2018 Volume 246 Issue 2 Pages 87-96
2018 Volume 246 Issue 2 Pages 87-96
MicroRNAs (miRNAs) are small noncoding RNA molecules that participate in normal B cell lineage development through posttranscriptional gene regulation. Antibody-mediated renal allograft rejection (ABMR) is emerging as one of the most common serious threats to renal transplant patients. In this study, we explored the role of miRNAs in the pathogenesis of ABMR. The differentially expressed miRNAs were identified by Affymetrix miRNA microarray analysis using B lymphocytes from 5 recipients and 5 volunteers. Based on quantitative RT-PCR, the expression levels of miR-107 were lower in the B lymphocytes from recipients than in those from volunteers. Computational analysis predicted that 3′-untranslated region of the autophagy-related protein 12 (ATG12) mRNA was targeted by miR-107, and we identified ATG12 as a target of miR-107 by Luciferase assay. Importantly, the expression levels of ATG12 in B lymphocytes of recipients were higher than those in the volunteer group, and miR-107 mimic significantly decreased ATG12 expression and formation of autolysosomes in B lymphocytes of recipients. Furthermore, we observed that levels of autophagy in B lymphocytes of transplant recipients were higher than those in B cells from volunteers. These findings suggest that miR-107 may contribute to the regulation of autophagy via targeting ATG12. Lastly, treatment with an miR-107 mimic caused the decrease in the secretion of IgG and IgM antibodies from B lymphocytes of transplant recipients, indicating that deregulated miR-107 could be involved in the pathogenesis of ABMR. Taken together, we propose that decreased miR-107 expression is associated with autophagy activation in B lymphocytes from patients with ABMR.
End-stage renal disease is a serious public health problem, and the global incidence rate continues to grow. Renal transplantation is the most important and most economical treatment for most patients with end-stage renal disease (Rodriguez Cubillo et al. 2016; Schaier et al. 2018; Yunhua et al. 2018). The continuous development of surgical techniques and the use of new immunosuppressive agents have significantly improved the short-term survival of renal transplant patients, but the long-term survival rate remains unsatisfactory. A large number of studies showed that antibody-mediated rejection (antibody-mediated renal allograft rejection, ABMR) is one of the most important mechanisms of rejection after renal transplantation (Drachenberg and Papadimitriou 2013; Zhao et al. 2017; Li et al. 2018). ABMR results in 30-50% of acute rejection episodes and more than 60% of late graft dysfunction. It is thought to be the leading cause of renal allograft rejection injury (Lachmann et al. 2017). Currently, ABMR prevention primarily involves inhibition of B cell maturation and elimination of antibodies in renal transplant patients, including via immunoadsorption, plasmapheresis and the use of rituximab, which is a chimeric mouse-human monoclonal antibody against the CD20 antigen on the surface of B lymphocytes (Pandey et al. 2013; Park et al. 2014; Kuhne et al. 2017). Nevertheless, these methods appear to be only partially or transiently successful and often lead to serious complications.
Autophagy degrades dysfunctional or unnecessary intracellular components, playing a critical role in protecting eukaryotic cells from various cell stresses (Chrisam et al. 2015). In this process, portions of cytoplasm components are sequestered into autophagosomes and delivered to lysosomes or vacuoles for degradation. Recent studies have demonstrated that autophagy plays a critical role in the regulation of the function of B cells (Alessandri et al. 2012; Li et al. 2014), and B cell activation strongly induces autophagy in the differentiation process of plasma cells (PC) in vitro (Pengo and Cenci 2013; Cenci 2014). Interestingly, autophagy generation was found not only in the differentiation process of plasma cells in vitro but also in one of bone marrow (BM) PCs in vivo (Pengo and Censi 2013). Despite the fact that autophagy is involved in the function of B lymphocytes, the mechanisms by which B cell activation induces autophagy require further clarification.
MicroRNAs (miRNAs) are a class of noncoding RNA (length, ~22 nt) that regulate gene expression at the post-transcriptional level. Accumulating evidence suggests that miRNAs not only play a key regulatory role in many biological processes but also participate in the regulation of autophagy, including inhibition of autophagy-related genes (Cecconi 2011; Fesler et al. 2017). In this study, we used a miRNA microarray to compare the expression profiles of cellular miRNAs between renal transplant recipients and volunteers, and found that the expression levels of miR-107 were significantly lower in renal transplantation patients than in healthy volunteers. To further explore the potential target proteins of miR-107, we utilized the bioinformatic tool TargetScan 7.2 and found that autophagy-related protein 12 (ATG12) was one of main targets. ATG12 contributes directly to the elongation of phagophores and to the maturation of autophagosomes; ATG12 is an important protein for regulation of autophagy (Ishibashi et al. 2011). Here, we hypothesized that decreased miR-107 expression is associated with autophagy activation via targeting of ATG12 in antibody-mediated renal allograft rejection.
Among all patients with ABMR, 19 patients who experienced renal transplant, including 11 males and 8 females, were selected from September 2015 to September 2017 in the Urology Department, Second Affiliated Hospital, Zhejiang University School of Medicine. Patients with infections based on clinical examination were excluded. Twenty healthy blood donors (12 males and 8 females selected from September 2016 to February 2018), who had no history of acute or chronic diseases, autoimmune diseases or treatment with immunosuppressive agents and had no microorganism infection within three months before blood collection, were selected as controls. The study was approved by the ethics committee of the Second Affiliated Hospital, Zhejiang University School of Medicine. After written informed consent was obtained, whole blood samples (10 ml) were collected from all participants.
Peripheral blood mononuclear cells were separated from blood samples by Ficoll-Paque density centrifugation (Biochrom, Berlin, Germany) at 2,500 r/min for 10 min at 4°C. Subsequently, B lymphocytes were enriched with a human CD19-positive selection kit (StemCell Technologies) in accordance with the manufacturer’s instructions.
Cell cultureB lymphocytes from blood samples, B cell lines Daudi and Raji from the cell bank at the Chinese Academy of Sciences, and HEK-293 human embryonic kidney cells were cultured in RPMI 1640 medium (Gibco, #11875-093) supplemented with 10% fetal bovine serum (FBS, Gibco, #10099-141) and 100 U/ml penicillin/streptomycin (ATCC® 302300™) in a humidified incubator containing 5% CO2 at 37°C.
Acridine orange and monodansylcadaverine stainingB lymphocytes were stained with acridine orange solution (1 mg/ml) or monodansylcadaverine (10 mM) from Sigma-Aldrich (China) for 10 min in RPMI 1640 medium with 10% FBS and 100 U/ml penicillin/streptomycin at 37°C. After being washed with phosphate buffered saline (PBS) 4 times, B lymphocytes were fixed in PBS with 3% paraformaldehyde for 30 min and analyzed by flow cytometry using a FACScan cytometer and CellQuest software.
Western blot analysisWestern blot analysis was performed following standard procedures. Antibodies against microtubule-associated protein 1 light chain 3 beta (MAP1LC3B) (L7543) is a central protein in the autophagy pathway where it functions in autophagy substrate selection and autophagosome biogenesis, and ATG12 (WH0009140) were obtained from Sigma-Aldrich in China (Mainland), and antibodies against β-actin (sc-10731) and sequestosome 1 (SQSTM1) (sc-28359) were obtained from Santa Cruz Biotechnology. SQSTM1 is a ubiquitin-binding protein that binds MAP1LC3B, thereby promoting autophagy by bringing SQSTM1-containing protein aggregates to the autophagosome for degradation.
Transmission electron microscopyB lymphocytes were harvested and processed as described previously (Zhang et al. 2015). Ultrathin sections were cut on a Reichert ultramicrotome, then stained with saturated uranyl acetate-lead citrate using standard procedures and examined on a Philips EM420 electron microscope.
RNA extraction and miRNA microarrayRNA was extracted from B lymphocytes with the miRNeasy Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. RNA yield and the A260/280 ratio were determined with a NanoDrop ND-1000 spectrometer (NanoDrop Technologies, Wilmington, DE), and RNA integrity numbers were measured with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Only RNA extracts with RNA integrity number values > 6 were included in further analyses. Differential expression analysis of miRNAs was performed using Affymetrix miRNA microarray technology (Feber et al. 2011; Smeets et al. 2011).
Quantitative RT-PCRQuantitative RT-PCR (qRT-PCR) analyses for miR-107 or ATG12 mRNA were performed with TaqMan miRNA assays (Ambion, #4440886) or PrimeScript RT-PCR kits (Takara, DRR037) in a Bio-Rad IQ5 (Bio-Rad Laboratories, Inc.). The reactions were performed using the following parameters: 95°C for 2 min followed by 40 cycles of 95°C for 15s and 60°C for 30s. RNU6-1 small nuclear RNA and β-actin mRNA were used as endogenous controls for data normalization. The primer sequences were as follows: ATG12, forward-5′-AGTAGAGCGAACACGAACCATCC-3′, reverse-5′-AAGGAGCAAAGGACTGATTCACATA-3′; and β-actin, forward-5′-TTCCTTCCTGGGCATGGAGTCC-3′, reverse-5′-TGGCGTACAGGTCTTTGCGG-3′. Relative expression levels were calculated using the comparative threshold cycle method.
Plasmid construction and luciferase assay experimentsThe pMIR-REPORT™ luciferase vector for the miR-107 target ATG12 was constructed according to the manufacturer’s instructions. The 3′-untranslated region (3'UTR) of ATG12 (195 bp) was amplified using cDNA from Daudi cells (primers for ATG12: forward-5′-ACTAGTTGTGATTTGTATTAA-3′, reverse-5′-AAGCTTCCCTTTAGTATA-3′). Another construct containing a mutated binding site was also generated as a control (TGCTGC to CATGTA). The amplified cDNA was digested with two restriction enzymes, HindIII (Takara, D1060) and SpeI (Takara, D1086), and ligated into the multiple cloning site of the luciferase vector (Ambion, AM5795). The resulting constructs were names pMIR-ATG12-wt and pMIR-ATG12-mut.
HEK-293 cells were cotransfected with 0.8 μg of firefly luciferase reporter vector (pMIR-ATG12-wt or pMIR-ATG12-mut), 0.04 μg of Renilla luciferase control vector (pRL-TK-Promega, #2241), and 100 nM miR-107 control (Ambion, #4464058), mimic (Ambion, #4464066), or inhibitor (Ambion, #4464084) with Lipofectamine 2000 (Invitrogen, #11668019). After transfection for 24 hours, luciferase activities were measured using the dual luciferase reporter assay system (Promega, E1910). The relative firefly luciferase activities were normalized against the Renilla luciferase activities.
Confocal microscopyB lymphocytes were cotransfected with miR-107 mimic, inhibitor or miRNA control (100 nM) along with the GFP-MAP1LC3B plasmid for 24 hours. A Radiance 2000 laser scanning confocal microscope was used to monitor the green fluorescence of GFP-MAP1LC3B formation assays, which has been used to monitor autophagy through indirect immunofluorescence or direct fluorescence microscopy, and the average number of MAP1LC3B puncta per cell (200 cells per sample) was determined by three different researchers.
Enzyme-linked immunosorbent assay (ELISA)The supernatants from in vitro culture systems were assessed with a DuoSet ELISA Development System (IgM, and IgG; R&D, Minneapolis, USA) according to the manufacturers’ instructions.
Statistical analysisStatistical analysis was performed with a two-tailed Student’s t test using SPSS 11.5 software. Differences were considered significant when the P value was less than 0.05.
To evaluate the role of miRNA in B lymphocyte function, we performed a screening of miRNA expression profiles of human peripheral blood B cell subpopulations using a miRNA microarray platform developed by Affymetrix. Among differentially expressed miRNAs, we identified 7 miRNAs (miR-107, miR-338-5p, miR-204, miR-142-3p, miR-140-3p, miR-486-5p and miR-155) whose expression changed more than 3-fold in B lymphocyte cells from recipients compared to those from volunteers (Fig. 1A). To confirm the microarray findings, the expression levels of miRNAs were detected with quantitative RT-PCR in the B lymphocytes of 19 recipient cases and 20 volunteers (Table 1), including the 10 specimens used in the microarray study. As expected, the expression levels of miR-142-3p, miR-140-3p, miR-486-5p and miR-155 were significantly higher in the recipient group than in the volunteer group, while the expression levels of miR-107, miR-338-5p and miR-204 were lower (Fig. 1B). The quantitative RT-PCR results were consistent with the microarray analysis results.

Differentially expressed miRNAs in the B lymphocytes of recipients and volunteers.
(A) Total RNA from the B lymphocytes of recipients and volunteers was used to perform the microarray assay. Hierarchical clustering analysis was conducted to show downregulated and upregulated miRNAs. Two subclasses, recipients (n = 5) and volunteers (n = 5), showed clustering results of 7 miRNAs that exhibited 3-fold changes in B lymphocytes. (B) Confirmation of microarray results by qRT-PCR. The expression levels of miR-107, miR-338-5p, miR-204, miR-142-3p, miR-140-3p, miR-486-5p and miR-155 were measured by qRT-PCR analysis in the B lymphocytes of recipients and volunteers. *P < 0.05.

Clinical characteristics of recipients and volunteers.
Data are presented as the mean ± SD.
BMI, body mass index; ABMR, antibody-mediated renal allograft rejection; HLA, human leukocyte antigen; eGFR, estimated glomerular filtration rate.
In a screen for miR-107 targets, ATG12 was identified as the putative miR-107 target gene using TargetScan (version 7.2, www.targetscan.org) (Fig. 2A). To prove that ATG12 is a target of miR-107, we generated two Luciferase report vectors that contain the putative miR-107 binding site within 3'UTR (pMIR-ATG12-wt) and mutant 3'UTR (pMIR-ATG12-mut). As shown in Fig. 2B, the relative luciferase activity was reduced by 70% following cotransfection with miR-107 mimic and pMIR-ATG12-wt compared with cotransfection with miR-107 control and pMIR-ATG12-wt. In contrast, no change of luciferase activity was observed in cells cotransfected with pMIR-ATG12-mut and miR-107 mimic. To confirm above results, an miR-107 mimic was transfected into B lymphocytes. As shown in Fig. 2C, ATG12 protein levels were lower with the miR-107 mimic than with the miR-107 control or inhibitor. Consistent with the inhibition of protein levels of ATG12, the mRNA levels of ATG12 also showed a 50% reduction with the miR-107 mimic (Fig. 2D). These data indicate that miR-107 decreased ATG12 expression levels probably through mRNA degradation.

ATG12 mRNA is a potential target of miR-107.
(A) Sequences of miR-107 and the potential binding site at the 3'UTR of ATG12. (B) Luciferase reporter assay. HEK293 cells were transiently cotransfected with the miR-107 mimic, inhibitor or control, together with luciferase reporter vectors (pMIR-ATG12-wt or pMIR-ATG12-mut). Luciferase activity was normalized to the activity of Renilla luciferase. (C and D) Western blot and qRT-PCR analyses of ATG12 expression in the B lymphocytes of recipients after transfection with miR-107 control, mimic or inhibitor. *P < 0.05.
To provide evidence that miR-107 negatively regulated autophagy, we examined the effect of miR-107on the formation of autolysosomes in the B lymphocytes of recipients after staining with AO or MDC. As shown in Fig. 3A, B, the miR-107 mimic caused significantly less formation of autolysosomes in the B lymphocytes of recipients compared with miR-107 inhibitor or the miRNA control, and the miR-107 inhibitor increased autolysosomes compared with the miRNA control. Consistent with this result, the ratio of microtubule-associated protein 1 light chain 3 beta-II (MAP1LC3B-II, the lipidated form of MAP1LC3B, which is attached to the autophagosome membrane) to β-actin was decreased by the miR-107 mimic in the B lymphocytes of transplant recipients (Fig. 3C). MAP1LC3B is initially synthesized in an unprocessed form, proMAP1LC3B, which is converted into a proteolytically processed form lacking amino acids from the C terminus, MAP1LC3B-I, and is finally modified into the PE-conjugated form, MAP1LC3B-II. In addition, the expression of SQSTM1 was increased by the miR-107 mimic in the B lymphocytes of transplant recipients. Furthermore, the miR-107 mimic decreased the number of GFP-MAP1LC3B puncta in the B lymphocytes of transplant recipients after transfection with the GFP-MAP1LC3B plasmids for 24 h (Fig. 3D) (P < 0.05). These results suggest that miR-107 exerts inhibitory effects on autophagy in the B lymphocytes of transplant recipients.

miR-107 exerts inhibitory effects on autophagy in the B lymphocytes of recipients.
(A and B) The quantification of autophagosomes in the B lymphocytes of recipients by acridine orange and MDC staining. The B lymphocytes of recipients were transfected with the miR-107 mimic, inhibitor or control at 100 nM for 48 h and then analyzed by flow cytometry. (C) Measurement of MAP1LC3B-II conversion, ATG12 and SQSTM1 expression in the B lymphocytes of recipients, after transfection with miRNA control, mimic or inhibitor. The cytosolic MAP1LC3B-I is conjugated to phosphatidylethanolamine (PE) in a ubiquitin-like reaction. This PE-conjugated to MAP1LC3B-I is known as MAP1LC3B-II, which serves as a specific marker for autophagy. (D) The number of GFP-MAP1LC3B puncta in each cell was counted using a confocal microscope after transfection with the miRNA control, mimic or inhibitor. *P < 0.05.
Autophagy plays an important role in B lymphocyte homeostasis and function (Pua and He 2009; McLeod et al. 2012), but the role of autophagy remains unclear in ABMR. To measure autophagy levels in B lymphocytes of recipient patients and healthy volunteers, we used flow cytometry to measure the formation of autolysosomes after staining with AO or MDC. Intriguingly, the percentage of autolysosome-accumulated cells in the B lymphocytes of recipients increased, to more than 70% of B lymphocytes, compared to that of volunteers according to the two staining assays (Fig. 4A, B). The ratio of MAP1LC3B-II to β-actin is an indicator of autophagy (Akin et al. 2014). As shown in Fig. 4C, the expression levels of autophagy protein MAP1LC3B-II and ATG12 of B lymphocytes in the recipient group were higher than those in the volunteer group, whereas the expression level of SQSTM1 protein (autophagy protein) in the ABMR group was lower than that in the volunteer group. The SQSTM1 protein serves as a link between MAP1LC3B and ubiquitinated substrates. SQSTM1 becomes incorporated into the completed autophagosome and is degraded in autolysosomes. Thus, activation of autophagy correlates with decreased levels of SQSTM1. Furthermore, the number of autophagosomes increased in the B lymphocyte of the recipient based on TEM analysis (Fig. 4D). These data indicate that autophagy was increased in the B lymphocytes of transplant recipients compared with those of volunteers.
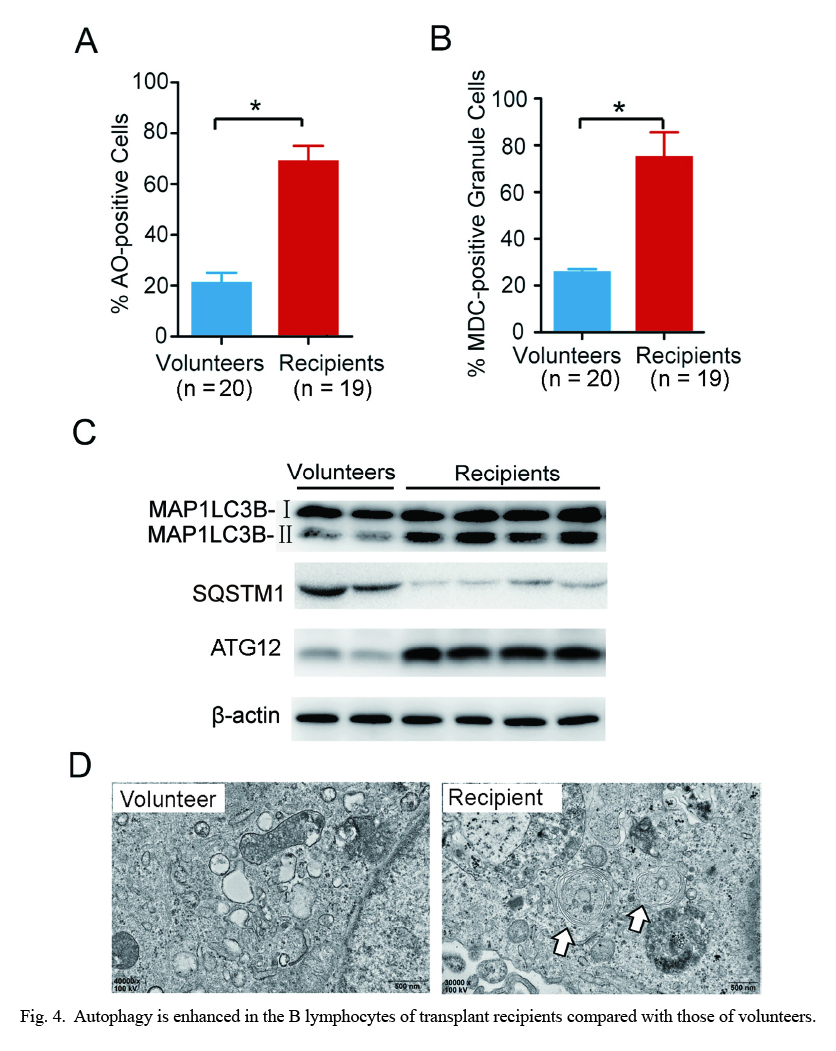
Autophagy is enhanced in the B lymphocytes of transplant recipients compared with those of volunteers.
(A and B) B lymphocytes were stained with acridine orange (AO, 1 mg/ml) or monodansylcadaverine (MDC, 10 mM) for 10 min and then immediately analyzed by flow cytometry. *P < 0.05. (C) Measurement of the MAP1LC3B-II conversion and SQSTM1 expression in B lymphocyte cells using Western blot analysis. (D) Representative TEM images of B lymphocyte cells. The white arrows indicate the autophagosomes.
To further study the role of miR-107 in the function of B lymphocytes, we measured immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies from B lymphocytes after transfection with the miR-107 mimic, control or inhibitor. B lymphocytes stimulated by antigens can differentiate into plasma cells, which can synthesize and secrete antibodies (immunoglobulins), including IgG and IgM antibodies. As shown in Fig. 5A, B, the miR-107 mimic significantly decreased the secretion of IgG and IgM antibodies in B lymphocytes from transplant recipients compared with the miR-107 control or inhibitor, and the miR-107 inhibitor increased the secretion of IgG and IgM antibodies compared with the miR-107 control. Similar results were observed in the B cell lines, Daudi and Raji (Fig. 5C-F). These results suggest that miR-107 may lead to the decrease in the production of IgG and IgM antibodies from B lymphocytes.

miR-107 causes the decrease in the secretion of IgG and IgM antibodies from B lymphocytes.
(A-F) Supernatant levels of immunoglobulins were detected by ELISA. B lymphocytes of recipients, Daudi and Raji cells were transfected with the miR-107 control, mimic or inhibitor for 72 h. *P < 0.05.
In this study, we found that microRNA-107 promoted antibody-mediated renal allograft rejection by targeting ATG12 in B lymphocytes. This novel model is supported by the following data: (1) the expression levels of miR-107 were lower in the B cells from transplant recipients than in those from volunteers, (2) miR-107 exerts inhibitory effects on autophagy in the B lymphocytes of transplant recipients by targeting ATG12, (3) autophagy was enhanced in the B lymphocytes of transplant recipients compared with those of volunteers and (4) miR-107 decreased the production of IgG and IgM antibodies from B lymphocytes.
Despite the fact that accumulating evidence demonstrates that autophagy is important both during B cell development and for efficient antibody secretion in healthy and diseased individuals, its role in antibody-mediated renal allograft rejection has not yet been reported. Here, we assessed whether the secretion of IgG and IgM antibodies from B lymphocytes was regulated by the inhibition of autophagy using miR-107 targeting ATG12, which can inhibit the autophagy. Remarkably, autophagy was increased in the B lymphocytes of recipients compared with those of volunteers. Consistent with this result, impaired antibody secretion in ATG5 (autophagy-related protein 5)-deficient B lymphocytes and autophagy regulated B cell function in several stages (Conway et al. 2013). These results indicate that autophagy regulates the function of B lymphocytes in ABMR.
In the present study, we found that miR-107 directly inhibited the expression of ATG12 and that miR-107 levels were lower in the recipient group than in the volunteer group, suggesting that the lower expression of miR-107 was the reason for autophagy enhancement in B lymphocytes from patients with ABMR. In addition, elevated miR-107 levels inhibited the production of antibodies from B lymphocytes. This may be the basis, at least in part, of B cell hyperactivity in patients with ABMR.
Studies of miRNA functions primarily focus on tumors and cancers, and there is limited knowledge regarding immune cell functions, especially in B lymphocytes (Ohyashiki et al. 2011; Haftmann et al. 2015). In the present study, in B lymphocytes from patients with ABMR, we chose miR-107, whose expression levels were lower than those of other miRNAs as determined by the miRNA microarray platform and qPCR analyses. Hence, we hypothesized that miR-107 plays an important role in B lymphocyte function. We found that miR-107 is a novel regulator of autophagy by targeting ATG12, a key autophagy-promoting protein, and that autophagy regulated B lymphocyte function in transplant recipients. Many miRNAs are also involved in regulating B cell function, including miR-338-5p, miR-204, miR-142-3p, miR-140-3p, miR-486-5p and miR-155 (Scian et al. 2011; Qayum et al. 2016). Vigorito et al. (2013) reported that a lack of miR-155 in B cells leads to a reduced germinal center (GC) response and not a secreted class-switched high-affinity IgG1 antibody. Other studies reported that miR-142 controlled B cell homeostasis by targeting the B cell-activating factor receptor, and miR-223 regulated naïve-to-GC B cell transitions and GC B cell-to-memory cell transitions (Yu et al. 2011; Kramer et al. 2015).
Overall, our study demonstrated that reduced miR-107 expression is associated with autophagy activation via targeting of ATG12 in antibody-mediated renal allograft rejection. Although the mechanism of ABMR remains to be determined, this study established a basis for future evaluations of the role of miR-107 in ABMR.
This study was supported by a grant from the National Natural Science Foundation of China (No. 81500571), the Natural Science Foundation of Zhejiang Province (No. LY14H050003 and LY18H050002), and the Public Welfare Project of Zhejiang Provincial Science and Technology Department (No. 2016C33154 and LGF18H160003).
The authors declare no conflict of interest.