2018 Volume 246 Issue 4 Pages 251-256
2018 Volume 246 Issue 4 Pages 251-256
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic vasculitis resulting in severe organ injuries. ANCA is a disease-labeled antibody of AAV, and myeloperoxidase (MPO) and proteinase 3 are the main targeted antigens of ANCA. Takotsubo syndrome, a transient cardiac dysfunction caused by emotional or physical stress, is characterized by ST-segment elevation and negative T waves in electrocardiogram, transient left ventricular asynergy, and absence of obstructive coronary disease. To the best of our knowledge, only two cases of coexistence of AAV and takotsubo syndrome have been reported. Herein, we report the case of AAV complicated with takotsubo syndrome. A 78-year-old Japanese woman presented with severe renal dysfunction, which was diagnosed as MPO-ANCA-associated systemic vasculitis. Despite the treatment with cyclophosphamide and glucocorticoid, the patient presented with severe respiratory failure due to alveolar hemorrhage and heart failure. Electrocardiography indicated newly developed T wave inversions. Echocardiography demonstrated severe left ventricular dysfunction with hypokinesis of the apical area. Moreover, coronary angiography revealed no noticeable stenotic or obstructive lesions. These findings indicate the onset of takotsubo syndrome. After immunosuppressive therapy, systemic vasculitis and takotsubo syndrome were improved. Although a coexisting case of AAV and takotsubo syndrome is rare, we have to consider the possible complication of takotsubo syndrome in case of presenting acute heart failure. Considering the present case and the previously reported coexisting cases of takotsubo syndrome and AAV, we propose that female sex, initiation of glucocorticoid therapy, and high titer of MPO-ANCA are potential risk factors of developing takotsubo syndrome.
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic inflammatory disease that develops systemic vasculitis, resulting in severe organ injuries (Jennette and Nachman 2017). ANCA is a disease-labeled antibody of AAV, and myeloperoxidase (MPO) and proteinase 3 are the main targeted antigens of ANCA (Jennette and Nachman 2017). AAV is categorized as three disease entities: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) (Jennette and Nachman 2017). MPA causes necrotizing crescentic glomerulonephritis, interstitial pneumonia, and alveolar hemorrhage, while GPA causes necrotizing multiple pulmonary nodules in addition to necrotizing crescentic glomerulonephritis (Jennette and Nachman 2017). Purpura and arthritis are also developed (Puéchal 2007). Furthermore, cholecystitis, gallbladder hemorrhage, hypertrophic pachymeningitis, and cerebral bleeding including subarachnoid hemorrhage have been reported as rare complications of AAV (Puéchal 2007; Ichinose et al. 2014; Decker et al. 2016; Kitaguchi et al. 2017). Coronary arteritis, pericarditis, and arrhythmia have been reported previously as the main cardiac complications in patients with AAV (Miloslavsky and Unizony 2014).
Takotsubo syndrome is a transient cardiac dysfunction caused by emotional or physical stress (Gianni et al. 2006). Takotsubo syndrome is characterized by the following findings: ST-segment elevation and negative T waves (T wave inversion) in electrocardiogram, transient hypokinesis, dyskinesis, or akinesis of the left ventricular mid-segments, with or without apical involvement, and absence of obstructive coronary disease (Gianni et al. 2006). Various pathogenic mechanisms of developing takotsubo syndrome have been reported previously. Although catecholamine-induced multivessel epicardial spasm (concerning sympathetic overactivity), microvascular coronary spasm, and direct catecholamine-mediated cardiomyocyte injury are thought to be the cause of takotsubo syndrome, its etiology and pathophysiology are still uncertain (Gianni et al. 2006; Miloslavsky and Unizony 2014).
Herein, we will describe the case of AAV that developed alveolar hemorrhage and takotsubo syndrome. To the best of our knowledge, only two cases of coexisting AAV and takotsubo syndrome have been reported (Sato et al. 2005; Tajima and Matsumoto 2006). Takotsubo syndrome may be rare but an important complication of patients with AAV.
A 78-year-old Japanese woman presented with severe fatigue and appetite loss and was later found to have severe renal dysfunction. The patient had no joint pains, skin lesion, leg edema, signs of neurologic impairment, or muscle weakness. The blood pressure was 157/58 mmHg. The main laboratory data are presented in Table 1. Urinary examination revealed urinary protein of 7.58 g/gCr, Hematuria was detected (more than 100 per high-power field), and granular and red blood cell casts were detected. Urinary β2 microglobulin was elevated at 48,900 μg/L, and N-acetyl-beta-D-glucosaminidase was elevated at 20.3 U/L. Blood examination revealed that renal function was severely deteriorated. Serum creatinine was 9.38 mg/dL, and blood urea nitrogen was 118.9 mg/dL. Hemoglobin level was 6.4 g/dL. Mild inflammation was detected (C-reactive protein 4.36 mg/dL). Then, MPO-ANCA was positive, with very high titers (> 300 U/mL). Anti-nuclear antibody, anti-double strand DNA antibody, proteinase 3-ANCA, and anti-GBM antibody were negative. Infiltration in the right lower lung lobe was detected by computed tomography, and bilateral kidney swelling was also detected. These findings indicated rapid progressive glomerulonephritis due to AAV. The patient was treated with intravenous cyclophosphamide (500 mg/body) and glucocorticoid (20 mg of daily oral dose of prednisolone). Because she had a history of pulmonary tuberculosis and underwent partial pulmonary resection, the initial dose of glucocorticoid was decreased. Her kidney dysfunction and systemic inflammation (C-reactive protein data) gradually improved after the start of the immunosuppressive therapy. However, 11 days from the start of the initial immunosuppressive therapy, the patient suddenly presented with wheezing and severe dyspnea. The blood pressure was 146/98 mmHg, heart rate was 107 beats/minute (sinus tachycardia), obvious coarse crackles were audible on bilateral lung area, heart sounds were regular, S3 or S4 was not audible, and slight systolic ejective murmur was audible. Chest radiography revealed extended left pulmonary infiltration, and electrocardiography indicated newly developed complete right bundle brock and T wave inversions of V2 to V6 (Fig. 1). Echocardiography demonstrated severe left ventricular dysfunction with hypokinesis of the apical area (Fig. 2). Creatine phosphokinase (normal range: 30-170 U/L) and brain natriuretic peptide (normal range: < 18.4 pg/mL) levels were 87 U/L and 379.5 pg/mL, respectively. Then, the respiratory condition rapidly deteriorated, which led to cardiac arrest. Fortunately, the patient was intubated, and the condition was restored; however, a large amount of fresh blood was detected in the intubation tube, indicating alveolar hemorrhage. Coronary angiography was performed to determine the cause of cardiac arrest, and no noticeable stenotic or obstructive lesions were found in the coronary arteries (Fig. 3). The patient was treated with intensive care and strong immunosuppressive therapy (1,000 mg of methylprednisolone administered intravenously for 3 consecutive days and one plasma exchange therapy). T wave inversion was gradually improved, and the cardiac function evaluated by echocardiography was improved in 2 weeks. At the time of admission, left ventricular ejection fraction (LVEF) was evaluated as 67%, but at the onset of takotsubo syndrome, the LVEF was 20%-25%. Then, 2 weeks from the onset of takotsubo syndrome, LVEF was improved to 63%. Although the patient initiated maintenance hemodialysis, the activity of AAV was well controlled. Accordingly, the patient was transferred to another hospital for rehabilitation.
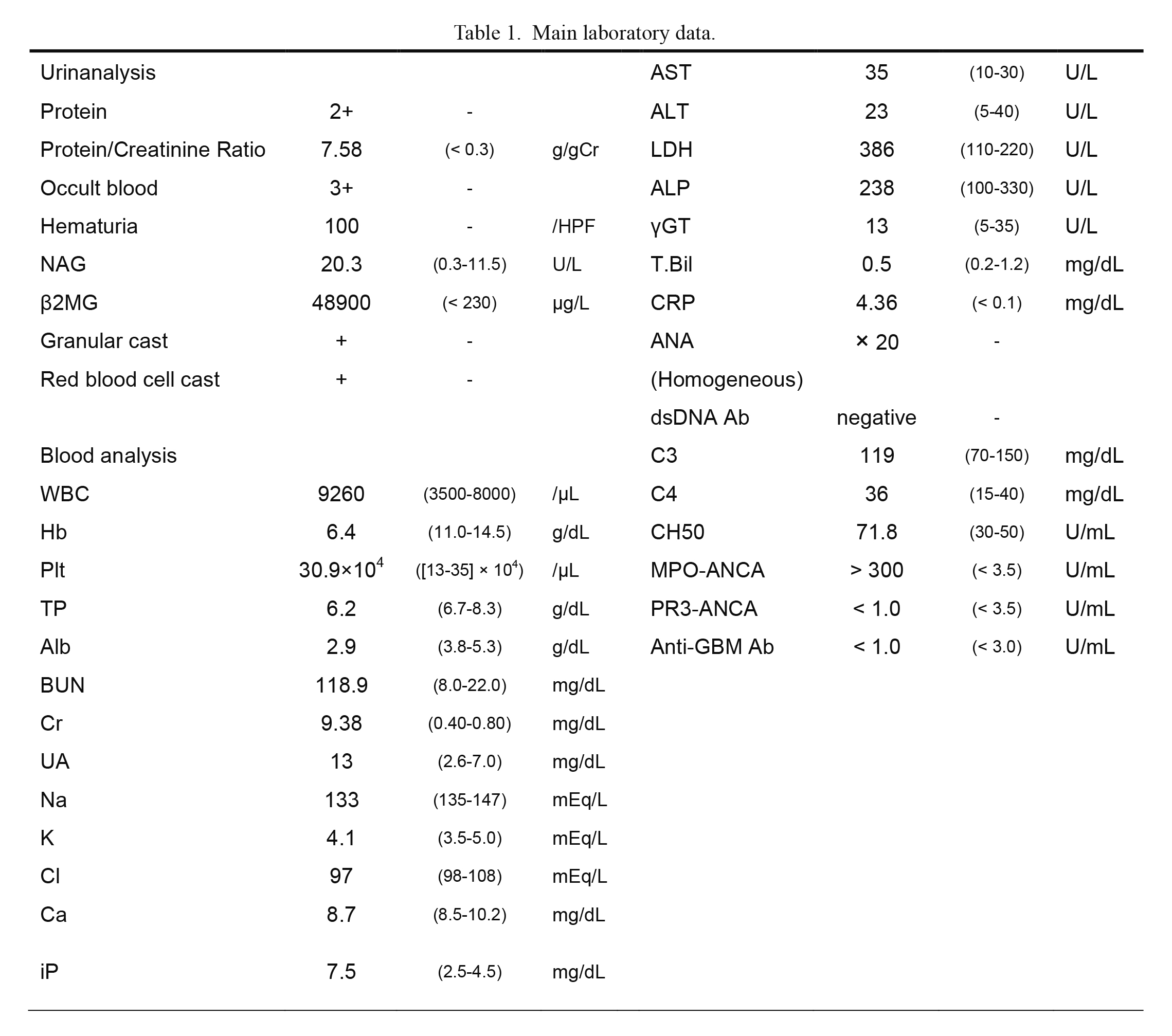
Main laboratory data.
Main laboratory data, normal range of the blood test in round brackets, and units are provided.
Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, anti-nuclear antibody; Anti-GBM antibody, anti-glomerular basement membrane antibody; AST, aspartate aminotransferase; BUN, blood urea nitrogen; C3, complement 3; C4, complement 4; Ca, calcium; CH50, complement hemolytic activity assay; Cl, chloride; Cr, creatinine; CRP, C-reactive protein; dsDNA ab, anti-double strand DNA antibody; g/gCr, gram/gram creatinine; Hb, hemoglobin; iP, inorganic phosphorus; K, potassium; LDH, lactate dehydrogenase; MPO-ANCA, myeloperoxidase-anti-neutrophil cytoplasmic antibody; Na, sodium; NAG, N-acetyl-β-D-glucosaminidase; Plt, platelet; PR3-ANCA, proteinase 3-anti-neutrophil cytoplasmic antibody; T.Bil, total bilirubin; UA, uric acid; WBC, white blood cell; β2MG, beta-2-microglobulin; γGT, gamma-glutamyl transpeptidase.
Conversion factors for units: Cr in mg/dL to μmol/L, × 88.4; BUN in mg/dL to mmol/L, × 0.357.

Changes in electrocardiographic (ECG) findings in the clinical course of the current case.
(A) ECG findings at admission. Slight T wave inversions are detected in II, III, aVF, V4, and V5. (B) ECG findings at the onset of takotsubo syndrome (11 days from the start of the initial immunosuppressive therapy). Complete right bundle brock and T wave inversions in I, aVL, and V2 to V6, are detected. Newly appeared representative T wave inversions in V2 to V6 are encircled. (C) ECG findings after 9 days from the onset of takotsubo syndrome. Large negative T waves are detected in I, II, III, aVF, and V3 to V6. Representative large T wave inversions in V3 to V6 are encircled. (D) ECG findings after 8 weeks from the onset of takotsubo syndrome. T wave inversions in V2 and V3 are improved and T wave inversions in V4 to V6 are getting smaller.
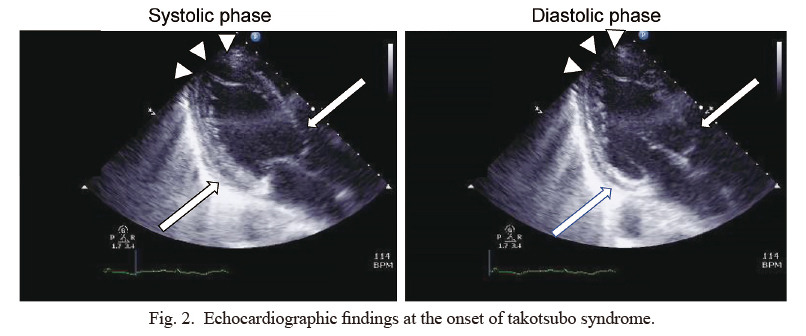
Echocardiographic findings at the onset of takotsubo syndrome.
Echocardiography demonstrates severe left ventricular dysfunction with hypokinesis of the apical area (arrowheads), while contraction of the cardiac base is preserved (arrows). Visual ejection fraction rate is almost 20%-25%. There is no pericardial effusion or any abnormalities in her valves.

Coronary angiography.
There is no noticeable stenosis or obstructive lesion in the left main trunk, left anterior descending coronary artery, and left circumflex coronary artery, and right coronary artery.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Coexisting case of AAV and takotsubo syndrome is rare. Thus far, there are only two previous case reports (Sato et al. 2005; Tajima and Matsumoto 2006). We present the clinical course of these cases in Table 2 (including the current case). All coexisting cases of both diseases developed in elderly and female patients, and all cases were positive of MPO-ANCA with high titers. In addition, takotsubo syndrome developed after the start of the initial immunosuppressive therapy, and all cases were treated with glucocorticoids. However, the mechanism of takotsubo syndrome occurrence after the start of the therapy is still unknown, and several hypotheses have been reported. Previous reports indicated the association between steroid hormone and takotsubo syndrome (Placci et al. 2015; Campean et al. 2016). The case reported by Placci et al. (2015) demonstrated that anabolic steroid abuse may cause takotsubo syndrome in a young male patient, and the other case reported by Campean et al. (2016) presented that fludrocortisone overdose for Addison’s disease may cause takotsubo-like syndrome. Takotsubo syndrome is developed in situations of strong emotional or physical stress. These AAV cases had presented severe physical condition. The case reported by Tajima and Matsumoto (2006) presented posterior reversible encephalopathy syndrome. In addition, the current case presented severe respiratory failure due to alveolar hemorrhage. Because the timing of developing takotsubo syndrome and alveolar hemorrhage is almost the same, alveolar hemorrhage may be associated with the development of takotsubo syndrome. As Seitz et al. (2012) reported that takotsubo syndrome developed after a massive hemoptysis due to cystic fibrosis, massive pulmonary bleeding may be a severe physical stress. In addition, the timing of the occurrence of takotsubo syndrome in the current case was later than in previous reports. One of the reasons of the late onset of takotsubo syndrome in the presented patient compared with the other two cases is alveolar hemorrhage, as alveolar hemorrhage might trigger the occurrence of takotsubo syndrome. Regarding emotional stress, this was the patient’s first hospital admission. Therefore, we are unable to exclude the possibility that the patient was emotionally stressed due to hospital admission.
As for the relationships between other autoimmune diseases and takotsubo syndrome, two cases of coexisting systemic lupus erythematosus and takotsubo syndrome (Lim 2008; Shim et al. 2013) and one case of coexisting systemic sclerosis and takotsubo syndrome have been reported (Melchiorre et al. 2008). Takotsubo syndrome complicated with autoimmune diseases is thought to be a rare condition.
As for heart involvement in AAV, EGPA was reported to cause cardiac complications in 15%-60% of cases (Miloslavsky and Unizony 2014). In addition to coronary arteritis, the most common cardiac manifestations are pericarditis and cardiomyopathy. These cardiac complications in EGPA often lead to severe condition. Heart involvement is less observed in patients with GPA and MPA than in patients with EGPA. The most common manifestations of GPA and MPA are pericarditis and arrhythmias, while myocarditis and coronary arteritis are less frequent (Miloslavsky and Unizony 2014). Because one of the previously considered pathophysiological mechanisms of developing takotsubo syndrome is microvascular coronary spasm (Gianni et al. 2006; Miloslavsky and Unizony 2014), AAV might cause microvascular inflammation, resulting in takotsubo syndrome.
In AAV cases, respiratory failure often developed because of various conditions. Various pulmonary diseases (such as alveolar hemorrhage, interstitial pneumonia, and bacterial and fungal pneumonia), heart failure, and excess body fluids due to renal failure often occur and cause dyspnea. Therefore, heart failure may be possibly due to takotsubo syndrome that is not appropriately diagnosed. Although whether there is a direct association between AAV and takotsubo syndrome is uncertain, when heart failure symptoms appear during the therapeutic course of AAV, we have to consider the possible complication of takotsubo syndrome. In particular, female sex, high titer of MPO-ANCA, and glucocorticoid therapy initiation are risk factors of developing takotsubo syndrome.

Coexisting cases of anti-neutrophil cytoplasmic antibody-associated vasculitis and takotsubo syndrome.
ANCA, anti-neutrophil cytoplasmic antibody; F, female; IVCY, intravenous cyclophosphamide; mPSL, methylprednisolone; MPO, myeloperoxidase; PRES, posterior reversible encephalopathy syndrome; PSL, prednisolone.
The authors declare no conflict of interest.