked to be fully relaxed with the lower leg exposed, positioned to standardize muscle length and angle at the measurement points. Assessment points were marked above the largest cross-section of the muscle belly and length was measured from anatomical landmarks so that identical points were measured on repeat measures. The probe was perpendicular to the skin surface overlying the muscles and pressure was exerted with constant force (0.18 N); then the device delivered a force of 0.4 N for 15 milliseconds on the underlying soft tissues, causing muscle deformation. Then, the resultant damped natural oscillations, caused by the viscoelastic properties of the biological tissue were recorded using a built in accelerometer at a sampling rate of 3,200 Hz. The device has excellent interrater reliability (ICC = 0.94-0.99) (Agyapong-Badu 2013) and good to excellent test-retest reliability (ICC = 0.80-0.93) (Bizzini and Mannion 2003).
Parameters measuredThe oscillation maximum frequency (Hz) (F = fmax) measures the tone, intrinsic tension of a muscle. It is obtained by a fast Fourier transform (FFT). The height of the dampened oscillations in the contracted state indicates the amount of tension in a muscle. The corresponding acceleration amax characterizes the resistance to an external force that deforms the initial shape of the tissue (force = m· amax, where m is the mass of the testing end) for the known deformation depth Δl. at the point of maximum compression of the muscle. The ratio amax ·m /Δl describes the dynamic stiffness of the tissue (N/m). Natural log of maximum displacement (tissue resistance measured in mG) divided by maximum displacement of the second period of oscillation which takes place due to the recuperation of stored residual mechanical energy in tissue expresses the dampening ratio due to the dissipation of mechanical energy in the tissue during one oscillation cycle (Gavronski et al. 2007; Agyapong-Badu et al. 2016).
Measurement of postural swayA force platform (Zebris FDM-S pressure platform, Zebris Medical GmbH, Germany) was used to assess the CoP displacement in the subjects. The size of the force platform was 3.2 × 6.8 m and consisted of 2,560 individually calibrated capacitive force sensors, each approximately 0.85 × 0.85 cm, underneath the platform and were sampled at 60 Hz. This enabled an analysis of the distribution of static forces beneath the feet during standing of the force plate. The MR 3.8 software (Noraxon Inc., Scottsdale, Arizona, USA) was used to integrate the force signals and graphic representation of the CoP. The following CoP measures during balance tasks were analyzed: CoP sway range in the anteroposterior (AP) and the mediolateral (ML) direction, CoP trace length, CoP trace and equivalent radius, CoP sway velocity and CoP sway equivalent area.
Balance tasksA single balance test was used that normally stresses balance significantly. The participants were asked to stand with their feet in tandem (feet in heel-toe position with the non-dominant foot in front) on a foam surface with their eyes closed for 10 seconds, while two trained researchers stood nearby to secure subjects in case of the falls.
ProcedureOnce participants agreed to sign the informed consent, the investigator obtained their demographics, including age, height, weight, menstrual cycle length, self-reported cycle, and subjective dysmenorrhea. Subjects were tested twice, once during ovulation, and once during the early follicular phase. On arriving at the laboratory, the participants rested comfortably in a regulated temperature room for 20 minutes to stabilize their body conditions. Muscle tone, elasticity, and stiffness of LG, PL, and TA were measured in a neutral position of the muscles. Subjects were then asked to perform the balance task on the force platform.
Statistical analysesThe GPower 3.1 software (Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany) was used to calculate the sample size needed using a moderate effect size of 0.85 between the two different menstrual cycle phases, with an alpha error probability of 0.05 and a power of 0.85 (Petrofsky et al. 2015; Lee and Yim 2016). A sample size of 15 participants was required to provide a statistical power of 86.4%. Taking into account potential drop outs, 19 participants were recruited.
The SPSS 23.0 software (IBM, Armonk, NY) was used to analyze the data, which were summarized as means ± SD. The assumption of normality of the continuous variables was examined using the Kolmogorov-Smirnov test. A paired t test was conducted to compare mean muscle tone, mechanical properties and postural sway characteristics between the follicular phase and ovulation. Pearson’s correlation analysis was used to examine the relationship between muscle mechanical properties and postural sway characteristics in each phase of menstrual cycle. The level of significance was set at α < 0.05.
Sixteen subjects completed the study. 19 subjects were recruited; however, 3 withdrew due to scheduling conflicts. The general characteristics of the subjects are described in Table 1.
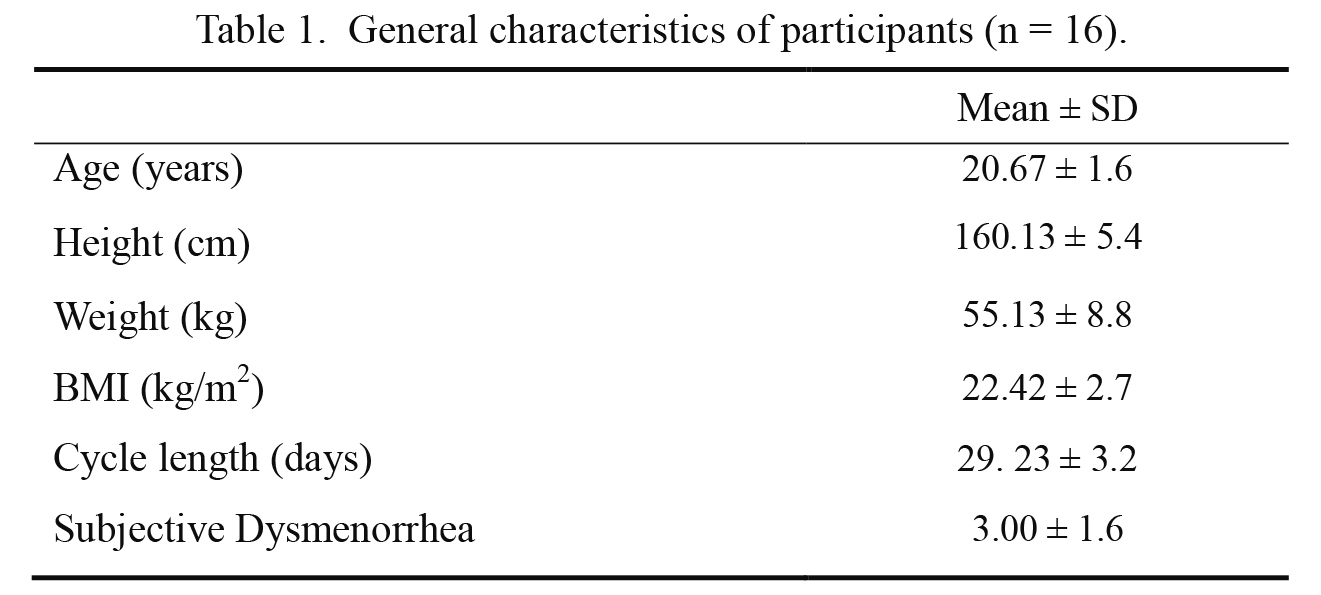
General characteristics of participants (n = 16).
SD, standard deviations.
The muscle oscillation frequency (representing muscle tone) for TA, PL, and LG was significantly greater in menstruating than ovulating women (p < 0.001, Table 2). Menstruating women had a significantly greater tone and stiffness of mean TA compared with ovulating women (p < 0.001). Elasticity was significantly lower (higher logarithmic decrement value) in ovulating women (p < 0.05). No significant difference was seen in mean PL elasticity between menstruating and ovulating women, but tone and stiffness were significantly greater in menstruating women (p < 0.01). For the LG muscle, a significant greater tone and lower elasticity were found in menstruating women (p < 0.01), however, no significant difference in mean muscle stiffness between the two phases was detected (p > 0.05).

Muscle tone, elasticity, and stiffness during follicular phase and ovulation in young women.
SD, standard deviations.
*** p < 0.001, ** p < 0.01, * p < 0.05, significant difference from the follicular phase.
The results of the determination of postural sway during the balance task comparing the follicular phase and ovulation are presented on Table 3. During the balance task, there was a significant increase in mean CoP sway in the AP, ML directions and velocity during ovulation compared with the follicular phase were observed during the balance task (p < 0.05, p < 0.001, p < 0.001, respectively). However, there were no significant differences in mean CoP and sway length and area between two phases of the menstrual cycle (p > 0.05).

Postural sway characteristics during follicular phase and ovulation in young women.
SD, standard deviations; CoP, center of pressure; AP, anteroposterior; ML, mediolateral.
*** p < 0.001, ** p < 0.01, * p < 0.05, significant difference from the follicular phase.
Table 4 shows the correlation between mechanical properties and the postural sway characteristics during ovulation and the follicular phase in women. At ovulation, there was a strong positive correlation between muscle oscillation frequency and elasticity of TA and CoP sway velocity (r = 0.78, r = 0.86, respectively; p < 0.01). However, LG and PL muscle tones were weakly correlated with postural CoP sway velocity (r = 0.39, r = 0.31, respectively; p < 0.01). A strong negative correlation was found between the TA and PL stiffness and CoP sway velocity as well as CoP sway area (r = −0.88 [TA & velocity], r = −0.72 [TA & area], r = −0.82 [PL & velocity], r = −0.80 [PL & area], respectively; p < 0.01). A weak negative correlation was observed between LG stiffness and CoP sway velocity (r = −0.35, p < 0.01).
At menstruation, a strong positive correlation was observed between TA muscle tone and CoP sway in the ML direction while a moderate positive correlation was seen between muscle oscillation frequency and elasticity of TA and CoP sway velocity and area (r = 0.68, r = 0.62, respectively; p < 0.05). In addition, PL elasticity was positively correlated with the CoP length (r = 0.55, p < 0.05). A weak correlation was found between PL muscle tone, and postural CoP sway velocity (r = 0.41, p < 0.05). A strong negative correlation was found between muscle stiffness of TA and CoP sway velocity (r = −0.78, p < 0.05). A weak negative correlation was observed between LG stiffness and CoP sway velocity (r = −0.35, p < 0.05).

Correlation coefficient of muscle tone, elasticity, and stiffness with postural sway characteristics follicular phase and ovulation in young women.
***p < 0.001, **p < 0.01, *p < 0.05, significant correlation.
The present investigation compared differences in tone, stiffness, and elasticity of ankle muscles and how they correlated to postural sway in women under 2 conditions. ovulation and menstruation. The major findings from this study were: 1) the tone in the ankle stabilizing muscles is lesser in ovulating women than in menstruating women; 2) TA and PL muscle stiffness is lower during ovulation than during menstruation; 3) TA muscle elasticity is greater during ovulation than during menstruation; 4) postural sway during the balance test is greater during ovulation than in the follicular phase; and 5) weak-to-strong correlation was observed between postural sway characteristics and mechanical properties of the ankle muscles.
A recent study found that there was significantly greater postural sway during ovulation than during the follicular phase of the menstrual cycle (Petrofsky and Lee 2015; Lee and Yim 2016). When women had a reduced capacity to control postural sway during ovulation, neuromuscular control of balance relied more on the TA than was seen during the follicular phase to compensate for ankle laxity. Decreased PL activity associated with increased TA activity to modulate the ankle joint laxity at ovulation may be affected by high estradiol concentrations (Lee and Yim 2016). This supports the study results that muscle elasticity is significantly increased and muscle tone and stiffness are significantly decreased with an increase in postural sway during ovulation. Further, previous studies that investigated gastrocnemius muscle tone, elasticity, and stiffness found a positive correlation between muscle tone and elasticity, and postural sway on standing on unstable surfaces in young men (Vain et al. 2015).
A logical explanation for many of the results seen here is the effect of estrogen. Khowailed et al. (2015) studied female runners and found more laxity at the ligaments of the knee at ovulation than at menstruation. This was compensated for by increased activity of the quadriceps and hamstring muscles to stabilize the knee at ovulation during running compared with the follicular phase of the menstrual cycle. Similar patterns were found in TA and PL muscles to stabilize the ankle at ovulation during balance tasks in a previous study (Lee and Yim 2016). Here a similar relaxation of the ligaments and tendons at the ankle would reduce muscle tension in the resting muscle and would not be compensated for during balance tasks. More laxity at the ankle would increase postural sway since there would be a larger mechanical lag between muscle activation of a change in tension at the ankle joint at ovulation. Another factor to be considered in this study and other studies is tissue temperature. Tendons and ligaments are warmest in the ovulatory phase of the menstrual cycle and coolest during menstruation (Petrofsky et al. 1976). Certainly, the increased laxity and relaxation of muscles noted here could be the result of higher tissue temperature during ovulation as well as the direct effect of estrogen. In a previous study, when leg temperature was stabilized throughout the menstrual cycle, there was still an effect of estrogen on tendon laxity but the effect was less, showing the combined effect of estrogen and temperature on tissues in the leg (Lee et al. 2013a).
Previous studies have examined the motor control needed to stabilize the ankle during standing and balance activities (Lee and Yim 2016). However, muscle force is modulated through the parallel and series elastic components in muscle and the laxity in tendons and ligaments. This is the first study to correlate these mechanical properties of tissue using a MyotonPRO, non-invasive digital palpation device to ankle stability during ovulation and the follicular phase in women.
However, this study has several limitations. All measurements were assessed only 2 points throughout only one menstrual cycle. More testing sessions may show larger menstrual cycle effects, thus maximizing the potential to observe an effect of estradiol which fluctuates throughout the menstrual cycle. Another limitation is that a self-reported menstrual cycle was used to detect ovulation. Sexual hormones such as estradiol and progesterone and even testosterone concentration in either blood or saliva should be measured to confirm the sexual hormone changes during the menstrual cycle. In addition, muscle tone, stiffness, and elasticity were measured only once in the relaxed position. In a previous study (Vain et al. 2015), researchers assessed these mechanical variables on subjects with them standing and in the relaxed position, which can show more precise correlations between variables. Finally, it would be useful to stabilize limb temperature to see the effect of body and shell temperature and estrogen independently.
Therefore, we recommend that women should be aware of these changes of muscle tone, mechanical properties, and its relation to postural sway throughout the menstrual cycle to prevent risk of lower extremity injuries during sports activities.
The authors declare no conflict of interest.
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT & Future Planning) (No. 2017R1C1B5017867).