Abstract
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of death worldwide. The named “destruction complex” has a critical function in the Wnt/β-catenin pathway regulating the level of β-catenin in the cytoplasm and nucleus. Alterations in this complex lead to the cellular accumulation of β-catenin, which participates in the development and progression of CRC. This study aims to determine the contribution of polymorphisms in the genes of the β-catenin destruction complex to develop CRC, specifically adenomatous polyposis coli (APC) (rs11954856 G>T and rs459552 A>T), axis inhibition protein 1 (AXIN1) (rs9921222 C>T and rs1805105 C>T), AXIN2 (rs7224837 A>G), and dishevelled 2 (DVL2) (2074222 G>A and rs222836 C>T). Genomic DNA from 180 sporadic colorectal cancer patients and 150 healthy blood donors were analyzed. The identification of polymorphisms was made by polymerase chain reaction followed by restriction fragment length polymorphism (PCR-RFLP) methodology. Association was calculated by the odds ratio (OR) test. Increased susceptibility to CRC was associated with the polymorphic variants rs11954856 (APC), rs222836 (DVL2), and rs9921222 (AXIN1). Decreased susceptibility was associated with the polymorphisms rs459552 (APC) and 2074222 (DVL2). Association was also observed with advanced Tumor-Node-Metastasis (TNM) stages and tumor location. The haplotypes G-T in APC (rs11954856-rs459552) and A-C in DVL2 (rs2074222-rs222836) were associated with decreased risk of CRC, while the G-T haplotype in the DVL2 gene was associated with increased CRC risk. In conclusion, our results suggest that variants in the destruction complex genes may be involved in the promotion or prevention of colorectal cancer.
Introduction
Worldwide, colorectal cancer (CRC) is the third most common cancer and the second leading cause of death in humans. In Mexico, the reported incidence of CRC for the year 2018 was 11.2 per 100,000 individuals (Bray et al. 2018; World Health Organization 2018). Although little is known about the CRC etiology, the accumulated evidence supports that the underlying mechanisms involved in CRC are mainly influenced by genetic factors and by lifestyle (Brandstedt et al. 2013; Mundade et al. 2014; Valle 2014). The occurrence and development of CRC result from a complicated process regulated by several genes and signaling pathways, which lead to dysfunctional cell proliferation and apoptosis (Mundade et al. 2014). During this process, oncogenes activation, tumor suppressor genes inactivation, and mismatch of DNA repair genes have been observed (Huelsken and Behrens 2002; Giles et al. 2003). Alterations of the Wnt signaling pathway leading to cytoplasmic and nuclear accumulation of β-catenin have been found in > 90% of CRC cases (Munemitsu et al. 1995; Colussi et al. 2013). The accumulation of β-catenin seems to play a critical role in the development of human cancer (Colussi et al. 2013).
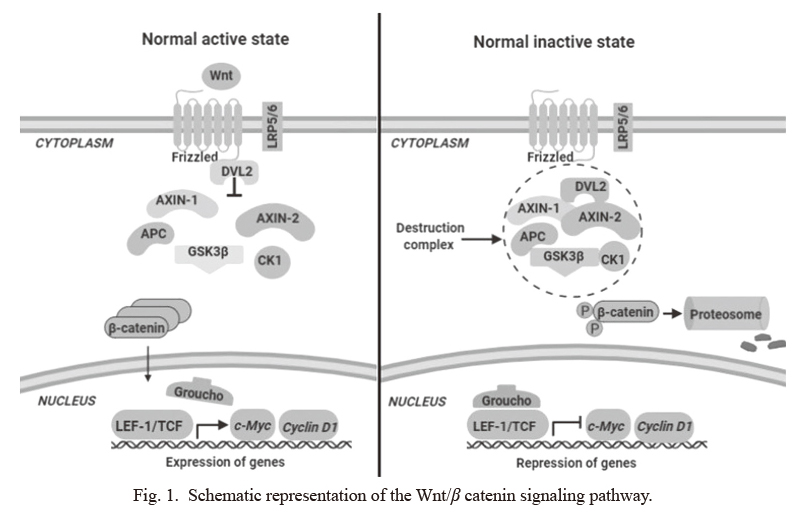
The activity of the Wnt/β-catenin pathway depends mainly on a protein complex named “β-catenin destruction complex” (β-CDC), which is formed by glycogen synthase kinase (GSK3β), adenomatous polyposis coli (APC), casein kinase 1α, axis inhibition protein (AXIN), and dishevelled 2 (DVL2) (Munemitsu et al. 1995). Changes in the activity of the Wnt/β-catenin pathway, induced by variations in their major components, contribute to the development of numerous types of cancer (Munemitsu et al. 1995; Polakis 2000; Colussi et al. 2013).
In the absence of Wnt ligands, β-CDC removes the newly synthesized β-catenin protein via the ubiquitin-proteasome pathway. In the presence of Wnt, this ligand binds to the Frizzled/LRP5/6 receptor complex and inhibits the activity of glycogen synthase kinase-3β (GSK3β), resulting in the cytoplasmic accumulation of β-catenin and its subsequent translocation to the nucleus. Within the nucleus, β-catenin integrates a transcriptional complex with the factors TCF and LEF, activating the transcription of several target genes such as c-Myc, CCND1, PPARδ, and MMP-7 (Munemitsu et al. 1995; Polakis 2000) (Fig. 1).
It is known that mutations in the β-CDC genes cause instability of the complex, reducing the degradation of β-catenin and increasing the transcription of target genes of the Wnt/β-catenin pathway and therefore rising the cancer risk (Ting et al. 2013; Paez et al. 2014). On the other hand, an significant number of studies have demonstrated association of polymorphic variants of β-CDC genes with different types of cancer (no CRC) and other diseases (Taniguchi et al. 2002; Wong et al. 2010; Letra et al. 2012; Mostowska et al. 2013, 2014; Feng et al. 2014; Lee et al. 2016; Yadav et al. 2016; Pu et al. 2017; Vijayan et al. 2018). Few studies have analyzed the association of polymorphic variants in β-CDC genes with colorectal cancer, and controversial results were observed for the APC gene (Menendez et al. 2004; Theodoratou et al. 2008; Picelli et al. 2010; Li et al. 2017; Parine et al. 2019). Polymorphisms in the DVL2, AXIN1, and AXIN2 genes have not been analyzed in CRC patients. At our knowledge, this is the first study exploring the association of a group of seven germline polymorphisms in the DVL2, APC, AXIN2 and AXIN1 genes of the β-CDC with colorectal cancer.
Materials and Methods
Study population
The study included 330 individuals; 180 patients (83 females and 97 males) diagnosed with colorectal adenocarcinoma according to clinical and pathological criteria of the Hospital de Especialidades, Centro Médico Nacional de Occidente (CMNO), Instituto Mexicano del Seguro Social, (IMSS) in Guadalajara, Jalisco. The CRC status was established according to the tumor-node-metastasis (TNM) classification. The control group included 150 healthy volunteers (63 females and 87 males). These volunteers were not matched by age with the group of patients. All subjects included in this study came from the metropolitan area of Guadalajara, Jalisco, Mexico. Blood samples were obtained after written informed consent. The study was approved by the Ethical Committee 1305 (R-2014-1305-8) of the Centro de Investigación Biomédica de Occidente, IMSS, and conducted following the national and international ethical standards. We used an epidemiologic questionnaire to collect personal data including age, sex, family history, drinking, and smoking status. Information about the clinical and pathological features of the patients was also obtained from the hospital records.
Genotyping analysis
Seven polymorphisms were selected to examine their potential genetic risk to develop CRC (Table 1). Genomic DNA was extracted from peripheral blood using the salting-out method (Miller et al. 1988). Genotyping of the APC (rs459552 and rs11954856), AXIN1 (rs9921222 and rs1805105), AXIN2 (rs7224837) and DVL2 (rs2074222 and rs222836) variants was performed by Polymerase Chain Reaction (PCR) followed by the appropriate restriction enzyme digestion (New England Biolabs, Ipswich, MA, USA), PCR-restriction fragment length polymorphisms (PCR-RFLP), using the primer pairs and conditions of analyses described by (Mostowska et al. 2012, 2014) (Table 2). One example of PCR-RFLP analysis is shown in Fig. 2. The genotyping quality was evaluated by repeated genotyping of 10% of samples randomly selected; the concordance between genotyping assays was 100%.
Statistical analysis
Allele and genotype frequencies were estimated by direct counting in both groups. The Chi-squared test assessed the Hardy-Weinberg equilibrium (HWE). Differences in allele and genotype distributions and the characteristics clinical between patients and controls were established by the Chi-squared test. Association of CRC with alleles or genotypes and the stratified analysis by TNM stage and tumor location was calculated by the odds ratio (OR) and corresponding 95% confidence intervals (CI) using an SPSS v17.0 software package (SPSS Inc., Chicago, IL, USA). A Bonferroni correction test was applied to adjusted the p values (P < 0.007 was considered significant). Interaction of the genetic polymorphisms in both loci of each gene was evaluated by the combined effect of the genotypes and analysis of the haplotypes. We compared the haplotypes frequency of each gene between patients with colorectal cancer with those of controls. The linkage disequilibrium and the haplotype frequencies were calculated using the Haploview 4.2 software. For the in silico functional assessment, the amino-acid predictions were performed by the PolyPhen2 (Polymorphism Phenotyping v2) and PROVEAN (Protein Variation Effect Analyzer) software. Additionally, we use the Ensembl and Clin Var software for intronic variants analysis.
Results
Characteristics of the studied population
Table 3 shows a comparative analysis of epidemiological and clinical data for the 180 CRC patients and 150 controls. There were no significant differences between CRC patients and controls concerning sex, smoking status, and drinking status. Only for age, a significant difference was observed (P = 0.001).
Genotype distribution and allele frequency in the β-CDC variants
Table 4 shows the association analysis results of seven β-CDC polymorphisms. Genotype frequencies of the single nucleotide polymorphisms (SNPs) analyzed in the control group were in agreement with the Hardy-Weinberg equilibrium (P > 0.05), suggesting genetic equilibrium for the enrolled population (data not shown).
The statistical analysis displayed an increased risk for CRC in individuals carrying the APC (rs11954856), AXIN1 (rs9921222), and DVL2 (rs222836) SNPs. Inversely, the APC (rs459552) and DVL2 (2074222) SNPs showed a protective effect against CRC in these individuals. On the other hand, AXIN2 (rs7224837) and AXIN1 (rs1805105) SNPs did not show association with CRC.
Analysis by TNM stage and tumor location
Analysis of each SNP regarding TNM staging is shown in Table 5. Patients with CRC were separated into two groups according to TNM staging (III + IV vs. I + II). In this analysis, patients with TNM stage III + IV were associated under a dominant pattern of allelic interaction in two SNPs: APC (rs11954856) and DVL2 (rs222836). On the other hand, a protective effect for the TNM stage III + IV was observed in patients carrying the same model of inheritance with the APC (rs459552) SNP. A comparison of these TNM staging groups with the control group showed significant differences for several polymorphisms. Using different models of allelic interaction, patients carrying the APC (rs11954856), DVL2 (rs222836), and AXIN1 (rs1805105 and rs9921222) SNPs showed a considerable risk for the TNM stage III + IV; meanwhile, patients carrying the APC (rs459552), and DVL2 (rs2074222) SNPs exhibited a protective effect to reach the TNM stage III + IV.
Results of the stratified analysis of each SNP by tumor location are shown in Table 6. A significantly protective effect for colon cancer was observed in patients with the presence of APC (rs459552), DVL2 (rs2074222), and AXIN1 (rs9921222) polymorphisms. Notably, a significant risk for rectal cancer was observed in patients with presence of APC (rs11954856) polymorphism.
Haplotypes in the β-CDC
Four different haplotypes in the APC, AXIN1, and DVL2 genes were found (Table 7). Significant differences were observed only for the haplotypes in APC and DVL2 genes. For the APC gene, the most frequent haplotypes were: T-A (CRC: 40%; controls: 25%), G-A (CRC: 31%; Controls: 30%) and T-T (CRC: 17%; controls: 19%). In this study, carriers of the G-T haplotype showed a protective effect (OR: 0.39; 95% CI: 0.22-0.70, P = 0.001).
For the DVL2 gene the most frequent haplotypes were G-T (CRC: 40%; controls: 22%), G-C (CRC: 25%; controls: 24%) and A-T (CRC: 21%; controls: 25%). Carriers of the G-T haplotype showed an increased risk for CRC (OR: 2.36; 95% CI: 1.45-3.85, P = 0.001) and carriers for the A-C haplotype showed a protective effect (OR: 0.40; 95% CI: 0.23-0.69, P = 0.001).
In silico functional prediction analysis of the β-CDC genetic variants
The in silico analysis demonstrated that all the genetic variants analyzed in this study were benign; they did not show a modifying impact on the protein and were classified as of undefined clinical significance.
Discussion
The sequencing of the human genome has allowed large-scale association studies in the genome, which in turn allows assessing the individual susceptibility to cancer. In CRC patients, recent studies have identified a significant number of mutations in several genes predisposing to cancer; among these, genes participating in the Wnt/β-catenin pathway have been frequently involved. The crucial role of β-CDCs in this signaling pathway suggests that changes in the components of their proteins should have a prominent effect on the development of cancer. In this study, we investigated the potential association of genes integrating the β-CDC with CRC. Some of these genes have previously been associated with genetic susceptibility to the development of CRC in different populations. In this study, we analyzed seven SNPs and haplotypes located in four genes whose protein products participate in the β-CDC: rs459552 (A>T) and rs11954856 (G>T) of the APC gene; rs9921222 (C>T) and rs1805105 (C>T) of the AXIN1 gene; rs2074222 (G>A) and rs222836 (C>T) of the DVL2 gene and rs7224837 (A>G) of the AXIN2 gene. This report represents the first association study that simultaneously analyzes several SNPs and haplotypes in the APC, AXIN1, and DVL2 genes.
The achieved results suggest that the genotypes in the (APC) rs11954856, (AXIN1) rs9921222 and (DVL2) rs222836 variants play a significant role in promoting CRC; meanwhile, the (APC) rs459552 and (DVL2) rs2074222 polymorphisms show a protective role for CRC. Stratification of the patients by tumor location showed that the DVL2 rs2074222 and 222836, APC rs459552, and AXIN1 rs9921222 polymorphisms are associated with colon cancer specifically; moreover, the APC rs11954856 and rs459552 polymorphisms were associated mainly with rectal cancer. On the other hand, the haplotypes found for the APC (rs11954856-rs459552) and DVL2 (rs2074222-rs222836) genes significantly modify both colon and rectal cancer susceptibility.
The APC protein participates in intercellular adhesion, cytoskeletal organization, cell cycle regulation, and apoptosis (Pronobis et al. 2015), APC provides binding sites for the AXIN1 and β-catenin proteins (Pronobis et al. 2015), down regulating the Wnt/β-catenin pathway. APC has been identified as a tumor suppressor gene that plays a crucial role in the early stages of human colorectal tumorigenesis (Menendez et al. 2004). Germline mutations in the APC gene, usually generating a stop codon, are responsible for the Familial Adenomatous Polyposis (FAP), an autosomal dominant inherited disease (Menendez et al. 2004). Although multiple germline missense polymorphisms in the APC gene have been reported, I1307K, E1317Q, and D1822V are considered the most recurrent variants; however, its contribution to the CRC risk is still controversial in different populations (Evertsson et al. 2001; Rozek et al. 2006).
In this study, rs459552 was the most common APC variant. Located between the 4th and 57th amino acid repeat region in the APC protein, rs459552 participate downregulating the β-catenin protein (Evertsson et al. 2001; Rozek et al. 2006; Feng et al. 2014). The APC rs11954856 variant is located before the exon 1 and plays a role in the transcriptional regulation (Yadav et al. 2016). This polymorphism has been associated with different types of cancer, including CRC (Mostowska et al. 2014; Yadav et al. 2016; Li et al. 2017). Li et al. (2017) demonstrated that the variant rs11954856 increases the expression of the APC gene in patients with CRC but also, although with less intensity, of β-catenin and the transcription factors LEF1, TCF7L1, and TCF7L2. Our results showed a significant association of both APC rs459552 and rs11954856 variants with the TNM stage and the tumor location in CRC patients. In the haplotypes analysis, we found a significant protective association of the G-T haplotype (rs11954856 and rs459552 SNPs) respecting to CRC. A similar result was reported by Yadav et al. (2016), who found this same G-T haplotype for gallbladder cancer.
The in silico analysis revealed that APC rs11954856 is an intronic variant with a modifying impact but with undefined clinical significance; however, it has been described that rs11954856 affects the transcriptional regulation of the Wnt/β-catenin signaling pathway (Li et al. 2017). On the other hand, the APC rs459552 polymorphism showed to be a missense variant predicted with a moderate modifying impact and undefined clinical significance. APC rs459552 has been analyzed by expression quantitative trait locus (cis-eQTL) affecting the REEP5 expression (receptor accessory protein 5), located nearly 30 kb downstream from APC (Hildebrandt et al. 2016).
DVL2 is a main phosphoprotein in the Wnt pathway, as it forms a dynamic recruitment platform for AXIN1, AXIN2, and other partners to transduce the Wnt/β-catenin signal (Metcalfe et al. 2010). Overexpression of DVL2 has been described in some neoplasms, including colon (Metcalfe et al. 2010), leukemia (Khan et al. 2016), and breast (Zhu et al. 2012). High DVL2 levels contribute to the nuclear accumulation of β-catenin at advanced stages of tumorigenic progression (Zhang et al. 2017). Recently, DVL2 variants (rs2074222 and rs222836) has been associated with pathologies related to craniofacial defects (Vijayan et al. 2018). The DVL2 variants are for the first time investigated concerning the association with CRC; our results showed a statistically significant association of the intronic (rs2074222) and exonic (rs222836) variants of the DVL2 gene with CRC, respecting to TNM stage, we observed that the rs222836 variant was associated to III+IV stage; with the tumor location, we observed that both variants rs2074222 and rs222836 were associated exclusively with colon localization. Furthermore, the DVL2 G-T and A-C haplotypes were significant associated with CRC. The in silico analysis showed that rs222836 is a variant with benign clinical significance; for the rs2074222 variant, no information was obtained.
The axis inhibitor (AXIN) is a multi-domain protein on which several cellular factors bind, suggesting that alterations in its sequence would potentially lead to functional anomalies. AXIN forms a complex with β-catenin, GSK3β, APC, protein phosphatase 2 (PP2A), catenin delta-1 (CTNND1), and DVL2, and promotes GSK3β-dependent β-catenin phosphorylation (Polakis 2000; Huelsken and Behrens 2002). The AXIN1 protein is a negative regulator of the canonical Wnt/β-catenin pathway and plays an essential role during embryogenesis (Xie et al. 2011). Mutations in AXIN1 have been involved in diseases associated with human development processes such as caudal duplication anomalies and medulloblastoma (Oates et al. 2006), and with hepatocellular and prostate cancer (Webster et al. 2000; Taniguchi et al. 2002), but not with CRC. Our results show, for the first time, an association of the AXIN1 rs9921222 (intronic) variant with susceptibility to CRC; respecting the TNM stage, we observed that both rs1805105 and rs9921222 polymorphisms were associated with III+IV stage. However, for these polymorphisms, we did not find any associated haplotype. The in silico analysis shows that rs1805105 is a synonymous variant with a slight modifier impact, whereas the intronic variant rs9921222 does not present a modifying impact.
Finally, AXIN2 encodes a protein with 60% of amino acid identity to AXIN1, and both proteins contain the same conserved domains for binding to APC, DVL2, GSK3β, CK1, and β-catenin (Roberts et al. 2011; Pronobis et al. 2015). Our results showed no statistically significant differences for the rs7224837 polymorphism or in the haplotype analysis. The in silico analysis showed that rs7224837 is an intronic variant with no modifying impact.
In conclusion, our findings suggest that multiple germline SNPs in genes of the Wnt/β-catenin pathway may be associated with CRC susceptibility. Specifically, this study provides the first evidence that the DVL2 rs222836 and AXIN1 rs9921222 polymorphisms might be a genetic risk factor for developing sporadic CRC; meanwhile, the DVL2 rs2074222 polymorphism could be considered as a protective genetic factor. Additionally, our results demonstrate that polymorphisms analyzed in the APC, DVL2 and AXIN1 genes are associated with advanced stages and with tumor location.
The results found in the analysis of haplotypes represent a more powerful approach than the analysis of individual polymorphisms (Gast et al. 2007; Naccarati et al. 2010); this approach ensures an increase in statistical power. Assignment of alleles to chromosomes (haplotypes) also provides essential information on recombination during meiosis, which is vital for finding disease-causing mutations by linkage methods (Naccarati et al. 2010). Thus, in our study, haplotypes based on different β-CDC genes explained the differences in the risk of CRC. The observed effects of the variants on the APC and DVL2 genes, in addition to the analyzed haplotypes, probably indicate that multiple polymorphisms can modify the risk of colorectal cancer, either directly or through interaction with environmental factors. Undoubtedly, additional studies with more diverse populations, more samples, and functional analysis of these polymorphisms are necessary to confirm and extend our findings; however, we suggest that these gene variants should be considered useful biomarkers of susceptibility or protection to sporadic colorectal cancer.
Acknowledgments
This study was supported by grants from the Fondo de Investigación en Salud. Instituto Mexicano del Seguro Social (FIS/IMSS/PROT/G15/1396 and FIS/IMSS/PROT/G18/1822). M.A. Rosales-Reynoso is a scholarship of the IMSS Foundation, Mexico.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Brandstedt,
J.,
Wangefjord,
S.,
Borgquist,
S.,
Nodin,
B.,
Eberhard,
J.,
Manjer,
J. &
Jirstrom,
K.
(2013) Influence of anthropometric factors on tumour biological characteristics of colorectal cancer in men and women: a cohort study. J. Transl. Med., 11, 293.
-
Bray,
F.,
Ferlay,
J.,
Soerjomataram,
I.,
Siegel,
R.L.,
Torre,
L.A. &
Jemal,
A.
(2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin., 68, 394-424.
-
Colussi,
D.,
Brandi,
G.,
Bazzoli,
F. &
Ricciardiello,
L.
(2013) Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int. J. Mol. Sci., 14, 16365-16385.
-
Evertsson,
S.,
Lindblom,
A. &
Sun,
X.F.
(2001) APC I1307K and E1317Q variants are rare or do not occur in Swedish colorectal cancer patients. Eur. J. Cancer, 37, 499-502.
-
Feng,
M.,
Fang,
X.,
Yang,
Q.,
Ouyang,
G.,
Chen,
D.,
Ma,
X.,
Li,
H. &
Xie,
W.
(2014) Association between the APC gene D1822V variant and the genetic susceptibility of colorectal cancer. Oncol. Lett., 8, 139-144.
-
Gast,
A.,
Bermejo,
J.L.,
Flohr,
T.,
Stanulla,
M.,
Burwinkel,
B.,
Schrappe,
M.,
Bartram,
C.R.,
Hemminki,
K. &
Kumar,
R.
(2007) Folate metabolic gene polymorphisms and childhood acute lymphoblastic leukemia: a case-control study. Leukemia, 21, 320-325.
-
Giles,
R.H.,
van Es,
J.H. &
Clevers,
H.
(2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta, 1653, 1-24.
-
Hildebrandt,
M.A.,
Reyes,
M.E.,
Lin,
M.,
He,
Y.,
Nguyen,
S.V.,
Hawk,
E.T. &
Wu,
X.
(2016) Germline genetic variants in the Wnt/beta-catenin pathway as predictors of colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev., 25, 540-546.
-
Huelsken,
J. &
Behrens,
J.
(2002) The Wnt signalling pathway. J. Cell Sci., 115, 3977-3978.
-
Khan,
A.S.,
Hojjat-Farsangi,
M.,
Daneshmanesh,
A.H.,
Hansson,
L.,
Kokhaei,
P.,
Osterborg,
A.,
Mellstedt,
H. &
Moshfegh,
A.
(2016) Dishevelled proteins are significantly upregulated in chronic lymphocytic leukaemia. Tumour Biol., 37, 11947-11957.
-
Lee,
S.H.,
Cho,
E.H.,
Ahn,
S.H.,
Kim,
H.M.,
Lim,
K.H.,
Kim,
B.J.,
Kim,
S.W.,
Kim,
T.H.,
Kim,
S.Y.,
Kim,
G.S.,
Kang,
M.I. &
Koh,
J.M.
(2016) Prediction of future osteoporotic fracture occurrence by genetic profiling: a 6-year follow-up observational study. J. Clin. Endocrinol. Metab., 101, 1215-1224.
-
Letra,
A.,
Bjork,
B.,
Cooper,
M.E.,
Szabo-Rogers,
H.,
Deleyiannis,
F.W.,
Field,
L.L.,
Czeizel,
A.E.,
Ma,
L.,
Garlet,
G.P.,
Poletta,
F.A.,
Mereb,
J.C.,
Lopez-Camelo,
J.S.,
Castilla,
E.E.,
Orioli,
I.M.,
Wendell,
S.,
et al. (2012) Association of AXIN2 with non-syndromic oral clefts in multiple populations. J. Dent. Res., 91, 473-478.
-
Li,
F.F.,
Zhao,
Z.X.,
Yan,
P.,
Wang,
S.,
Liu,
Z.,
Zhang,
Q.,
Zhang,
X.N.,
Sun,
C.H.,
Wang,
X.S.,
Wang,
G.Y. &
Liu,
S.L.
(2017) Different effection of p.1125Val>Ala and rs11954856 in APC on Wnt signaling pathway. Oncotarget, 8, 70854-70864.
-
Menendez,
M.,
Gonzalez,
S.,
Blanco,
I.,
Guino,
E.,
Peris,
M.,
Peinado,
M.A.,
Capella,
G. &
Moreno,
V.
(2004) Colorectal cancer risk and the APC D1822V variant. Int. J. Cancer, 112, 161-163.
-
Metcalfe,
C.,
Ibrahim,
A.E.,
Graeb,
M.,
de la Roche,
M.,
Schwarz-Romond,
T.,
Fiedler,
M.,
Winton,
D.J.,
Corfield,
A. &
Bienz,
M.
(2010) Dvl2 promotes intestinal length and neoplasia in the ApcMin mouse model for colorectal cancer. Cancer Res., 70, 6629-6638.
-
Miller,
S.A.,
Dykes,
D.D. &
Polesky,
H.F.
(1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res., 16, 1215.
-
Mostowska,
A.,
Biedziak,
B.,
Zadurska,
M.,
Dunin-Wilczynska,
I.,
Lianeri,
M. &
Jagodzinski,
P.P.
(2013) Nucleotide variants of genes encoding components of the Wnt signalling pathway and the risk of non-syndromic tooth agenesis. Clin. Genet., 84, 429-440.
-
Mostowska,
A.,
Hozyasz,
K.K.,
Wojcicki,
P.,
Lasota,
A.,
Dunin-Wilczynska,
I. &
Jagodzinski,
P.P.
(2012) Association of DVL2 and AXIN2 gene polymorphisms with cleft lip with or without cleft palate in a Polish population. Birth Defects Res. A Clin. Mol. Teratol., 94, 943-950.
-
Mostowska,
A.,
Pawlik,
P.,
Sajdak,
S.,
Markowska,
J.,
Pawalowska,
M.,
Lianeri,
M. &
Jagodzinski,
P.P.
(2014) An analysis of polymorphisms within the Wnt signaling pathway in relation to ovarian cancer risk in a Polish population. Mol. Diagn. Ther., 18, 85-91.
-
Mundade,
R.,
Imperiale,
T.F.,
Prabhu,
L.,
Loehrer,
P.J. &
Lu,
T.
(2014) Genetic pathways, prevention, and treatment of sporadic colorectal cancer. Oncoscience, 1, 400-406.
-
Munemitsu,
S.,
Albert,
I.,
Souza,
B.,
Rubinfeld,
B. &
Polakis,
P.
(1995) Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. USA, 92, 3046-3050.
-
Naccarati,
A.,
Pardini,
B.,
Polakova,
V.,
Smerhovsky,
Z.,
Vodickova,
L.,
Soucek,
P.,
Vrana,
D.,
Holcatova,
I.,
Ryska,
M. &
Vodicka,
P.
(2010) Genotype and haplotype analysis of TP53 gene and the risk of pancreatic cancer: an association study in the Czech Republic. Carcinogenesis, 31, 666-670.
-
Oates,
N.A.,
van Vliet,
J.,
Duffy,
D.L.,
Kroes,
H.Y.,
Martin,
N.G.,
Boomsma,
D.I.,
Campbell,
M.,
Coulthard,
M.G.,
Whitelaw,
E. &
Chong,
S.
(2006) Increased DNA methylation at the AXIN1 gene in a monozygotic twin from a pair discordant for a caudal duplication anomaly. Am. J. Hum. Genet., 79, 155-162.
-
Paez,
D.,
Gerger,
A.,
Zhang,
W.,
Yang,
D.,
Labonte,
M.J.,
Benhanim,
L.,
Kahn,
M.,
Lenz,
F.,
Lenz,
C.,
Ning,
Y.,
Wakatsuki,
T.,
Loupakis,
F. &
Lenz,
H.J.
(2014) Association of common gene variants in the WNT/beta-catenin pathway with colon cancer recurrence. Pharmacogenomics J., 14, 142-150.
-
Parine,
N.R.,
Azzam,
N.A.,
Shaik,
J.,
Aljebreen,
A.M.,
Alharbi,
O.,
Almadi,
M.A.,
Alanazi,
M. &
Khan,
Z.
(2019) Genetic variants in the WNT signaling pathway are protectively associated with colorectal cancer in a Saudi population. Saudi J. Biol. Sci., 26, 286-293.
-
Picelli,
S.,
Zajac,
P.,
Zhou,
X.L.,
Edler,
D.,
Lenander,
C.,
Dalen,
J.,
Hjern,
F.,
Lundqvist,
N.,
Lindforss,
U.,
Pahlman,
L.,
Smedh,
K.,
Tornqvist,
A.,
Holm,
J.,
Janson,
M.,
Andersson,
M.,
et al. (2010) Common variants in human CRC genes as low-risk alleles. Eur. J. Cancer, 46, 1041-1048.
-
Polakis,
P.
(2000) Wnt signaling and cancer. Genes Dev., 14, 1837-1851.
-
Pronobis,
M.I.,
Rusan,
N.M. &
Peifer,
M.
(2015) A novel GSK3-regulated APC: Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient betacatenin destruction. Elife, 4, e08022.
-
Pu,
Y.,
Mi,
X.,
Chen,
P.,
Zhou,
B.,
Zhang,
P.,
Wang,
Y.,
Song,
Y. &
Zhang,
L.
(2017) Genetic association of polymorphisms in AXIN1 gene with clear cell renal cell carcinoma in a Chinese population. Biomark. Med., 11, 947-955.
-
Roberts,
D.M.,
Pronobis,
M.I.,
Poulton,
J.S.,
Waldmann,
J.D.,
Stephenson,
E.M.,
Hanna,
S. &
Peifer,
M.
(2011) Deconstructing the sscatenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol. Biol. Cell, 22, 1845-1863.
-
Rozek,
L.S.,
Rennert,
G. &
Gruber,
S.B.
(2006) APC E1317Q is not associated with colorectal cancer in a population-based case-control study in northern Israel. Cancer Epidemiol. Biomarkers Prev., 15, 2325-2327.
-
Taniguchi,
K.,
Roberts,
L.R.,
Aderca,
I.N.,
Dong,
X.,
Qian,
C.,
Murphy,
L.M.,
Nagorney,
D.M.,
Burgart,
L.J.,
Roche,
P.C.,
Smith,
D.I.,
Ross,
J.A. &
Liu,
W.
(2002) Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene, 21, 4863-4871.
-
Theodoratou,
E.,
Campbell,
H.,
Tenesa,
A.,
McNeill,
G.,
Cetnarskyj,
R.,
Barnetson,
R.A.,
Porteous,
M.E.,
Dunlop,
M.G. &
Farrington,
S.M.
(2008) Modification of the associations between lifestyle, dietary factors and colorectal cancer risk by APC variants. Carcinogenesis, 29, 1774-1780.
-
Ting,
W.C.,
Chen,
L.M.,
Pao,
J.B.,
Yang,
Y.P.,
You,
B.J.,
Chang,
T.Y.,
Lan,
Y.H.,
Lee,
H.Z. &
Bao,
B.Y.
(2013) Common genetic variants in Wnt signaling pathway genes as potential prognostic biomarkers for colorectal cancer. PLoS One, 8, e56196.
-
Valle,
L.
(2014) Genetic predisposition to colorectal cancer: where we stand and future perspectives. World J. Gastroenterol., 20, 9828-9849.
-
Vijayan,
V.,
Ummer,
R.,
Weber,
R.,
Silva,
R. &
Letra,
A.
(2018) Association of WNT pathway genes with nonsyndromic cleft lip with or without cleft palate. Cleft Palate Craniofac. J., 55, 335-341.
-
Webster,
M.T.,
Rozycka,
M.,
Sara,
E.,
Davis,
E.,
Smalley,
M.,
Young,
N.,
Dale,
T.C. &
Wooster,
R.
(2000) Sequence variants of the axin gene in breast, colon, and other cancers: an analysis of mutations that interfere with GSK3 binding. Genes Chromosomes Cancer, 28, 443-453.
-
Wong,
H.L.,
Peters,
U.,
Hayes,
R.B.,
Huang,
W.Y.,
Schatzkin,
A.,
Bresalier,
R.S.,
Velie,
E.M. &
Brody,
L.C.
(2010) Polymorphisms in the adenomatous polyposis coli (APC) gene and advanced colorectal adenoma risk. Eur. J. Cancer, 46, 2457-2466.
-
World Health Organization
(2018) Colorectal cancer. http://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf [Accessed: October 1, 2019].
-
Xie,
R.,
Jiang,
R. &
Chen,
D.
(2011) Generation of Axin1 conditional mutant mice. Genesis, 49, 98-102.
-
Yadav,
A.,
Gupta,
A.,
Yadav,
S.,
Rastogi,
N.,
Agrawal,
S.,
Kumar,
A.,
Kumar,
V.,
Misra,
S. &
Mittal,
B.
(2016) Association of Wnt signaling pathway genetic variants in gallbladder cancer susceptibility and survival. Tumour Biol., 37, 8083-8095.
-
Zhang,
C.,
Li,
C.,
Chen,
X.,
Zhou,
Y.,
Yin,
B.,
Ni,
R.,
Zhang,
Y. &
Liu,
J.
(2017) Overexpression of dishevelled 2 is involved in tumor metastasis and is associated with poor prognosis in hepatocellular carcinoma. Clin. Transl. Oncol., 19, 1507-1517.
-
Zhu,
Y.,
Tian,
Y.,
Du,
J.,
Hu,
Z.,
Yang,
L.,
Liu,
J. &
Gu,
L.
(2012) Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS One, 7, e37823.









