2018 Volume 246 Issue 2 Pages 65-71
2018 Volume 246 Issue 2 Pages 65-71
Actigraphy is an easy and noninvasive method used to monitor human ultradian cycles. However, to our knowledge, it has been not applied to experiments with rodents. Therefore, using actigraphy, we assessed the ultradian cycles and behavior of rats. Rats with or without allergic rhinitis wore an actigraphy device, and triaxial acceleration was recorded. The counts that represent physical activity were lower from 8:00 to 20:00 than those from 20:00 to 8:00 in control rats, suggesting that their sleep phase was from 8:00 to 20:00 and their awake phase from 20:00 to 8:00. The counts from 8:00 to 10:00 were significantly higher in allergic rhinitis rats than in control rats (p < 0.01), suggesting the presence of difficulty with sleep induction in rats with allergic rhinitis. The counts from 18:00 to 20:00 were also significantly higher in allergic rhinitis rats than in control rats (p < 0.05), suggesting the presence of early awakening in rats with allergic rhinitis. Moreover, the counts were significantly higher in allergic rhinitis rats than in control rats from 20:00 to 8:00. These results suggest that rats with allergic rhinitis experienced hyperactivity disorder during the daytime. Additionally, hyperreactivity and difficulty with sleep induction were observed in 6-hydroxydopamine-lesioned rats, an animal model of attention-deficit hyperactivity disorder. This study shows for the first time that actigraphy can be successfully used for behavioral analysis in rodents. These rat models could be useful for analyzing the mechanisms involved in sleep disturbances and hyperactivity disorder.
Actigraphy, which is an easy and noninvasive method used to monitor human ultradian cycles, has been employed as an objective tool for the ambulatory monitoring of sleep and activity in attention-deficit hyperactivity disorder (ADHD) (De Crescenzo et al. 2016). Actigraphy has been employed with animals such as monkeys (Sri Kantha et al. 2009), sheep (Brutcher and Nader 2013), dogs (Barthélémy et al. 2011), and horses (Martin et al. 2010). However, actigraphy has been not employed with rodents, such as rats and mice, although experiments with rodents are necessary for detailed study. In fact, rodents have been often used in experiments on sleep and behavior (Gradwohl et al. 2017; Xie et al. 2018). For the investigation of sleep using rodents, electroencephalograms (EEGs) and electromyograms (EMGs) have been recorded under anesthesia after surgery (Gradwohl et al. 2017; Xie et al. 2018). However, this technique is invasive, and as measuring EEG and EMG without surgery and anesthesia is challenging, such investigations cannot take place under real-life conditions.
Allergic rhinitis is a common condition encountered worldwide. Although the primary symptoms of allergic rhinitis are sneezes, nasal discharge, and nasal obstruction, it has also been associated with conditions such as sleep disturbance (Craig et al. 2004; Chirakalwasan and Ruxrungtham 2014), resulting in reduction of quality of life (QOL). Sleep disturbance caused by allergic rhinitis should be seriously considered, since it may induce tiredness, lethargy, impaired memory, irritability, despondency, and others. Hoehle et al. (2017) reported that sleep may have the greatest negative impact on QOL in patients with allergic rhinitis. Furthermore, it has been reported that ADHD is common among children with allergic rhinitis (Yang et al. 2016; Feng et al. 2017; Miyazaki et al. 2017).
Using actigraphy, Rimmer et al. (2009) identified sleep disturbances in patients with allergic rhinitis, which may impair QOL. Yuksel et al. (2009) showed that actigraphy could be used as an objective tool to evaluate sleep disturbance in patients with allergic rhinitis. However, there have been few detailed studies investigating sleep and behavior in patients with allergic rhinitis.
ADHD is a common behavioral illness characterized by persistent symptoms of inattention, hyperactivity, and impulsivity. Although 6-hydroxydopamine (6-OHDA)-lesioned rats have been used as an animal model of ADHD (Heffner and Seiden 1982; Davids et al. 2002; Caballero et al. 2011), it is expected that the underlying etiologies for ADHD are elucidated, and that a novel treatment for ADHD is developed.
To the best of our knowledge, an easy and noninvasive method has been not established for the study of behavior and sleep in a rodent model with allergic rhinitis or ADHD. To this end, in this study, we investigated behavior and sleep in rats with allergic rhinitis or ADHD using actigraphy.
Seven-week-old male Wistar rats (Japan SLC, Shizuoka, Japan) were immunized, and rats with allergic rhinitis were established using a method similar to that previously reported (Kumar et al. 2008; Ren et al. 2017). Rats were intraperitoneally sensitized with 0.5 mg of ovalbumin (OVA, Sigma-Aldrich, St. Louis, MO) and 50 mg of Al(OH)3 dissolved in 1 mL of physiological saline every day on days 1 through 14. Subsequently, rats were intranasally challenged with 20 μL of OVA solution (20 μg, dissolved in physiological saline) every day from day 15 to day 21. On day 21, the numbers of sneezes and nasal rub movements were counted for 30 min immediately after the challenge, and sera were collected on the same day. Control rats were also established. Briefly, rats were intranasally challenged with only physiological saline on days 15 through 21 after intraperitoneal sensitization with 0.5 mg of OVA and 50 mg of Al(OH)3 on days 1 through 14. Physical activities of five rats in each group were measured after the intranasal challenge on day 21. Rats were sacrificed, and samples were collected on day 23. This study was approved by the Research Ethics Committee of Nagoya City university. Rats were housed in an environmentally-controlled animal facility at our university. The protocols were in accordance with the Guidelines for the Care and Use of Animals of our university. Every effort was made to minimize the discomfort of the animals.
ActigraphyThe actigraphy device, GT3X® (ActiGraph, Pensacola, FL), was used to measure physical activity in this study, which includes an accelerometer that is able to record triaxial acceleration (x, y, and z axes). The sampling frequency of the actigraph was set at 30 Hz. Physical activity and sleep/wakefulness were measured using actigraphy with a dynamic range of ± 2.5 G-forces. Rats carrying the actigraphy device on their back secured in place through an elastic belt for 24 hours as shown in Fig. 1. The weight of the actigraphy device was 19 g. One day before the recording, the actigraph was set for habituation, and intranasal challenge with OVA (20 μg) and behavioral analysis by the actigraphy were performed. Data were post-processed with the Data Analysis Software ActiLife 6® provided by ActiGraph. The counts, i.e., the number of accelerations detected by the actigraph every 2 hours, were calculated and used to evaluate rat activity in this study.

Installation of the actigraphy device.
A rat carries the actigraphy device on their back, secured with an elastic belt.
The heads were decalcified and sectioned. Three-micrometer thick sections of nasal tissue were stained with Luna staining. The number of eosinophils in the nasal mucosa of the nasal septum was counted microscopically in a field of view at 400 × magnification.
Measurement of OVA-specific IgESerum titers of OVA-specific IgE were measured with enzyme-linked immunosorbent assay (ELISA) using a modification of a previously reported method (Suzuki et al. 2008, 2017). ELISA 96-well plates were coated with 100 μL anti-rat IgE monoclonal antibody (Yamasa, Tokyo, Japan). Non-specific binding was blocked using blocking buffer, and sera were added. Biotinylated OVA was added to the well and the plates were incubated with avidin-peroxidase. The TMB microwell peroxidase substrate system (KPL, Gaitherburg, MD) was applied according to the manufacturer’s instructions. After washing, the optical density was measured at 450 nm.
Establishment of a rat model of ADHD6-OHDA-lesioned rats were prepared by the method previously reported (Heffner and Seiden 1982). On postnatal days 3, Wistar rat pups received subcutaneous injection of desipramine (25 mg/kg body weight, Sigma-Aldrich, St. Louis, MO) to protect adrenergic neurons from the effects of 6-OHDA. Under hypothermal anesthesia, animals were randomly given an injection of 6-OHDA (100 µg, Sigma-Aldrich) in 0.9% sodium chloride containing 0.1% ascorbic acid or only 0.9% sodium chloride containing 0.1% ascorbic acid into the right lateral ventricle. On postnasal day 6, all rats again received same treatment, desipramine followed by 6-OHDA or vehicle, as rats received on postnasal day 3, into the left lateral ventricle. Physical activities of five rats in each group were measured on postnasal day 42. This study was approved by the Research Ethics Committee of Nagoya City university. Rats were housed in an environmentally-controlled animal facility at our university. The protocols were in accordance with the Guidelines for the Care and Use of Animals of our university. Every effort was made to minimize the discomfort of the animals.
Data analysisData are expressed as mean ± standard error of the mean. Statistical comparisons between the groups were performed using Student’s t-test. A probability of p < 0.05 was considered statistically significant.
There was no significant difference in OVA-specific IgE level between rats who were and were not intranasally challenged with OVA after sensitization with OVA, although OVA-specific IgE was detected in both groups (Fig. 2). Eosinophilia was only seen in rats that were intranasally challenged with OVA (Fig. 3). The numbers of sneezes and nasal rub movements in rats that were intranasally challenged with OVA were significantly higher than those in control rats (Fig. 4, p < 0.0001). These results suggested that allergic rhinitis was successfully established in rats who received intranasal OVA challenge after OVA sensitization (allergic rhinitis rats).

IgE in sera.
Ovalbumin-specific IgE was measured in sera of rats that received intranasal challenge with ovalbumin in physiological saline (allergic rhinitis rats) or physiological saline alone (control rats).

Eosinophilia in the nasal septum of rats with allergic rhinitis.
Typical sections of the nasal septum in rats that received intranasal challenge with ovalbumin in physiological saline (A, allergic rhinitis rat) or physiological saline alone (B, control rat). Arrows show eosinophils (A), although eosinophils are not observed in B.
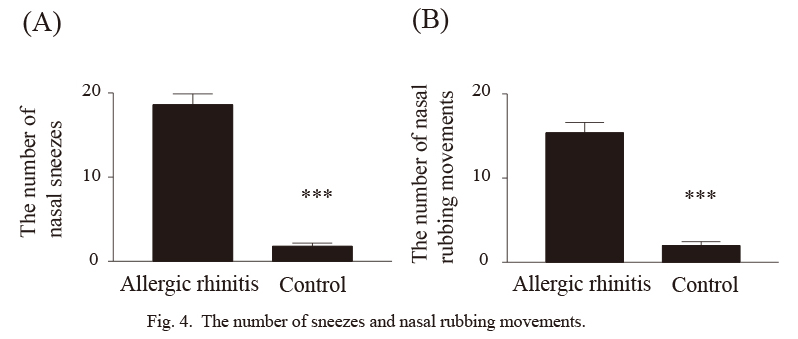
The number of sneezes and nasal rubbing movements.
The number of sneezes and nasal rubbing movements immediately after intranasal challenge with ovalbumin in physiological saline (allergic rhinitis rat) or physiological saline alone (control rat) were counted in allergic rhinitis rats and control rats (***p < 0.0001 versus allergic rhinitis rats).
The counts, i.e., the number of accelerations detected by the actigraphy, were measured every 2 hours in allergic rhinitis and control rats (Fig. 5). The counts from 8:00 to 20:00 were lower than those from 20:00 to 8:00 in control rats, suggesting that their sleep phase was from 8:00 to 20:00 and their awake phase from 20:00 to 8:00. The counts (451.6 ± 29.0) from 20:00 to 8:00 were significantly higher than those from 8:00 to 20:00 (199.2 ± 16.5) in control rats (p < 0.0001).
Next, differences in counts between allergic rhinitis and control rats were examined. The counts from 8:00 to 10:00 were significantly higher in allergic rhinitis rats than in control rats (p < 0.01), suggesting the presence of difficulty inducing sleep in rats with allergic rhinitis. The counts from 18:00 to 20:00 were also significantly higher in allergic rhinitis rats than in control rats (p < 0.05), suggesting the presence of early awakening in rats with allergic rhinitis. Therefore, these results suggest that rats with allergic rhinitis experienced sleep disturbances. Furthermore, the counts in allergic rhinitis rats were significantly higher than those in control rats from 20:00 to 22:00 (p < 0.01), from 22:00 to 24:00 (p < 0.05), from 0:00 to 2:00 (p < 0.05), from 2:00 to 4:00 (p < 0.05), from 4:00 to 6:00 (p < 0.05), and from 6:00 to 8:00 (p < 0.01). These results suggest that rats with allergic rhinitis experienced hyperactivity disorder during the daytime.
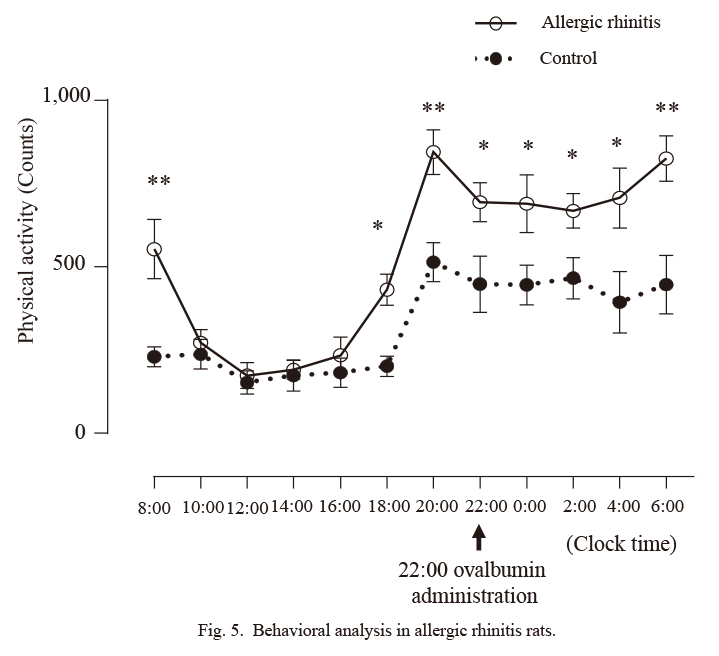
Behavioral analysis in allergic rhinitis rats.
The counts, i.e., the number of accelerations detected by the actigraphy, were measured every 2 hours in allergic rhinitis and control rats (**p < 0.01 and *0.05 versus control rats).
The counts were measured every 2 hours in 6-OHDA-lesioned and control rats (Fig. 6). Counts of 6-OHDA-lesioned rats and control rats were compared. The counts from 8:00 to 10:00 were significantly higher in 6-OHDA-lesioned rats than in control rats (p < 0.01), suggesting the presence of difficulty inducing sleep in 6-OHDA-lesioned rats. And the counts in 6-OHDA-lesioned rats were significantly higher than those in control rats from 22:00 to 24:00 (p < 0.01), from 0:00 to 2:00 (p < 0.01), from 2:00 to 4:00 (p < 0.01), from 4:00 to 6:00 (p < 0.01), and from 6:00 to 8:00 (p < 0.05). These results suggest that 6-OHDA-lesioned rats showed hyperactivity disorder during the daytime.

Behavioral analysis in 6-OHDA-lesioned rats.
The counts, i.e., the number of accelerations detected by the actigraphy, were measured every 2 hours in 6-OHDA-lesioned and control rats (**p < 0.01 and *0.05 versus control rats).
For sleep-wake cycle recording in rodents, EEG and EMG have been used after surgery (Gradwohl et al. 2017; Xie et al. 2018). In rodents, to record EEG, steel-screw cortical electrodes are screwed through the skull under anesthesia, and wires are inserted into the muscles to record EMG. Hence, the techniques used for sleep-wake cycle recording with EEG and EMG in rodents are invasive. On the other hand, it is not necessary for rodents to receive anesthesia, surgery, or insertion of electrodes and wires in experiments with actigraphy. Therefore, sleep-wake cycle recording with actigraphy, which we used in this study, is a noninvasive and safer method. Additionally, sleep-wake cycle recording with EEG and EMG is performed under anesthesia, which is not required when using actigraphy, and thus actigraphy can be employed for examinations under real-life conditions. Furthermore, we showed that actigraphy can be applied to experiments with rats. Considering these, sleep-wake cycle recording with actigraphy may be useful for investigations with rodents.
In this study, counts from 8:00 to 20:00 were lower than those from 20:00 to 8:00 and counts from 20:00 to 8:00 were significantly higher than those from 8:00 to 20:00 (p < 0.0001) in control rats. These results suggest that, in rats, the sleep phase is from 8:00 to 20:00 and the awake phase is 20:00 to 8:00. Xie et al. (2018) also showed wakefulness from 20:00 to 8:00 and slow-wave sleep from 8:00 to 20:00 using EEG and EMG. Thus, the actigraphy results are in line with those obtained with EEG and EMG.
Actigraphy has been developed for humans, and it has been applied to large animals, such as monkeys (Sri Kantha et al. 2009), sheep (Brutcher and Nader 2013), dogs (Barthélémy et al. 2011), and horses (Martin et al. 2010). On the other hand, it has been not applied to small animals such as mice and rats. The reason for this may be associated with the size of the actigraph. In this study, rats, not mice, were used to monitor ultradian cycles with actigraphy because of the size of the actigraph. However, the actigraph will become smaller with future technological advances. If the actigraphy device becomes smaller, it may also be used in mice, and, at that time, behavioral analysis should be performed with mice as well as with rats.
Difficulty inducing sleep and early awakening have been reported in patients with rhinitis (Hellgren et al. 2007; Bengtsson et al. 2017). In this study, we compared outcomes of actinograph in rats with and without allergic rhinitis, and showed the existence of difficulty inducing sleep, early awakening, and sleep disturbance in rats with allergic rhinitis. One of possibilities that can explain the discrepancies of actinography is allergic responses and symptoms. Although allergic rhinitis induces nasal obstruction, nasal obstruction increased the risk of insomnia (Sundbom et al. 2013). Nasal resistance by nasal obstruction has been shown to contribute to the upper airway resistance (Chirakalwasan and Ruxrungtham 2014). When there is an increase in nasal resistance, it decreases the air-flow through increase pressure surrounding the airway (Chirakalwasan and Ruxrungtham 2014). And allergic rhinitis and nasal obstruction has been reported to be associated with obstructive sleep apnea (Georgalas 2011; Chirakalwasan and Ruxrungtham 2014). There are also possibilities that allergic symptoms such as pruritus, sneezes, and nasal discharge cause sleep disturbance (difficulty with sleep induction, early awakening, etc) (Chang et al. 2014). Additionally, it has been reported that cytokine and inflammation are associated with sleep disturbance. Vgontzas et al. (2002) reported that the daytime increase of interleukin (IL)-6 and tumor necrosis factor secretion may explain difficulty with sleep induction. Lee et al. (2014) documented that cytokine polymorphisms associated with wake after sleep onset. Rubinstein (1995) reported that nasal inflammation plays a role in upper airway obstruction in obstructive sleep apnea. Sakami et al. (2002) also reported a link between insomnia and a shift toward dominance of T helper type 2 (Th2) immune response which induce allergic responses. And Gay et al. (2015) showed that sleep onset insomnia was associated with higher IL-13 plasma level, although IL-13, which is one of Th2 cytokines, is associated with allergic responses and symptoms (Miyahara et al. 2006; Gour and Wills-Karp 2015). Furthermore, IL-4, one of Th2 cytokines, inhibits spontaneous sleep in rabbits, (Kushikata et al. 1998) although IL-4 is strongly associated with allergy and allergic rhinitis (Gour and Wills-Karp 2015). These suggest that inflammation and cytokines, which are caused by allergic rhinitis, induce sleep disturbance. Therefore, it is important to investigate the association between allergic responses and sleep with rats with allergic rhinitis, because it is not easy to collect human nasal specimens.
Nasal steroids improved the subjective quality of sleep, and are reportedly useful for patients with mild obstructive sleep apnea (Georgalas 2011). Antihistamine drugs are often prescribed for patients with allergic rhinitis, although antihistamine drugs affect sleep (Thakkar 2011). It is therefore also important to examine the effects of these drugs on sleep. However, it is not easy to directly investigate the association between these drugs and sleep in humans. Such investigations are facilitated through the use of animals, especially rodents; experiments with allergic rhinitis rats using actigraphy may contribute to this end.
Allergic rhinitis reduces QOL (Craig et al. 2004), and sleep is strongly associated with QOL (Hoehle et al. 2017). Considering these, allergic rhinitis may lead to reduction of QOL, tiredness, fatigue, irritability, memory deficits, excessive daytime somnolence, and depression due to sleep disturbance as well as nasal symptoms.
ADHD has been reported in children with allergic rhinitis (Yang et al. 2016; Feng et al. 2017; Miyazaki et al. 2017). The counts in allergic rhinitis rats were significantly higher than those in control rats from 20:00 to 22:00 (p < 0.01), from 22:00 to 24:00 (p < 0.05), from 0:00 to 2:00 (p < 0.05), from 2:00 to 4:00 (p < 0.05), from 4:00 to 6:00 (p < 0.05), and from 6:00 to 8:00 (p < 0.01) in this study, indicating hyperactivity disorder during the daytime. Melamed and Heffron (2016) showed that ADHD and allergic rhinitis may have common mechanism and that nerve growth factor link allergic rhinitis to ADHD. Although there have been no clear evidences how allergic rhinitis induce hyperactivity disorder, this may be one possibility.
Many reports have shown hyperactivity disorder in 6-OHDA-lesioned rats (Heffner and Seiden 1982; Davids et al. 2002; Caballero et al. 2011). Heffner and Seiden (1982) reported that rats given 6-OHDA treatment displayed more than 6-fold increase in locomotor hyperactivity compared with controls on postnasal day 46. In the present study, hyperactivity disorder was also observed in 6-OHDA-lesioned rats using actigraphy. Additionally, actigraph patterns suggested difficulty with sleep induction in 6-OHDA-lesioned rats, which is consistent in part with the previous report, showing the presence of difficulty with sleep induction in ADHD (Hvolby 2015).
Here, we show the presence of difficulties inducing sleep, early awakening, and hyperactivity in rats with allergic rhinitis. Actigraphy also showed the existence of hyperactivity disorder and difficulties inducing sleep in 6-OHDA-lesioned rats. These findings provide insight into the mechanisms underlying sleep disturbance and hyperactivity disorder and may contribute to the development of new therapies for these conditions. However, our investigation was not sufficient to entirely uncover the implicated mechanisms, and further experiments with rodents using actigraphy are warranted.
The authors declare no conflict of interest.