2018 Volume 244 Issue 4 Pages 291-296
2018 Volume 244 Issue 4 Pages 291-296
Ovarian cancer is the fourth leading cause of cancer death in women and the most fatal gynecologic malignancy. Placenta growth factor (PGF), a member of the vascular endothelial growth factor, plays an important role in angiogenesis. The overexpression of PGF was observed in several types of cancers, but the clinical significance of PGF in epithelial ovarian cancer (EOC) is still unknown. To explore the prognostic value of PGF among patients with serous EOC, we analyzed the expression of PGF in 89 EOC specimens by immunohistochemistry. The scoring system of immunohistochemistry was based on the staining intensity and the percentage of PGF-positive cells in each EOC tissue. According to the immunohistochemical score, 34 patients with score ≥ 6 were defined as high PGF expression, and other 55 patients were the group with low PGF expression. The prognostic significance of PGF expression was analyzed. EOC patients with higher IHC scores of PGF expression are significantly associated with positive lymphatic invasion and poorer response to chemotherapy. Patients with higher IHC scores of PGF expression had poorer response to chemotherapy and lower overall survival rate. Additionally, the positive lymph node metastasis, advanced TNM stage, and poorer response to chemotherapy were all remarkably correlated to poorer prognosis. In conclusion, patients with higher PGF in EOC tissues were more predisposed to positive lymphatic invasion, poorer response to chemotherapy and unfavorable prognosis of patients with serous EOC. We propose that PGF expression may be predictive of chemoresistance and poor prognosis of serous EOC.
Ovarian cancer is the fourth leading cause of cancer death in women and the most fatal gynecologic malignancy with about 200,000 newly diagnosed patients and 15,000 deaths (Jeruss and Woodruff 2009; Zaid et al. 2013). As a heterogeneous group of neoplasms, ovarian cancers mainly consist of malignant epithelial tumors (Cho and Shih Ie 2009). The histological subtypes generally include serous, endometrioid, clear cell and mucinous adenocarcinomas (Basu et al. 2014). Unfortunately, partially because of no specific symptoms at the early stage, approximately 70% of patients are present with a diagnosis of ovarian carcinoma in an advanced stage (Barnes et al. 2002). In the past decades, with the progress of modern surgeries and adjuvant chemotherapies, the overall prognosis of patients with ovarian cancers have improved remarkably, but most patients would experience the relapse and the overall survival rate remains unsatisfactory. The 5-year survival rates of patients with chemoresistance are only 25%-35% (Colombo et al. 2014). The current standard treatment for epithelial ovarian cancer (EOC) is the aggressive surgical cytoreduction followed by adjuvant chemotherapy. New treatment approaches and modified chemotherapies rely on the exploration of new biomarkers and drug targets. Thus, the prognostic and predictive biomarkers for EOC are still in urgent need.
Placenta growth factor (PGF), a member of the vascular endothelial growth factor (VEGF) sub-family, plays an important role in angiogenesis and endothelial cells growth (Rolny et al. 2011). PGF could be secreted out and trigger downstream signaling pathway mainly by binding to VEGFR-1 or neuropilin (Rolny et al. 2011). PGF is highly expressed in placenta throughout all stages of gestation. As a key member of angiogenesis, PGF is expressed in endothelial cells of many organs including ovary (De Falco 2012). The aberrant expression of PGF is associated with many kinds of human diseases. For example, serum PGF is a potential biomarker for preeclampsia during pregnancy. In both early and late onset preeclampsia, maternal serum levels of PGF are lower in women presenting with preeclampsia (Khalil et al. 2008). The elevation of PGF expression was reported in many types of cancers, including gastric cancer, colorectal cancer, hepatocellular carcinoma, breast cancer and lung cancer (Dewerchin and Carmeliet 2014; Song et al. 2016). In ovarian cancer cells, PGF has been demonstrated to promote cell invasion via activating zinc finger E-box-binding homeobox 2 (ZEB2) and promote metastases via suppressing miR-543 and up-regulating MMP7 (Song et al. 2015, 2016). However, all the previous studies were limited in experiments in vitro, and the clinical significance of PGF in EOC is still unknown.
In this study, we enrolled 89 patients with serous EOC and investigated the expression of PGF with immuhohistochemistry (IHC). By univariate and multivariate analysis, we evaluated the prognostic significance of PGF among patients with serous EOC.
This study was approved and supervised by the Ethics Committee of the Affiliated Hospital of Shandong Medical College and the Ethics Committee of Linyi People’s Hospital. There are total 353 patients who underwent surgery of EOC in the Affiliated Hospital of Shandong Medical College and the Linyi People’s Hospital from 2009 to 2013. Total of 89 patients were selected following the inclusion criteria: (1) the pathological type was serous ovarian cancer and the first operation was in advanced-stage (stage IIIB to IV), (2) there were available follow-ups and tissues for IHC, (3) there were no chemotherapies or radiotherapies before surgery, (4) patients got systemic platinum-based chemotherapy after the operation, and (5) there were no severe complications and other tumors occurred post-operationally. The age of patients ranged from 41 to 73 years old, with the average age as 54.3 years old. The written informed consents were obtained from patients or their families. Optimal Debulking surgery was defined as a residual tumor no more than 1 cm in diameter according to previous study (Zaid et al. 2013). The TNM stage of patients was according to the AJCC/UICC staging system.
ImmunohistochemistryThe paraffin-embedded specimens of serous EOC were sliced into 4-μm sections for IHC according to previous studies (Xu et al. 2014; Yang et al. 2014; Liu et al. 2017b). Briefly, 5% bovine serum albumin was used to incubate the specimen for 1 hour to block unspecific antigen binding after the endogenous peroxidase inactivation antigen retrieval. The primary rabbit polyclonal PGF antibody (Cat No. ab9542, Abcam, Cambridge, UK) at dilution 1:200 was applied overnight at 4℃, and the corresponding secondary antibody (Sangon Biotech, Shanghai, China) was used at room temperature for 2 hours after rinsing with phosphate buffered saline. The final signal visualization was achieved by application of 3,3′-diaminobenzidine. The slides were counterstained with hematoxylin finally.
Evaluation of IHC resultsThe results of IHC were evaluated and scored by two senior pathologists who were blind to the clinical data. The score system of IHC was dependent on the staining intensity and positive cells percentage according to previous studies (Liu et al. 2017a; Xu et al. 2017). The score of staining intensity was defined as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The score of positive cells percentage was defined as follows: 1, < 25% of positive cells; 2, 25%-50% of positive cells; 3, 50%-75% of positive cells; and 4, 75%-100% of positive cells. Thus, the final IHC score was calculated as the product of the score of staining intensity multiplied by the score of positive cells percentage, from 0 to 12. The optimal cut-off of PGF expression was identified by the point with the highest sum of sensitivity and specificity in ROC curve, which was 5.0 in our test. The total cohort was divided into the group with high expression of PGF (score ≥ 6) and the group with low expression of PGF (score ≤ 4) with the optimal cut-off.
Statistical analysisAll the significant differences were analyzed with SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA). The correlation between the expression of PGF and the clinicopathological parameters was analyzed with Fisher test. The survival curves were estimated with the Kaplan-Meier method and the statistical significance of different groups was accessed by the log-rank test. The Cox’s regression proportional hazards model is applied to identify the independent prognostic factors and the hazard ratio (HR) with 95% confidence interval (95% CI) was used to evaluate the prognostic value. P-value < 0.05 was considered statistically significant.
The expression of PGF in serous ovarian cancer was detected with IHC. In EOC tissues, PGF was mainly observed in the intracellular cytoplasm. According to the cut-off, the cohort of our study was divided into the groups of low PGF expression and high PGF expression (Fig. 1A, B), which accounted for 61.8% (55/89) and 38.2% (34/89), respectively.

Representative immunohistochemical images of high- and low-expression of PGF in EOC.
A. Image of low expression of PGF. The staining intensity was weak and the positive cells percentage was < 25%. The total IHC score was 1, and this case was defined as low expression of PGF.
B. Image of high expression of PGF. The staining intensity was strong and the positive cells percentage was 50%-75%. The total IHC score was 9, and this case was defined as high expression of PGF.
The correlations between PGF expression and the clinicopathological factors were analyzed by the Fisher test (Table 1). In our study, high expression of PGF was significantly associated with positive lymph node metastasis (P = 0.004), indicating that high PGF may promote the progress of lymphatic invasion. Interestingly, patients with high expression of PGF usually had poorer response to chemotherapy (P = 0.001), suggesting that high PGF played a key role in the resistance of chemotherapy, which could help stratify patients more preciously and explore the underlying mechanisms of drug resistance of EOC.

Correlation between PGF and the clinicopathological features.
*Fisher test.
PGF, placenta growth factor.
The relationship between the expression of PGF and the overall survival rate was evaluated by univariate analysis with Kaplan-Meier method and the difference between the subgroups was analyzed with log-rank test (Table 2). High expression of PGF was demonstrated to be significantly associated with lower overall survival rate of EOC (P = 0.026) (Fig. 2A). The 5-year overall survival rates of patients with low and high expression of PGF were 37.9% and 10.2% respectively. In addition, the positive lymph node metastasis (P = 0.005), advanced TNM stage (P = 0.031), and poorer response to chemotherapy (P = 0.027) were all remarkably correlated to the poorer prognosis (Fig. 2B-D).

Prognostic value of PGF and clincopathological features.
*Calculated by Log-Rank test.
&Calculated by Cox-regression model.
PGF, placenta growth factor; HR, hazard ratio; CI, confidence interval.
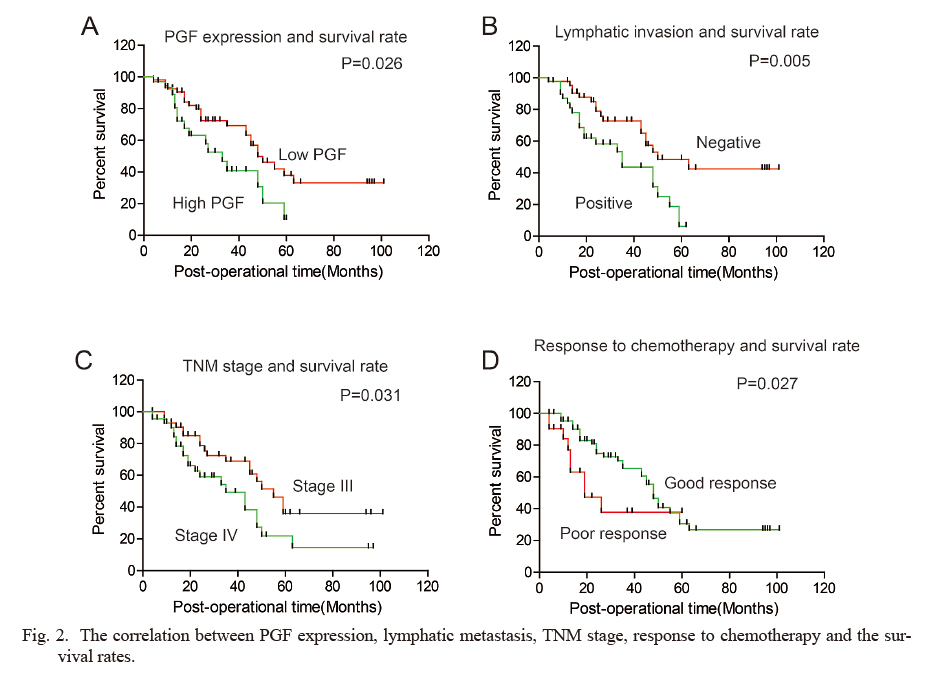
The correlation between PGF expression, lymphatic metastasis, TNM stage, response to chemotherapy and the survival rates.
The overall survival curves were stratified by PGF expression (A), lymphatic metastasis (B), TNM stage (C) and response to chemotherapy (D). The statistical differences were calculated with the log-rank test.
The prognostic factors were screened out by the univariate analysis, and the independent prognostic risks were confirmed by multivariate analysis with the Cox-regression model (Table 2). The lymphatic status, TNM stage, response to chemotherapy and expression of PGF were enrolled into the Cox-regression model. In our study, the positive lymph node metastasis and advanced TNM stage were identified as the independent prognostic risks of EOC. The poorer response to chemotherapy seemed to be the independent prognostic factors, with a statistical insignificant tendency (P = 0.053). However, the high-expression of PGF was not confirmed as an independent prognostic risk in our study (P = 0.518). This may be resulted from that PGF had significant interaction with lymphatic metastasis and response to chemotherapy, which were also enrolled into the multivariate analysis. PGF probably induced unfavorable prognosis via promoting lymphatic invasion or poor response to chemotherapy.
Combination of cytoreductive surgery and platinum-based therapy is the standard first-line therapy in the treatment of EOC now (du Bois et al. 2003). However, ovarian cancer is a kind of malignancy with high heterogeneity, and epithelial serous ovarian cancer is the most common pathological type. Identification of prognostic biomarkers and their mechanisms to affect prognosis at the molecular level could attribute to the drug target and targeted treatments. But the exploration of biomarkers should be based on the separate pathological type, which is more important in ovarian cancer. PGF is a member of VEGF family and there are three VEGF receptors, which are VEGFR1 (FLT1), VEGFR2 (KDR, FLK1) and VEGFR3 (FLT4). Among these receptors, PGF could only interact with VEGFR1, which is also a candidate biomarker in EOC (Kendall and Thomas 1993). We observed that high expression of PGF was associated with the survival rate of patients with EOC in our study, without involving the underlying molecular mechanisms, which has been explored previously (Song et al. 2015, 2016). In previous study, VEGF-c was proved to promote ovarian cancer progression through paracrine and autocrine mechanisms (Decio et al. 2014). As a secretory growth factor, we also hypothesized that PGF may lead to the poorer prognosis in a paracrine or autocrine way synergetic with VEGFR1. But the hypothesis needs further validation with experiments because PGF could also bind to neuropilin-1 and neuropilin-2 in a heparin-dependent manner.
In our study, we demonstrated that high expression of PGF was significantly associated with the unfavorable prognosis in patients with advanced stage epithelial serous ovarian cancer, indicating that the anti-PGF therapy may be a potential treatment therapy to patients with EOC. However, it is interesting that multivariate analysis did not define PGF expression as an independent prognostic factor in EOC, suggesting that high PGF expression may lead to poorer prognosis via influencing other factors, such as lymph node metastasis and chemotherapy response. Recently, many approaches have achieved significant progresses for the treatment of EOC. These approaches include proteins trapping, siRNA encapsulated in nanoparticles and humanized antibodies or specific small-molecular inhibitors (Landen et al. 2005; Yap et al. 2009). The most promising drug of EOC now is EGF antibody bevacizumab, which is in a phase 3 clinical trial (Burger 2011). Moreover, PARP inhibitors are also potential targeted drugs and are in pre-phase 3 now clinical trial (Fong et al. 2010). As to PGF signaling, there is an anti-PGF monoclonal antibody named RO5323441, which has been shown preclinical activity in several cancer models (Wang et al. 2017). The pharmaceutical value of RO5323441 in EOC treatment should be further investigated, but we considered RO5323441 as a potential targeted drug in EOC treatment based on our clinical findings.
In summary, we detected the expression of PGF in 89 cases of serous EOC and demonstrated that high expression of PGF is significantly associated with poorer response to chemotherapy and unfavorable prognosis of patients. Thus, PGF is a prognostic biomarker, and detecting PGF could stratify the patients of high-risk and potential chemoresistance. The present findings also suggest the possibility that anti-PGF therapy is a promising therapy to treat EOC.
The authors declare no conflict of interest.