2019 年 248 巻 3 号 p. 143-150
2019 年 248 巻 3 号 p. 143-150
Indium is mainly used as indium-tin oxide (ITO), which has a unique character of transparency, and is a requisite in making liquid crystal displays. Pulmonary toxicity of indium compounds in humans were not recognized until the last 2 decades. Several initial human cases of indium-related lung disease, named indium lung, were reported in Japan, with their main pathologic findings being interstitial pneumonia, emphysema and cholesterol crystals-containing granulomas. In 2010, three cases with alveolar proteinosis were reported from the United States and China. As of March 2019, more than 10 cases of interstitial pneumonia-dominant indium lung have been reported. Cross-sectional studies in indium workers indicate that the serum indium concentration (sIn) is closely related to the exposure period, the extent of interstitial as well as emphysematous changes of the lung on high-resolution computed tomography (HRCT) and serum biomarkers of interstitial pneumonia, including KL-6 and surfactant protein-D (SP-D). Longitudinal studies have shown it is possible to reduce the sIn as well as the interstitial shadows on HRCT; however, emphysematous lesions increased progressively in heavily exposed workers, even after cessation of exposure. Early detection is required to prevent irreversible changes. The first case of lung cancer associated with indium lung developed in a nonsmoking ex-worker. He had been diagnosed with indium lung and stopped working in indium processing 17 years before. This suggested there is a need for appropriate screening to detect for complications of lung cancer at early stages for those with indium lung.
Indium is mainly used as indium-tin oxide (ITO), a mixture of 90% indium oxide (In2O3) and 10% tin oxide (SnO2). Its production has been increasing over the past two decades as the primary use of ITO is for flat panel displays, such as liquid crystal displays (LCD). ITO plates, called targets, are used as a source of transparent membranous electrodes “sputtered” on the surface of the flat panel displays. Fig. 1 illustrates the manufacturing process of an ITO target, which consists of many processes, including mixing of In2O3 and SnO2, and sintering and surface grinding of the ITO plates, most of which could generate respirable particles containing In2O3 and/or ITO.
The pulmonary toxicity of indium compounds inhaled or instilled into the trachea has been reported in animal experiments (Blazka et al. 1994; Uemura et al. 1997; Tanaka et al. 2002); however, it was not until 2003 that the first case of severe interstitial pneumonia was reported in a worker who had been engaged in surface grinding of ITO targets for three years (Homma et al. 2003; Fig. 2). Since then, there have been several case reports (Homma et al. 2005; Taguchi and Chonan 2006; Nakano et al. 2007; Takeuchi 2008; Chonan et al. 2010) and two reviews on indium lung (Tanaka et al. 2010; Omae et al. 2011). In this current review we aimed to clarify: 1) the typical clinicopathological features of indium lung initially reported in Japan, and compare them with those reported from the United States of America and China; 2) the results of the longitudinal, as well as cross-sectional studies carried out in indium processing workers, examining the potential effects of improvements in the working environment; and 3) the possible carcinogenicity of indium compounds in humans. In addition, the review will examine the progress of research on indium lung, in general.

Manufacturing process of indium-tin oxide (ITO) targets.
Every step could generate respirable particles of indium oxide or ITO.
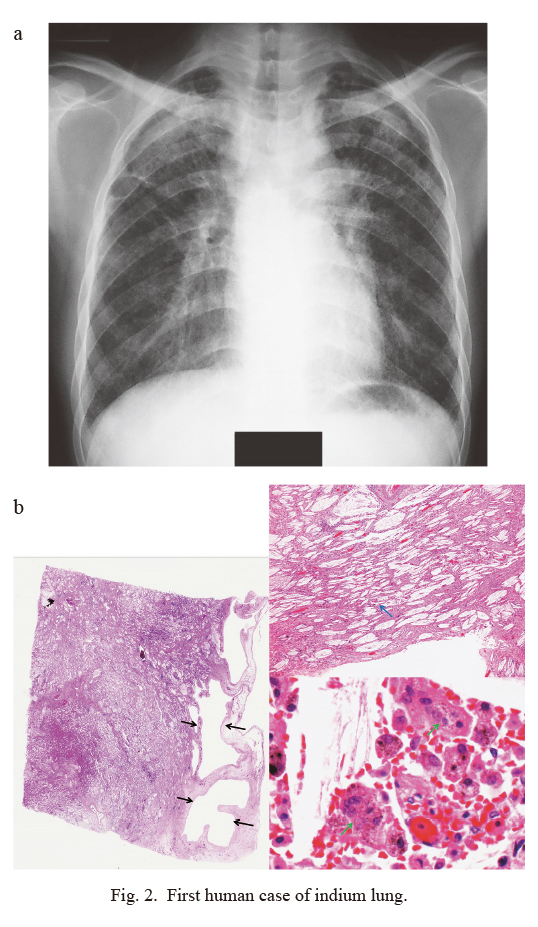
First human case of indium lung.
a) Chest radiograph.
Severe interstitial pneumonia was diagnosed in a 27-year-old male who had been engaged in surface grinding of ITO targets for three years (Homma et al. 2003). The film shows diffuse reticulonodular shadows with ground glass-like opacity in the right upper and lower lung fields.
b) Autopsy specimen.
Affected alveolar areas are replaced by fibrosis and associated with thick-walled bullae (left, black arrows, panoramic view) and cholesterol granulomas (right upper, blue arrow, HE, × 20). Brownish-colored particles are engulfed by macrophages and giant cells (right lower, green arrows, HE, × 800).
The first case of indium lung described was followed by reports of several milder cases of interstitial pneumonia and/or pulmonary fibrosis (IP/PF), all from Japan (Homma et al. 2003; Homma et al. 2005; Taguchi and Chonan 2006). Serum indium levels (sIn) were increased in all these cases (Normal levels: 0.06 ± 0.03 ng/mL, Mean ± SD), accompanied by an increase in the serum biomarker of interstitial pneumonia, Krebs von den Lungen-6 (KL-6; Kohno et al. 1989). Furthermore, all the cases showed pathological findings of cholesterol granulomas, which are comprised of macrophages, giant cells and cholesterol crystals, with the latter presumably derived from the denatured surfactant and macrophages. It was postulated that alveolar macrophages, which have engulfed cholesterol crystals, formed the multinucleated giant cells and cholesterol granulomas that are characteristic of, although not specific to, indium lung. Fine, brownish colored particles, probably containing indium, were also observed in the macrophages and giant cells found in most of the cases. Figs. 3, 4 and 5 show high-resolution computed tomography (HRCT) images and pathological findings obtained from three different patients.
The common features of these cases include 1) occupational history of handling indium compounds, 2) increase in sIn, 3) interstitial (and emphysematous) changes of the lung on HRCT, 4) pathological confirmation of cholesterol (crystals-containing) granulomas and particles engulfed by alveolar macrophages and giant cells, 5) elevation of serum biomarker of interstitial pneumonia including KL-6 and SP-D, and 6) decrease in diffusing capacity of the lung for carbon monoxide. Table 1 shows the characteristics of the indium lung compared to the well-known pneumoconioses, silicosis and asbestosis. Based on these findings it was hypothesized that inhaled indium could cause a new interstitial pulmonary disease, indium lung.
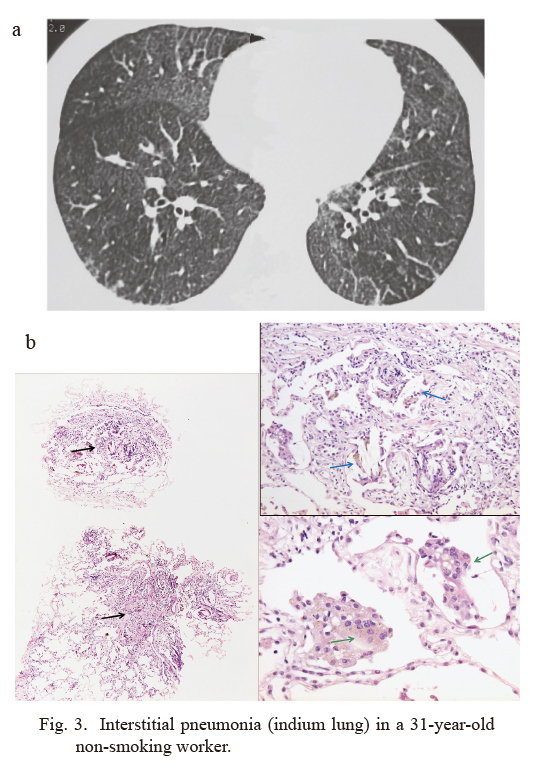
Interstitial pneumonia (indium lung) in a 31-year-old non-smoking worker.
a) High-resolution computed tomography (HRCT) image.
This worker had been engaged in indium processing for 12 years (Taguchi and Chonan 2006). The images show diffuse fine reticulonodular or ground glass-like shadows, i.e. interstitial shadows, in the lower lung fields.
DLCO (diffusing capacity of the lung for carbon monoxide) = 77% predicted. Serum indium concentration (sIn) = 40 ng/mL. KL-6 = 1,930 U/mL. See text for normal values.
b) Trans-bronchial lung biopsy (TBLB) specimen.
Patchy spots of fibrosis (left, black arrows, HE, × 40) with multiple cholesterol granulomas in fibrosis (right upper, blue arrows, HE, × 200) are present. Many brownish colored particles may be noted in giant cells (right lower, green arrows, HE, × 400)

Interstitial pneumonia (indium lung) in a 39-year-old ex-smoker.
a) HRCT images of upper and lower lung fields.
This worker had been engaged in indium processing for 12 years (Taguchi and Chonan 2006). Image shows diffuse fine reticulonodular shadows with small low attenuation areas suggestive of emphysematous changes. This worker had an 18 pack∙year smoking history. sIn = 127 ng/mL, KL-6 = 3,570 U/mL.
b) Specimen obtained by Trans-bronchial lung biopsy (TBLB).
TBLB specimen contains many cholesterol granulomas (blue arrows, HE, × 200) with infiltration of inflammatory cells.

Chronological changes in a case of indium lung: from interstitial to emphysematous lesions.
a) HRCT taken at three levels (upper, middle, and lower lung fields).
These are the data of a non-smoking patient with indium lung, who had worked as a surface grinder of ITO targets from 1992 to 2000 (Taguchi and Chonan 2006). The images, taken in 2002 when the patient was aged 28 years (left), are characterized by widely distributed interstitial shadows, i.e., ground glass-like opacity (GGO), and reticulonodular shadows whereas in the images of 2012 (right) low attenuation areas and bullae are prevalent with little GGO. Between 2002 and 2012 the values of sIn, DLCO, and KL-6 changed from 98.8 to 58.2 ng/mL, from 78% to 49% predicted, and from 1,190 to 639 U/mL, respectively.
b) Lung specimen obtained by surgery for a left relapsing pneumothorax.
Left panoramic view shows bullae (red arrows), emphysema, and patchy nodular lesions (black arrows) and the repaired site for the rupture of bullae (triangle, HE). The nodular lesions are mainly composed of cholesterol granulomas (right upper, blue arrow, HE, × 200), and brownish colored particles are noted (iron and suspected indium, right lower, green arrows, HE × 800) in giant cells.

Characteristics of indium lung as compared to silicosis and asbestosis.
Confronted with the development of a severe interstitial pneumonia in an ITO worker, Chonan et al. (2007), as industrial physicians, carried out a cross-sectional study in 108 male indium workers in the factory where the five cases, i.e., the first human case with indium lung and the subsequently reported four cases originated (Homma et al. 2003; Homma et al. 2005; Taguchi and Chonan 2006). They found interstitial and emphysematous changes in 23 (21%) and 14 (13%) workers on HRCT, respectively. Notably, serum KL-6 levels were also abnormally high (> 500 U/mL) in 40 (37%) workers. Those workers with serum indium concentrations in the highest quartile had significantly longer exposure periods, greater HRCT changes, lower diffusing capacity of the lung for carbon monoxide and higher KL-6 levels compared with those in the lowest quartile. Hamaguchi et al. (2008) also obtained similar results in a study of four ITO manufacturing or recycling plants in Japan different from the one examined by Chonan et al. (2007). By combining the data of Chonan et al. (2007), Hamaguchi et al. (2008), and other eight indium handling factories, Nakano et al. (2009) analysed data from more than five hundred workers, who were either currently or formerly exposed to indium. They found dose-dependent increases in serum KL-6, surfactant protein (SP)-D, and SP-A which strongly correlated with the increase in serum indium levels which strengthened the relationship between exposure to indium and the pulmonary effects observed. Nakano et al. (2009) also proposed a cut-off value for serum indium of 3 ng/ml to prevent early effects on the lungs, and this recommendation has been included in a technical guideline issued by the Japanese Ministry of Health, Labour and Welfare (MHLW) (2010). Based on the case reports and the cross-sectional epidemiologic studies, the concept of indium lung has been delineated. Table 2 describes the chronology regarding indium lung in Japan.

Chronology of investigation of indium lung in Japan.
In 2010, there were two case reports from the US (Cummings et al. 2010) and one from China (Xiao et al. 2010) on indium-related lung disease; however, distinct from the Japanese cases, the main pathological findings from these cases was pulmonary alveolar proteinosis (PAP). Moreover, serum autoantibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) was elevated in one of the US cases, and the authors suggested inhaled ITO might induce PAP through an autoimmune mechanism. This is consistent with the findings that autoimmune diseases are sometimes linked with occupational dust exposure, such as the association between silica exposure and rheumatoid arthritis (Costabel and Nakata 2010).
Surfactant lipids and proteins are synthesized, stored, and secreted into the alveoli by type II alveolar epithelial cells, 70-80% of which is cleared by reuptake into alveolar type II cells for reuse or catabolism, with the remainder being catabolized by alveolar macrophages. GM-CFS is necessary for the maturation of alveolar macrophages that digest 30% of the surfactant protein in the alveoli (Trapnell et al. 2003); therefore, macrophage immaturity could cause an imbalance between the production and the catabolism of surfactants. Accumulation of surfactants could then result in PAP and the formation of cholesterol crystals. To confirm this hypothesis, 17 ITO workers from the Japanese cases who had elevated levels of both indium and KL-6 in their serum were assessed for antibodies against GM-CSF; however, they were not detected (Masuko et al. 2011). Therefore, it was concluded that inhalation of ITO was unlikely to induce GM-CSF autoantibodies, although it is possible that ITO causes PAP through dysfunction of alveolar macrophages by another mechanism, such as heavy exposure swamping the capacity of macrophages to digest the surfactants.
Until 2010, there had been reports on eight cases of mainly IP/PF from Japan, and three cases of mainly PAP from the US and China. A workshop was held in 2010 by the National Institute for Occupational Safety and Health (NIOSH) to explore common and different features of the two groups of indium-related lung disease (Cummings et al. 2012). Common characteristics were symptoms comprising of cough, dyspnea and occasionally clubbed fingers, the histopathological feature of cholesterol crystal-containing granulomas, and the presence of fine particles in alveolar macrophages.
The diagnostic latency, i.e., the time from the first exposure to diagnosis, was 4-13 years for IP/PF and 1-2 years for PAP, which in both cases was relatively short compared with classic pneumoconiosis. As US patients had a shorter diagnostic latency, this may suggest PAP cases were subject to earlier and heavier pathological events.
In many cases of IP/PF, there were localized PAP-like findings, histopathologically characterised by the accumulation of periodic acid-Schiff-positive materials in alveoli; alternatively, in two cases of PAP, progression to fibrosis was radiologically diagnosed over several years. It was hypothesized that the disease caused by indium may begin with PAP and then progress to fibrosis, and emphysema in some cases (Cummings et al. 2012).
The possibility exists that the three cases reported from the US and China may not be typical cases of indium lung (Cummings et al. 2010; Xiao et al. 2010). The blood indium level was undetectable in the second case of the US workers, which might suggest the possibility of idiopathic PAP, rather than an ITO related form. For the Case 1 US worker, the blood indium was not available, although the existence of indium in the lung was assessed by scanning electron microscope and energy dispersive X-ray analysis on a biopsied specimen. The Chinese case appeared to have been exposed to massive amounts of silica, which could have caused secondary PAP (Trapnell et al. 2003). On the other hand, 2 additional cases that were reported by the NIOSH group at the workshop probably had the features of IP reported from Japan, which may suggest that the IP/PF pattern is more prevalent than the PAP pattern.
In 2011, the results of chronic inhalation toxicity studies in rats and mice were published, where low concentrations (0.01-0.1 mg/m3) of ITO were inhaled for two and 13 weeks (Nagano et al. 2011a). Similar studies were also extended to 104 weeks in carcinogenicity and chronic toxicity studies (Nagano et al. 2011b). In addition to carcinogenesis in rats in these studies, non-neoplastic lesions, including alveolar proteinosis, infiltration of alveolar macrophages and inflammatory cells were observed, which was followed by alveolar fibrosis (Nagano et al. 2011a, b). These results are consistent with the hypothesis proposed at the workshop, and it is plausible that PAP plays some role in the pathogenesis of indium lung; however, the process of progression from PAP to IP/PF is not yet clear. Further research is needed to determine whether these 2 pathologic changes represent different stages in the same spectrum of disease or result from different mechanisms.
Faced with the report of the first human case of indium lung, the MHLW announced provisional regulations on inhaled indium levels in 2004. After issuing risk communications and recruiting public comments, the MHLW issued a technical guideline for preventing health hazards for ITO workers (MHLW 2010). In the guidelines the target level of environmental indium was set at 0.01 mg/m3, provided that a high-quality dust respirator was used, even though an acceptable concentration based on the animal carcinogenicity studies (Nagano et al. 2011a, b) was considered to be 3 × 10−4 mg/m3. Employers are required to provide ITO workers with a medical examination at the start of employment and for every six months thereafter. The medical examination includes a questionnaire on respiratory symptoms, measurement of serum indium and KL-6 concentrations and a computed tomography scan (CT) of the lungs. Restrictions on working can be implemented, following the advice of a doctor, when blood indium is ≥ 3 ng/mL or KL-6 is ≥ 500 U/mL and associated with symptoms, or there is an abnormality caused by indium in radiological or functional tests. In 2013 MHLW enacted the rules and restrictions regarding workers handling indium, with obligations for employers based on the guidelines.
Since the report of the first case, the working environment has been improved by a combined effort of industry, government and researchers. Indium concentrations in the air at the factory where the first victim occurred have decreased from 0.01-0.02 mg/m3 (Geometric Mean) in 2001 to 0.0001-0.0012 mg/m3 in 2016.
From the beginning of the cross-sectional studies, the researchers have been aware of the need for longitudinal studies, since the natural history and the long-term effects of environmental improvements were not known for this peculiar disease. Amata et al. (2015) conducted longitudinal surveillance of 84 indium workers for nine consecutive years in the factory where the first cases of indium lung had been reported. In association with an improvement in the work environment and work practices, serum indium concentration(sIn), KL-6, and SP-D levels decreased significantly. Notably, the biological half-life of sIn was estimated to be 8.09 years. Interstitial lesions assessed by HRCT had also regressed partially, whereas emphysematous lesions increased progressively in workers with high sIn values; the progression of emphysematous changes was not related to their smoking history. Consistent with these results, the annual reduction rate of FEV1/FVC (forced expiratory volume in one second/forced vital capacity) was greater in heavily exposed workers, as compared with that in lightly exposed workers.
Nakano et al. (2014) reported similar results in a five-year longitudinal cohort study in which progression of emphysematous changes was observed among those with sIn levels higher than 20 ng/mL, especially smokers. It appeared that inhaled indium compounds could cause chronic pulmonary inflammation involving alveolar macrophages and inflammatory cells, leading to paracicatricial, paraseptal or bullous emphysema (Honma 1999), and smoking facilitated the formation of emphysematous lesions. It is uncertain whether PAP could cause emphysema.
Carcinogenicity of inhaled indium phosphide (National Toxicology Program 2001) and ITO (Nagano et al. 2011b) has been reported in chronically exposed rats; however, carcinogenicity in humans is unclear. Amata et al. (2019) have recently described the development of a lung adenocarcinoma in a 46-year-old, non-smoking, former indium worker, who was transferred to a non-indium section 16 years ago, having an IP/PF type of indium lung, and had been examined once or twice a year. He visited the authors’ clinic complaining of left chest discomfort, and a large, 32 × 30 mm diameter nodule was found by chest X-ray and CT in the left upper lung. Video-assisted thoracoscopy revealed many small nodules on the pleura, and together with lung biopsy from the left lower lobe, pleural dissemination from the primary cancer in the left upper lobe of the lung was diagnosed. The progression of carcinoma may have occurred in the sub-pleural fibrotic zone, such as reported in cases of lung cancer complicated with the usual interstitial pneumonia (Watanabe et al. 2017). In considering the pathogenesis of lung cancer in this case, it should be noted that serum KL-6 and sIn were elevated in the 17 years since the patient stopped working in indium processing, decreasing very slowly, which suggests that elevated pulmonary inflammation may have facilitated the carcinogenesis. An early detection strategy against newly developed lung cancer is needed for indium workers, especially for those who have been heavily exposed.
The difference between the two main pathological findings of indium lung, those that have mainly an IP/PF pattern and are Japanese in origin and those with an alveolar proteinosis pattern found in the US cases, has been discussed already. However, the role of smoking in the pathogenesis of emphysematous lesions has yet to be explored more extensively. Since there are non-smoker cases of indium lung with emphysema, it is clear that inhaled indium compounds can evoke emphysema alone; however, the interaction between indium and smoking in generating emphysema is unclear. It is plausible that smoking and indium interact in the formation of emphysema, and so this issue is important in maintaining the health of indium workers. Even without evidence for a link, a strong recommendation to quit smoking should probably be implemented.
The case reports as well as the results of both cross-sectional and longitudinal studies suggest that inhaled indium compounds could be retained in the lungs for more than ten years. Small particles of indium are likely processed by macrophages, and then gradually dissolved in the blood and excreted in the urine. Some part of the inhaled indium may also be expectorated by the mucociliary transport system of the airway. While in the lungs, indium compounds could evoke interstitial and emphysematous changes, and also lung cancer; however, the longer time course (> 15 years) of these adverse effects is not known. In addition, distribution and elimination rates of inhaled indium compounds have not been reported in humans.
In modern industry, where an old substance rather than a totally novel one is used in a different manner, unexpected adverse effects sometimes occur. As industrial physicians, we have to be aware of novel work-related diseases brought about by historically used substances, such as indium.
The authors appreciate Dr. Akira Hebisawa’s valuable comments on the pathology. The authors also thank Dr. Takefumi Saito for allowing us to access the pathological data of one of the patients, and Dr. Yuki Yabuuchi for his clinical contributions.
This study was supported in part by MHLW (No. 180303).
The authors declare no conflict of interest.