2019 Volume 248 Issue 4 Pages 273-284
2019 Volume 248 Issue 4 Pages 273-284
Lamotrigine, a frequently used antiepileptic drug, inhibits voltage-gated sodium-channels. By suppressing the release of glutamate and aspartate, lamotrigine acts as a membrane stabilizer, and it is also effective in bipolar disorder and migraine. However, lamotrigine is known to induce tremor among 4-10% of patients. We examined the lamotrigine-induced tremor in 28 epilepsy patients (age: 38.06 ± 13.56 years; 24 females and 4 males) receiving lamotrigine monotherapy and compared the data to 30 age- and sex-matched controls (age: 33.06 ± 10.71 years; 25 females and 5 males). Tremor was visually assessed by clinical tremor rating scales. Quantitative characteristics (intensity, center frequency and frequency dispersion) which are regularly used to differentiate various tremor syndromes were measured by validated, sensitive biaxial accelerometry in resting, postural and intentional positions. Regularity of repetitive finger and hand movements and reaction time were also determined. Data were statistically analyzed. Clinical tremor rating scales detected pathological tremor in three patients (10%), while accelerometry revealed tremor in seven patients (25%). Center frequency of patients with pathological tremor was similar to controls, but the frequency dispersion was significantly lower and tremor intensity was significantly higher in both postural and intentional positions. Rhythmic movements and reaction time were normal. Our results show that objective measurements detect pathological intention tremor in 25% of epilepsy patients receiving lamotrigine monotherapy. Quantitative characteristics suggest the involvement of the cerebellum in the pathomechanism of lamotrigine-induced tremor. Determining the parameters of drug-induced tremor syndromes might help to understand the complex action of tremor generator networks.
Lamotrigine (LTG) is one of the most widely used antiepileptics due to its effectiveness in partial-onset seizures, generalized tonic-clonic seizures, bipolar disorders, migraine, and Rett syndrome. It is the best tolerated and it has the longest retention rate among 17 antiepileptic drugs (AEDs) both in mono- and polytherapy settings (Alsfouk 2018). LTG is known to cause tremor in 4-10% of patients (Morgan and Sethi 2005), which is often reversible on the withdrawal of the drug (Perucca and Meador 2005; Yang et al. 2010). LTG may cause disabling tremor when co-administered with valproic acid (VPA) (Reutens et al. 1993). Other hyperkinetic disorders like chorea (Zesiewicz et al. 2006), myoclonus (Zaccara et al. 2004), and blepharospasm (Verma et al. 1999) were also observed in LTG-treated patients.
Lamotrigine mainly acts on voltage-dependent sodium channels inhibiting presynaptic glutamate release (Kuo and Lu 1997; Köhling 2002), but it also influences serotonergic (Vinod and Subhash 2002; Goldsmith et al. 2003), dopaminergic (Ahmad et al. 2005), and gamma-amino-butyric acid (GABA)-ergic (Cunningham and Jones 2000) receptors. Although tremor might be associated with brain lesions causing symptomatic epilepsy or antiepileptic surgery (Lee et al. 2014), the tremorogenic effect of LTG is present even in patients with bipolar disorder (Calabrese et al. 1999) and Rett syndrome (Stenbom et al. 1998) treated with LTG monotherapy. LTG is, therefore, likely to influence tremor genesis independently from the epileptic activity, but the pathomechanism of lamotrigine-induced tremor is unclear (Yang et al. 2010).
It has been shown that the pathomechanism of essential tremor (ET) is partly associated with abnormal function of the GABAergic neurotransmitter system (Helmich et al. 2013). It is therefore not surprising that drugs which increase GABAergic transmission are effective in treating ET. Besides ethanol, several antiepileptics are used in the treatment of ET, including topiramate, gabapentin (Deuschl et al. 2011) and levetiracetam (Bushara et al. 2005). VPA also increases GABAergic mechanisms, however it induces pathological tremor more frequently than other anticonvulsants (Alonso-Juarez et al. 2017). Moreover, VPA is associated with drug-induced Parkinsonism (Sasso et al. 1994; Brugger et al. 2016; Morgan et al. 2017). Antiepileptics which mainly inhibit sodium channels without significant GABAergic effect like carbamazepine, phenytoin, and lamotrigine, might also cause tremor, but the data on their tremorogenic action are controversial. Phenytoin is known to cause hyperkinetic movements including tremor (Duarte et al. 1996) even at non-toxic serum levels (Liihdorf and Lund 1977). Although carbamazepine is effective in treating cerebellar tremor (Sechi et al. 1989), pathological tremor is a common side effect of long-term carbamazepine medication (Koliqi et al. 2015).
Despite the extensive research, there are no conclusive data on how antiepileptic drugs induce tremor. The fact that AEDs acting on both GABAergic and glutamatergic systems induce tremor raises the possibility that there might be a neurochemical imbalance rather than a specific GABAergic deficit or glutamatergic excess which could drive the system into pathological synchronization. Alternative mechanisms of drug induced tremor also cannot be excluded. A recent report showed that in patients with bipolar disorder after 12-week of lamotrigine monotherapy the volume of the cerebellum decreased (Bauer et al. 2018). Cerebellar signs and symptoms, like ataxia, nystagmus, vertigo, as LTG side-effects were also noted in epilepsy patients (Moreira et al. 2007; Thome-Souza et al. 2012). These findings raise the possibility that lamotrigine-induced tremor might be a result of cerebellar dysfunction.
The cerebellum, which contains 60-80% of all human neurons (Herculano-Houzel 2009), represents an important hub in the human central nervous system. Its anterior lobe receives spinocerebellar afferents and thus, it acts as a predictor, adjuster, and timekeeper of executed movements. The posterior lobe has connections with several cortical associative areas like the cingulate and parahippocampal gyri, the frontal, parietal, and temporal cortices. Via these connections the cerebellum is involved in cognitive-emotional functions and its lesions might produce cognitive-affective symptoms. Lesions of the anterior lobe might cause motor cerebellar signs like limb ataxia and tremor, which illustrate the deterioration of correct timing and impaired prediction of consecutive movements.
The cerebellum is the key structure of the tremor network as all tremor syndromes have been linked to the cerebellum to various extent (Elble 2000). Focal lesions of the cerebellum might cause intentional (Bhatia et al. 2018) and postural (Holmes 1922) tremor. In animal experiments (Flament and Hore 1988) and human studies (Kovács et al. 2019), cerebellar lesions caused most frequently low-frequency tremor, but high-frequency tremor was also described (Cole et al. 1988; Milanov 2001; Kovács et al. 2019). In ET, loss of Purkinje cells and pathological recurrent axon collaterals were detected (Louis 2016), and neuroimaging (Cerasa and Quattrone 2016), physiological (Filip et al. 2016) as well as clinical data (Benito-Leon and Labiano-Fontcuberta 2016; Bhatia et al. 2018) also proved the involvement of the cerebellum. Increased cerebellar activation was detected during Parkinsonian rest tremor (Helmich et al. 2012; Wu and Hallett 2013). These findings suggest the dysfunction of the cerebellar network in neurodegenerative tremor syndromes.
According to the literature, the cerebellum acts as a time keeper at frequencies both lower and higher than 2 Hz (Spencer and Ivry 2013). The cerebellum is known to cause disorders of timing and thus cerebellar dysfunction might impair regularity of alternating hand movements and/or finger tapping.
The aim of our study was to measure the objective neurophysiologic characteristics (intensity, center frequency, frequency dispersion) of tremor and the regularity of fine repetitive hand and finger movements in epilepsy patients receiving lamotrigine monotherapy. Determining the quantitative parameters of LTG-induced tremor might help to understand its pathomechanism and might contribute to the elucidation of pathological tremor generator networks.
We have examined 30 epilepsy patients (26 females and 4 males) taking lamotrigine monotherapy and 30 age- and sex-matched healthy controls (25 females and 5 males). Two female patients had to be excluded; one due to slight mental retardation and the other because of pathological tremor which occurred prior to LTG-treatment and was probably related to 20-year-long phenytoin therapy. Control subjects had no history of any neurological disorder and did not take any medication regularly. Epilepsy patients on lamotrigine monotherapy were randomly selected from the Epilepsy Outpatient Unit of the National Institute of Clinical Neurosciences, Budapest, between March and October 2018. We used the following exclusion criteria: lamotrigine treatment for less than 5 years if the patient was treated earlier with any AEDs known to cause tremor (e.g., phenytoin, valproic acid); any other medication known to affect tremor (beta-blockers, benzodiazepines, lithium); signs of upper motor neuron lesion; signs of Parkinsonism; previous history of any kind of tremor; previous brain surgery; disabling hand conditions (rheumatoid arthritis, traumatic injuries, hand operations); elevated serum lamotrigine level within one year prior to the examination (normal range: 12-55 µmol/L); structural abnormalities detected by brain MRI affecting the motor or the sensory cortex, the thalamic nuclei, the basal ganglia, the cerebellum, or the mesencephalon; Mini Mental State Examination (MMSE) score lower than 24; abnormal serum thyroid-stimulating hormone (TSH), serum ammonia and vitamin B12 levels; abnormal hematological panel.
Patients were examined by an expert neurologist (A. Kovács), and the following data were collected: age, handedness, duration of LTG treatment, daily dose of LTG, serum LTG level at the time of assessment, frequency of seizure/year, duration of epilepsy disorder, type of epilepsy, magnetic resonance imaging (MRI) findings, previously taken AEDs, other drugs taken by the patient, history of tremor, quality of life related to tremor, any brain or hand surgery/injury in the past, any psychiatric co-morbidities, and playing musical instruments or professional dancing carrier in the past. Vital parameters (heart rate, blood pressure, O2 saturation) were measured. Blood pressure and heart rate directly do not influence tremor recording outcomes, however, high blood pressure and elevated heart rate could point to elevated catecholamine levels which might cause enhanced physiologic tremor. TSH, vitamin B12, ammonia levels and routine hematological panel were also assessed. MMSE was performed before tremor recording. All patients underwent a routine neurological examination.
The study was performed according to the Declaration of Helsinki. The experimental procedure was approved by the Ethical Committee of the National Institute of Clinical Neurosciences. All individuals gave written informed consent.
Tremor recordingTremor is a sinusoidal, rhythmic involuntary movement. Physiologic tremor is present in all human beings. It consists of central and peripheral components like mechanical-reflex oscillations, which are influenced by inertia, and stiffness (Elble 1996). The amplitude of the physiologic tremor is hardly noticeable. It is known that stress might increase the amplitude of physiologic tremor that is called enhanced physiologic tremor.
For clinical tremor rating we used part A of the Fahn-Tolosa-Marin (FTM) scale because it is recommended by the Movement Disorders Society, and it is suitable for the assessment of both postural and kinetic/intentional tremor (Elble et al. 2013). FTM scale estimates rest and kinetic tremor intensity of the limbs on a 0-4 scale (Fahn et al. 1988). However, estimation of tremor intensity itself is not sufficient for the differentiation of physiologic and pathological tremor. Pathological tremor has to be characterized on the basis of rhythmicity (Elble and McNames 2016), and thus objective quantitative tremor analysis is necessary.
There are various approaches for objective analysis of human tremor; however, the optimal protocols for evaluating various tremor syndromes have not been elaborated yet (Elble and McNames 2016). The most common methods are electromyography (EMG), accelerometry, gyroscope recordings, and laser-based measurements. Accelerometry has been used for 50 years in human motion analysis (Elble and McNames 2016), and it has proved to be a simple, reliable and convenient technique (Grimaldi and Manto 2010) providing a precise linear measure of tremor, in contrast to subjective, non-linear clinical ratings. There are three main types of accelerometers: a) piezoelectric; b) piezoresistive and c) capacitive (Elble and McNames 2016). The suitability of an accelerometer depends on its technical properties and on the amplitude and frequency characteristics of the tremor (Elble and McNames 2016). Accelerometers record a combination of linear acceleration, gravity and additive noise (Luinge and Veltink 2005). Unlike gyroscopes, they are not sensitive to rotational acceleration. One of the limitations of accelerometry is that it might record gravitational artifacts. If a uniaxial accelerometer is mounted at the axis of the rotation, its output will be entirely gravitational artifact. Therefore, bi- or triaxial accelerometers are recommended and they should be mounted on the same body part of all patients to measure inertial acceleration free of gravitational artifact. Accelerometry has been used for decades together with EMG, because EMG can capture burst duration and the pattern of muscle contraction which might help in differentiating Parkinsonian, ET, and dystonic tremor syndromes (Grimaldi and Manto 2010). Nowadays, applying new mathematical algorithms in data analysis, accelerometry became a valid method for diagnosing and differentiating various tremor syndromes (Papapetropoulos et al. 2010; Wastensson et al. 2013; di Biase et al. 2017).
Accelerometric recordings provide tremor time series which might be mathematically analyzed in several ways. The most commonly used method is fast-Fourier transformation. The Fourier theory states that any periodic signal can be represented as the weighted sum of sines and cosines of different frequencies. The Fourier transform mathematically decomposes a signal into sines and cosines. The amplitudes of sine and cosine waves give the amplitude of oscillation at the given frequency. Amplitude plotted against frequency results in power spectrum (Elble and McNames 2016). Analysis of the characteristics of the power spectrum of tremor, such as center frequency, peak frequency, and frequency dispersion, helps in distinguishing physiological and pathological tremor types. The frequency of physiologic tremor is 8-12 Hz (Elble 1996). Pathological tremors usually have lower frequencies; however, the characteristic frequency values of various tremor syndromes might overlap (Bhatia et al. 2018). As noted above, pathological tremor has to be determined on the basis of rhythmicity (Elble and McNames 2016). Pathologically increased rhythmicity usually results in narrow spectral peak with a half-bandwidth of 2 Hz or less (Elble and McNames 2016).
In the present study, we used accelerometry because it is the most frequently applied feasible, valid, and sensitive tremor recording method (Elble and McNames 2016). Our Coordination-Tremor-Balance Test System (CATSYS), CATSYS 2000 biaxial tremor recording system (Danish Product Development Ltd., Snekkersten, Denmark), is validated, sensitive (Edwards et al. 1997; Papapetropoulos et al. 2010), and standardized (Després et al. 2000) and provides all data that are necessary to differentiate physiologic and pathological tremors (Edwards et al. 1997; Papapetropoulos et al. 2010).
Accelerometry was carried out in a soundproof room, where subjects were seated comfortably. Data were collected in the morning within 2-4 hours after patients took their regular lamotrigine medication. Subjects were asked to refrain from drinking coffee and/or alcohol 24 hours before the examination, since these are known to affect tremor, motor performance and alertness. Subjects were asked to have breakfast, because hunger might cause stress and thus, enhanced physiologic tremor. Subjects were instructed to have a minimum of 6 hours sleep before the examination as this is required for maintained alertness. Shorter sleep might affect reaction time.
Tremor was recorded for 20 s in four different positions: (1) at rest (rest tremor; RT): the forearm and the hand were fully supported on a table; (2) in postural position with open eyes: the arm and hand were held against gravity in an outstretched, horizontal, prone position with eyes open; (3) in postural position with closed eyes (postural tremor; PT): the arm and hand were held against gravity in an outstretched, horizontal, prone position with eyes closed; (4) in a static precision task (intention tremor; IT): the patient with his index finger pointed to the tip of an arrow, stable on the screen. Tremor at rest and in postural position was recorded simultaneously on the right and left sides, while in intentional position registrations were carried out consecutively.
Tremor was registered using a biaxial micro-accelerometer (weight: 10.5 g, sensitivity: > 0.3 m/s2), fixed between the second and the third metacarpal bone, 2 cm proximal to the metacarpophalangeal joint as it has been described previously (Farkas et al. 2010). Accelerometry signals of the two axes were digitized at 128 Hz. Data between 0.9 and 25 Hz were analyzed.
Regularity and maximum frequency of repetitive finger- and alternating hand movements were examined using a touch sensitive drum as previously described (Farkas et al. 2010). For finger tapping measurements, subjects hit the drum with the index finger while the wrist was supported. Alternating hand movements were investigated with the drum on the table. Subjects were instructed to perform pronation/supination movements for 10 s, keeping precise pace with the 2.5 Hz acoustic signal generated by the computer. The time offset (ms) between the signal and the subject’s beat was measured. Moreover, subjects were instructed to perform finger tapping or hand pronation/supination paced by gradually accelerating (from 1.6 to 7.5 Hz) acoustic signal. To compensate for random errors, the subject was allowed to miss one hit if the next two were recorded. The last legal hit determined the maximum frequency. This principle distinguishes well between controlled and chaotic movements.
Simple motor reaction time (ms) was measured in a traditional stimulus-response test using a handle switch. The computer gave random auditory signals to which the subject had to press the handle switch with the thumb. Reaction times shorter than 0.1 s or longer than 0.5 s were excluded.
Outcome measuresTremor parameters were derived from fast-Fourier power spectra. The following parameters described and used in previous studies (Després et al. 2000; Farkas et al. 2010) were automatically calculated by the built-in software of our CATSYS tremor recording system: (1) Tremor intensity (TI, m/s2), which is related to tremor amplitude, was calculated as the root-mean square of acceleration (Farkas et al. 2010). (2) Frequency dispersion (FD, Hz) which reflects the regularity of tremor was defined as the half width of the frequency band centered on the peak frequency containing 68% of the total power. Frequency dispersion of physiologic tremor was broad (3-4 Hz), while it was reduced (0.5-1 Hz) in pathological tremors like Parkinsonian or essential tremor (Farkas et al. 2006). (3) Center frequency (CF, Hz) reflects the frequency below which lays 50% of the power in the spectrum. CATSYS provides this parameter, because a computer-based system must use a clearly defined algorithm for calculation of tremor frequency characteristics in all subjects (Edwards and Beuter 1999). However, when there is no apparent peak frequency, or the spectrum is bi- or trimodal, the calculated CF may lie at a frequency without much power, unrelated to any oscillatory component of the signal (Farkas et al. 2006). In our study, controls and patients with physiologic tremor had bimodal spectra with a low frequency peak (2-3 Hz) and a high frequency peak (7-8 Hz), whereas patients with pathological tremor had unimodal spectra with a single peak about 7-8 Hz and low frequency dispersion. Therefore, among patients with pathological tremor, peak frequency and center frequency were overlapping.
Regularity of repetitive movements was quantified by the standard deviation of the time-offset values (tap-to-cue variability, ms). Maximum frequency of repetitive movements was defined as the last legal hit paced by the gradually accelerating signal (Hz). Reaction time was calculated as an average value of ten trials of the simple stimulus-response test (ms).
Definition of pathological tremorTremor frequency itself does not differentiate tremor syndromes since tremor with different etiology might have the same frequency (Bhatia et al. 2018; Kovács et al. 2019). Tremor intensity is high in enhanced physiologic tremor; thus, intensity itself cannot be used to define pathological tremor. It has been shown that low frequency dispersion is a sensitive parameter of pathological tremor (Beuter et al. 2005; Farkas et al. 2006; Kovács et al. 2019). Therefore, we considered the tremor pathological if frequency dispersion was a low outlier from the control group. It was calculated according to the following formula: FD q25 – (q75 – q25) × 1.5 where q75 represents the upper quartile, q25 the lower quartile and q75 – q25 the interquartile range. Based on this formula, resting tremor was considered pathological if FD was lower than 3.82 Hz, postural tremor if FD was lower than 2.63 Hz and intentional tremor if FD was lower than 2.52 Hz (Table 1). Normal range for tremor intensity and frequency, alternating hand movement, finger tapping and reaction time was calculated the same way. Maximum frequency of pronation-supination and finger tapping was considered pathological if it was a low outlier, whereas regularity of alternating hand movement or finger tapping and reaction time were pathological if they were high outliers (higher than q75 + (q75 – q25) × 1.5). Threshold values for each parameter are presented in Table 1.
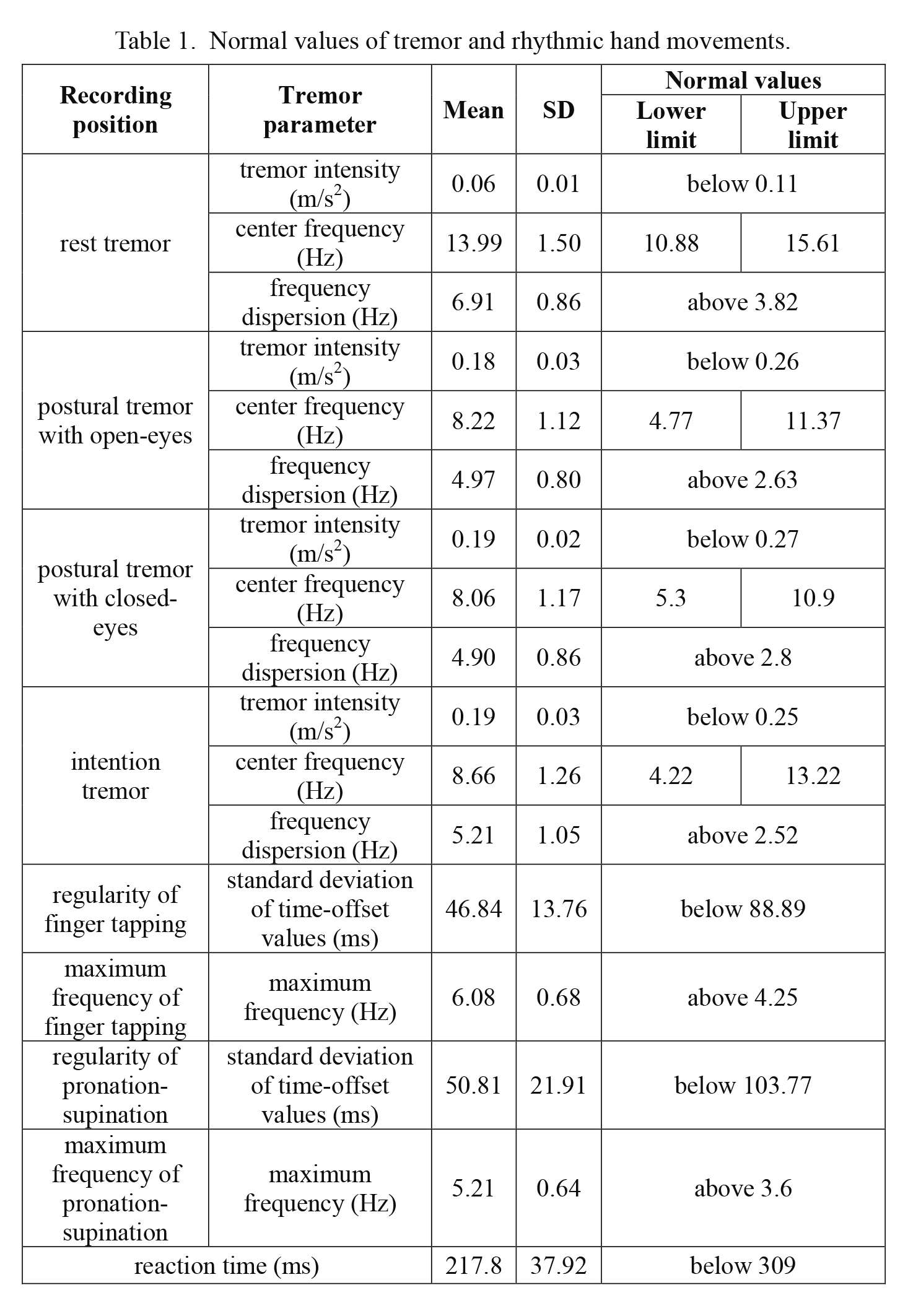
Normal values of tremor and rhythmic hand movements.
Calculations were based on the data of the 30 healthy controls. Data of dominant hand are shown.
Statistical analysis was performed using the Statistica software package (Statsoft Inc., version 8.0, Tulsa, OK, USA). Due to small sample size, the distribution of data was controlled using Shapiro-Wilks test. Parametric and non-parametric tests were used. To compare tremor parameters in patients with tremor, without tremor and controls, ANOVA and Kruskal-Wallis tests were used. To study group differences, Scheffé’s or Dunn’s post-hoc tests were applied. To study the correlation between lamotrigine serum level and tremor parameters, as well as between age and tremor parameters, Pearson-test was carried out. We applied logistic regression to test the factors that might increase the risk of developing pathological tremor. The alpha level to determine significance was 0.05.
We have included 28 patients (38.06 ± 13.56 years; 24 females (85.7%) and 4 males (14.3%)) and 30 age- and sex-matched controls (33.06 ± 10.71 years; 25 females (83.3%) and 5 males 16.7%)) into the study. In the patient group 25/28 and in the control cohort 26/30 subjects were right-handed. We measured the parameters on both hands, but because there was no difference between the data of the dominant and non-dominant sides, the results of the dominant hands are presented. Clinical characteristics of patients are presented in Table 2.
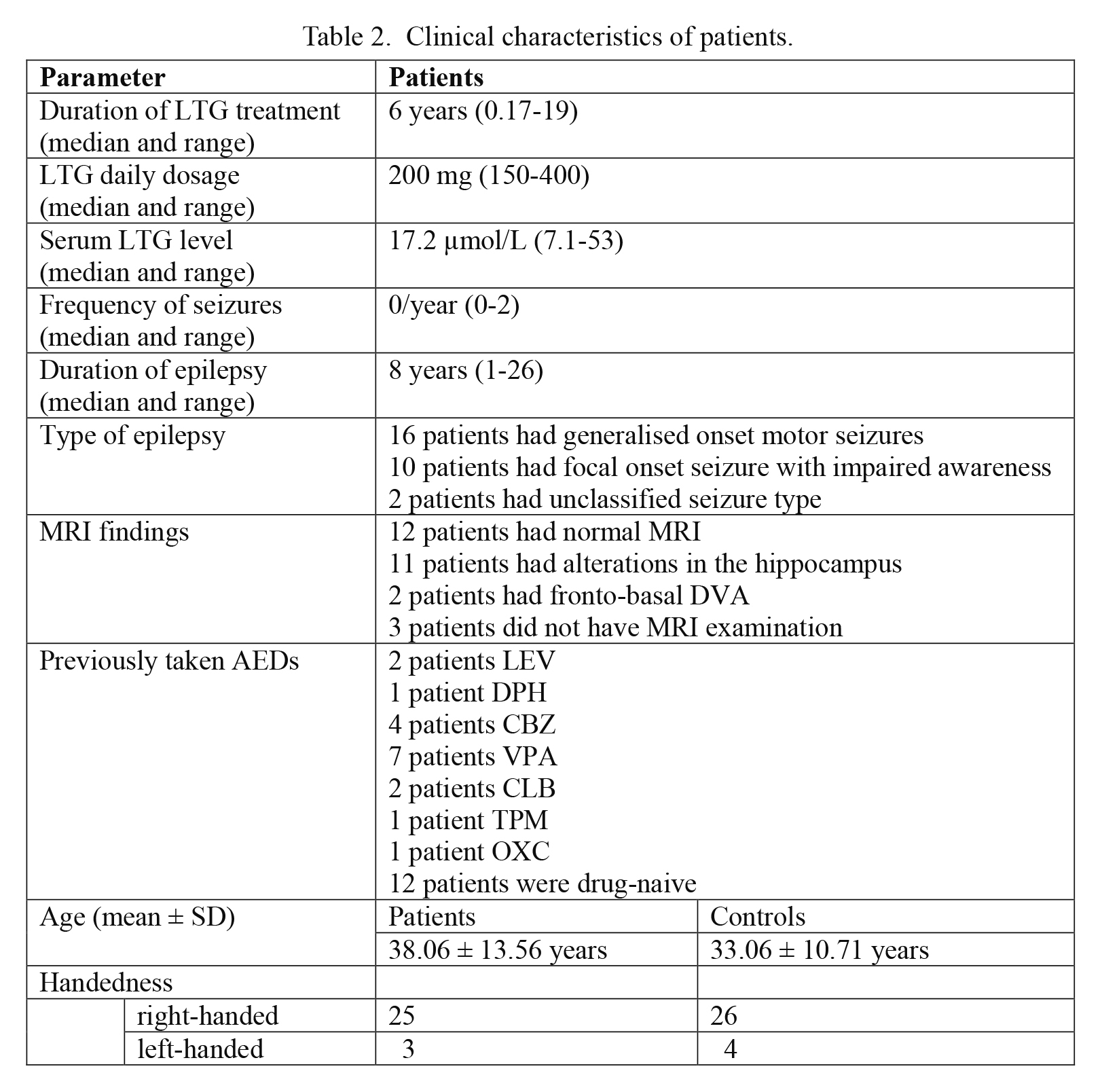
Clinical characteristics of patients.
LTG, lamotrigine; AEDs, antiepileptic drugs; LEV, levetiracetam; DPH, phenytoin; CBZ, carbamazepin; VPA, valproic acid; CLB, clobazam; TPM, topiramate; OXC, oxcarbazepine.
Ten patients (35.71%; 8 females and 2 males) out of 28 reported occasional upper limb action tremor, while one patient (3.57%) had head tremor and myocloni of the upper limb. The upper limb tremor limited the daily activities (eating and playing string instruments) of three patients (10.71%), and in one of them (3.57%) it was so severe that he wanted to stop taking lamotrigine three months after starting the medication.
Clinical examination did not reveal rest tremor in any patient. In one patient (3.57%) postural tremor was detected. Three patients (10.71%) had disabling postural and intentional tremor with severity of 2 and 3 points on the FTM scale part A. In the rest of the patients, tremor severity was rated 0 to 1 point on FTM.
Objective tremor measurementsPathological rest tremor was not detected in any subject. Objective tremor assessment revealed pathological action tremor in 7 patients (25%; 6 females and 1 male). Three patients (10.71%) had only pathological intentional tremor. Pathological postural and intentional tremor was detected in 4 patients (14.28%). As there was no statistically significant difference between parameters in postural position with open and with closed eyes, data recorded in postural position with closed eyes are presented.
Among the patients, 7 (25%) developed bilateral pathological upper limb tremor with a normal CF in postural (8.42 ± 0.80 Hz) and in intentional (8.07 ± 1.17 Hz) positions. Average FD of PT was low but in the normal range (3.13 ± 1.45 Hz) while it was abnormally decreased in intentional position (1.76 ± 0.39 Hz) (Fig. 1). Average TI was slightly but not pathologically elevated in PT (0.27 ± 0.05 m/s2) while it was pathologically higher than normal in IT (0.37 ± 0.05 m/s2) (Table 3).
The rest of lamotrigine-monotherapy receiving patients (n = 21; 75%) had physiologic tremor with all tremor parameters in the normal range (Table 3).
Parameters derived from alternating hand movements and finger tapping were not altered in any case (Table 3). Reaction time was normal in all patients (Table 3).
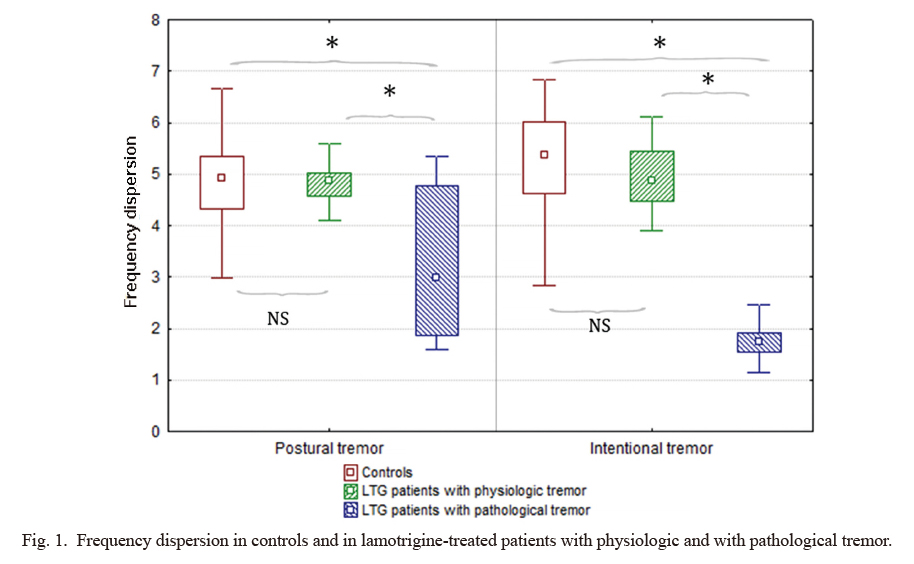
Frequency dispersion in controls and in lamotrigine-treated patients with physiologic and with pathological tremor.
Frequency dispersion, which quantifies the regularity of tremor and thus it is a reliable marker of tremor pathology, was significantly different among the three groups. Lamotrigine-treated patients with pathological tremor (defined by frequency dispersion lower than 2.6 Hz; n = 7) had significantly lower frequency dispersion than those who were treated with LTG but had physiologic tremor (defined by normal frequency dispersion values; n = 21) or than controls (n = 30) both in postural and intentional position.
LTG, lamotrigine; NS, non-significant difference between the two groups.
*Significant difference between the two groups.
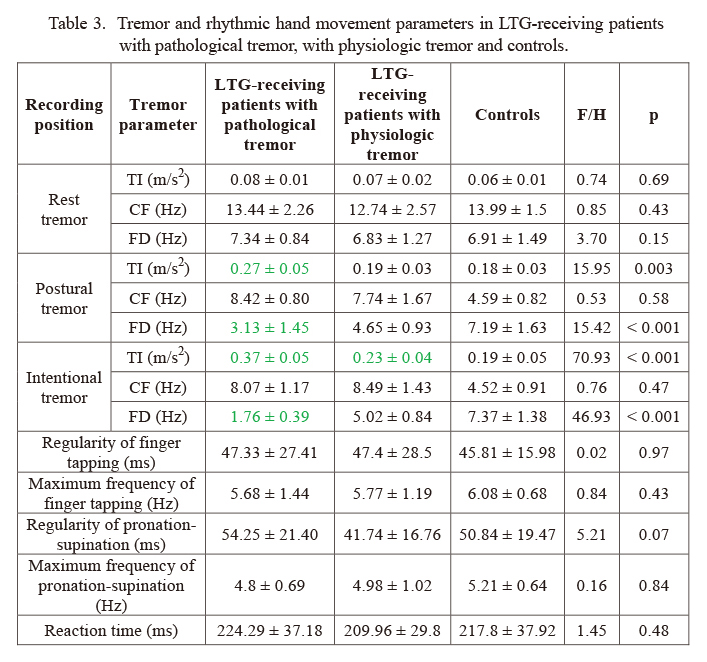
Tremor and rhythmic hand movement parameters in LTG-receiving patients with pathological tremor, with physiologic tremor and controls.
Values marked with green were significantly different from controls.
TI, tremor intensity; CF, center frequency; FD, frequency dispersion.
Tremor parameters in postural and intentional position were statistically compared among three groups: LTG-receiving patients with pathological tremor, LTG-receiving patients with physiologic tremor and controls. CF values of controls and of the LTG-receiving groups were statistically not different. However, tremor intensity and frequency dispersion had significant group effect: PT TI (H (2; 55) = 15.95, p = 0.003) (Fig. 2), PT FD (F (2; 51) = 15.42, p < 0.001) (Fig. 1), IT TI (F (2; 55) = 70.93, p < 0.001) (Fig. 2), IT FD (F (2; 55) = 46.93, p < 0.001) (Fig. 1). Post-hoc tests revealed that there was significant difference in PT TI (p < 0.001), PT FD (p < 0.001) and IT FD (p < 0.001) between LTG-receiving patients with pathological tremor and those with physiologic tremor as well as between LTG-receiving patients with pathological tremor and controls. There was no difference between LTG-receiving patients with physiologic tremor and controls. TI in intentional position was significantly higher in LTG-receiving patients with physiologic tremor than in controls (p = 0.009).
Correlations between serum lamotrigine levels or age and various tremor parameters were tested. Pearson-test revealed moderate but statistically significant correlation between age and frequency dispersion of postural tremor (r (19) = –0.5, p = 0.02) (Fig. 3), as well as between serum LTG levels and frequency dispersion of intentional tremor (r (19) = –0.51, p = 0.02) (Fig. 3). There was no significant correlation between age and serum LTG levels.
Contingency tables showed no association between type of epilepsy and pathological tremor, other antiepileptics in the past and pathological tremor, positive MRI findings and pathological tremor.
Statistical analysis showed that serum lamotrigine level increases the risk of pathological tremor: the odds ratio for 1 µmol/L increase in serum lamotrigine level was 1.32 (Table 4). This model predicted correctly 60% of patients with pathological tremor and 100% of patients with physiologic tremor.
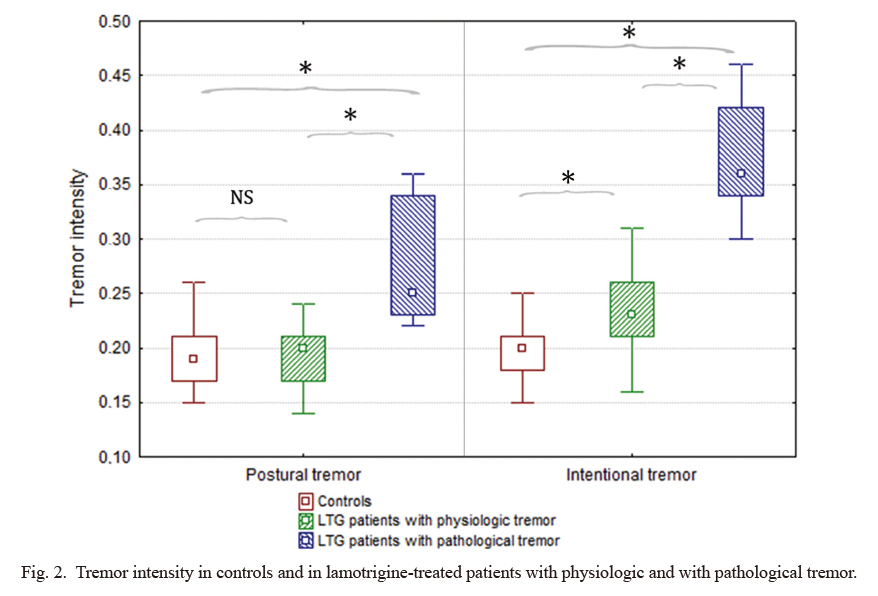
Tremor intensity in controls and in lamotrigine-treated patients with physiologic and with pathological tremor.
Tremor intensity, which quantitatively reflects tremor amplitude, was significantly different among the three groups. Post-hoc tests revealed that lamotrigine-treated patients with pathological tremor (defined by frequency dispersion lower than 2.6 Hz; n = 7) had significantly higher postural tremor intensity than those who were treated with LTG but had physiologic tremor (defined by normal frequency dispersion values; n = 21) or than controls (n = 30). Intention tremor intensity was significantly higher in both lamotrigine groups compared to controls. For numeric data, see Table 3.
LTG, lamotrigine; NS, non-significant difference between the two groups.
*Significant difference between the two groups.
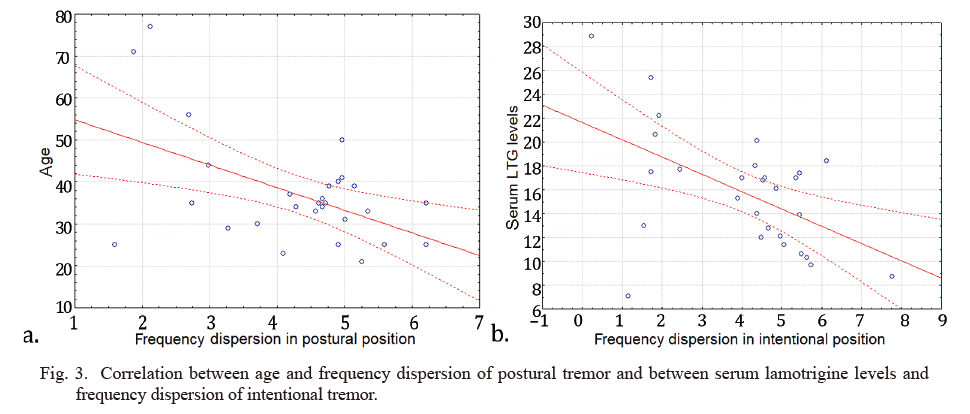
Correlation between age and frequency dispersion of postural tremor and between serum lamotrigine levels and frequency dispersion of intentional tremor.
Pearson-test revealed moderate correlation between age and frequency dispersion of postural tremor (n = 28; r (19) = –0.52, p = 0.02) (a), as well as between serum lamotrigine levels and frequency dispersion of intentional tremor (n = 28; r (19) = –0.51, p = 0.02) (b).
There was no significant correlation between age and serum lamotrigine levels.
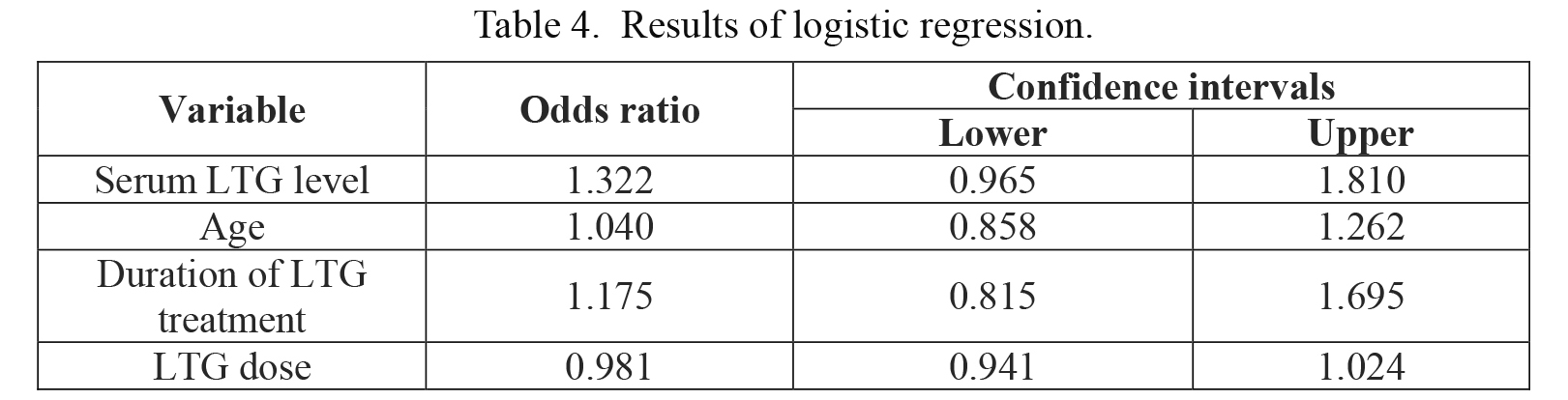
Results of logistic regression.
LTG, lamotrigine.
To the best of our knowledge, this is the first quantitative study on the occurrence and quantitative neurophysiologic parameters of tremor induced by lamotrigine monotherapy in epilepsy patients.
Our accelerometric recordings revealed pathological tremor in 25% of patients. These data show 2.5-5 times higher prevalence than proposed by earlier reports based on clinical visual assessment: 4% by Morgan and Sethi (2005) and 10% by DRUGBANK (2019). Our findings suggest that computerized quantitative tremor assessment is needed for the early recognition of lamotrigine-induced pathological tremor.
Center frequency of LTG-induced tremor was similar to that of physiologic tremor. Frequency dispersion was significantly lower in LTG-induced tremor than in physiologic tremor in both postural and intentional position. This was an expected finding as patients with pathological tremor were selected based upon frequency dispersion. However, pathological values of FD were seen in intentional position only. Tremor intensity showed similar alterations. TI of LTG-induced tremor was significantly higher than in physiologic tremor in both postural and intentional position but pathological values of TI were reached in intentional position only. Moreover, TI was significantly higher in LTG-treated patients who had normal CF and FD tremor than in controls. The above presented data demonstrate that LTG-induced tremor is a pathological, mostly intentional tremor with milder manifestation in postural position.
The pathomechanism of LTG-induced tremor is unclear. As LTG mainly acts on sodium channels inhibiting presynaptic glutamate release, it would be expected that LTG decreases tremor through membrane stabilization. Our findings however, do not support this hypothesis. Since LTG-induced tremor is mainly intentional tremor, our data suggest that cerebellar pathways might be involved in its pathomechanism.
Cerebellar tremor has been equated to intentional tremor according to the latest consensus statement on tremor of the Movement Disorder Society (Bhatia et al. 2018). However, postural tremor has also been described in patients with cerebellar lesions (Holmes 1922; Kovács et al. 2019). Focal cerebellar lesions were associated with low frequency tremors both in animal experiments (Poirier et al. 1974) and humans (Kovács et al. 2019). However, rare cases of high frequency tremor were also noted (Cole et al. 1988; Kovács et al. 2019). Non-focal, degenerative cerebellar diseases like essential tremor have higher frequency. Essential tremor was linked to degenerative processes in the cerebellum (Louis and Vonsattel 2008; Shill et al. 2008; Nicoletti et al. 2010; Broersma et al. 2015).
Various cerebellar symptoms, like nystagmus, ataxia, vertigo and tremor were reported as side effects of chronic co-medication of VPA and LTG at stable doses. Although serum levels were not provided, it might be hypothesized that these cerebellar signs were related to toxicity which ceased after 25-50 mg dose reduction of LTG (Moreira et al. 2007). It was demonstrated that glutamate is necessary for oligodendrocyte proliferation and differentiation in the developing cerebellum (Yuan et al. 1998). However, it was also shown that excess in glutamate release may lead to apoptotic cell death (McDonald et al. 1998) and volume decrease as well as remodeling in the frontal cortex (Musazzi et al. 2011). It can be assumed that LTG might prevent cerebellar Purkinje cells from excitotoxicity by decreasing glutamatergic release. However, there is some evidence that LTG might be associated with cerebellar volume loss. Patients with bipolar depression treated with 200 mg/day LTG for 12 weeks, showed volume reduction in the cerebellum, amygdala and nucleus accumbens, but only those who clinically responded to the treatment (Bauer et al. 2018). It is unknown why some patients exhibited cerebellar volume loss, some others did not. In our study, we did not perform volumetric measurements; therefore, we cannot provide further evidence for LTG associated cerebellar volume loss. However, our data demonstrate that LTG-induced tremor in our patients was not a result of toxicity.
LTG serum level and frequency dispersion of intentional tremor, as well as age and frequency dispersion of postural tremor showed moderate correlation. This is in accordance with previous findings which demonstrated that intolerability increases with serum level and intolerability is much higher in older patients than in younger ones (Arif 2011). Our study failed to show correlation between age and serum level. This is possibly due to our small sample size. However, individual data revealed that there are many non-tremulous patients with high serum level and there are tremulous patients with low serum LTG level. Therefore, correlation in our data did not exceed 50%. The logistic regression model showed that LTG serum level has the most important influence on the emergence of pathological tremor among all factors studied (LTG serum level, age, LTG dosage, and duration of LTG treatment). The model predicted all patients with physiologic tremor correctly, and it predicted 60% of patients with pathological tremor. This suggests that other factors might also influence tolerability and development of tremor as a side effect. LTG is primarily metabolized through glucuronidation (Cohen et al. 1987). In contrast to cytochrome P450, knowledge about uridine-5′diphosphate-glucuronosyltransferase (UGT) pharmacogenetics is lagging, in large part because of the absence of isoform-specific probe substrates (de Wildt et al. 1999). Inter-individual differences of tolerability at similar LTG serum levels might be a consequence of unknown UGT mutations/polymorphisms. To prove this possibility, further pharmacogenetic studies are needed.
To the best of our knowledge, this is the first study on the regularity of repetitive hand and finger movements, and reaction time in epilepsy patients treated with lamotrigine. Previous animal experiments showed that LTG directly inhibits tyrosine hydroxylase in the striatum of mice and thus, it inhibits dopamine synthesis (Vriend et al. 1997). Blepharospasm as a rarely reported side effect of LTG was also attributed to LTG-associated dopaminergic dysfunction in the basal ganglia (Verma et al. 1999). On the contrary, the MPTP model of Parkinsonism suggested that antiglutamatergic drugs might protect substantia nigra cells from destruction and thus, they might prevent disease progression (Lange et al. 1997). Our patients had normal repetitive hand/finger movements and reaction time, and furthermore none of the patients developed rest tremor. Therefore, our data support that lamotrigine, as an antiglutamatergic drug, does not cause Parkinsonism. Our findings suggest that the pathomechanism of LTG-induced tremor is independent from dopaminergic pathways.
In conclusion, we have demonstrated that objective measurements detect pathological intention tremor in 25% of epilepsy patients treated with lamotrigine monotherapy. Since drug-induced tremor might become irreversible, we recommend early and regular assessment for timely detection of LTG-related tremor. Tremor frequency was similar to that of physiologic tremor (around 8 Hz), but frequency dispersion was about half of the normal values (around 1.75 Hz). The tremorogenic effect of lamotrigine showed significant interindividual variability, unexplained by lamotrigine serum level, age, and duration of drug administration. Alternating hand movements and finger tapping were not affected. Our data suggest that in spite of contradictory previous reports, lamotrigine probably does not affect dopaminergic pathways and does not cause Parkinsonian signs. Our results raise the possibility of cerebellar involvement in the pathomechanism of lamotrigine-induced tremor.
We are very grateful to Dr. András Horváth for his valuable comments on the manuscript.
The authors declare no conflict of interest.