Abstract
Myopia is the most common refractive disorder in Eastern Asia. The development of myopia is associated with the cooperation of various ocular tissues. Exosomes in the aqueous humor (AH) have been implicated to modulate intracellular communications by transferring exosomal miRNAs and proteins between cells. These exosomal miRNAs and proteins are likely involved in the pathogenesis of various eye diseases. In this study, we aimed to explore human exosomal miRNA profiles and their roles in myopia development. AH samples were collected from 16 patients (8 myopia and 8 control) undergoing routine cataract surgeries. Exosomes were isolated from AH of each individual using the ExoQuick solution. The numbers and sizes of exosomes were not significantly different between the myopia and control groups. The individual exosomes of the same group were pooled to purify RNA. Unexpectedly, the myopia group contained 2.78-fold total RNA amount than that in the control group. Thereafter, miRNA profiles were analyzed using the OpenArray system. We thus found 15 myopia-specific miRNAs and four myopia-absent miRNAs. By using bioinformatics analysis, we identified six well-known myopia-associated genes that are potential targets of five myopia-specific miRNAs (has-miR-582-3p, has-miR-17-5p, has-miR-885-3p, has-miR-19b-3p, and has-miR-450b-5p). These genes are cholinergic receptor muscarinic 2 (CHRM2), cyclic nucleotide-gated channel beta 3 (CNGB3), vascular endothelial growth factor A (VEGFA), adenosine A2a receptor (ADORA2A), insulin-like growth factor 1 (IGF1), and lumican (LUM). Moreover, CHRM2 may be a target of myopia-absent miRNA (has-miR-378a-5p). In conclusion, we show the expression profiles of AH-derived exosomal miRNAs and their potential roles in myopia development.
Introduction
Myopia is one of the most common eye diseases, which causes the refractive image formed by the cornea and lens to fall in front of the photoreceptors of the retina (Carr and Stell 1995). Myopia can be classified into refractive, such as corneal diopter, or axial, which is caused by growth of the axial axis (Meng et al. 2011). Several risk factors that lead to myopia, such as familial inheritance, long-term near work, and less time spent in outdoor activities, have been reported (Wong et al. 2000, 2001; Morgan et al. 2006; Pan et al. 2012). However, its molecular mechanism of pathogenesis remains unclear. Myopia is considered a mild eye disease since the optical errors can be corrected by wearing glasses/contact lenses and undergoing refractive surgery (Morgan et al. 2012). Additionally, several treatment modalities can slow the progression of myopia, such as the use of orthokeratology contact lenses, soft bifocal contact lenses, and pharmaceutical agents, such as atropine and pirenzepine (Holden et al. 2014; Smith and Walline 2015). Recently, the prevalence rate of myopia has sharply increased worldwide, especially in Eastern Asian countries (Morgan and Rose 2005; Wu et al. 2016). For example, in Taiwan, the prevalence rate in elderly individuals aged > 65 years is only 19.4% (Cheng et al. 2003), whereas the relative prevalence rate in younger individuals aged 16-18 years is as high as 84% (Lin et al. 2004). Highly nearsighted individuals have increased risk for several vision-threatening problems, such as retinal detachment, choroidal neovascularization, cataract, and glaucoma (Russo et al. 2014). Myopia has become a global public health problem, leading to visual impairment and blindness.
The aqueous humor (AH) is a clear fluid that is continually secreted by the non-pigmented epithelial cells of the ciliary body and comes in contact with the anterior surface of the lens, iris, and corneal endothelial cells, before draining out of the eye via the trabecular meshwork (Goel et al. 2010). The main role of the AH is to provide nutrition, remove excretory products from metabolism, transport neurotransmitters, and contribute to the regulation of homeostasis for these ocular tissues (Goel et al. 2010). Recent studies have suggested that there are some potential modulators in AH, such as hormones and growth factors, which may be involved in cellular communication between ocular tissues (Coca-Prados and Escribano 2007; Zhang et al. 2009; Lee et al. 2011). These modulators are associated with the pathogenesis of various eye diseases (Duan et al. 2008; Liu et al. 2016; Ji et al. 2018). For example, high levels of matrix metalloproteinase, inhibitors of metalloproteinases, and transforming growth factor-β2 in AH are significantly associated with the axial length of the eyes in patients with myopia (Jia et al. 2014; Zhang et al. 2016).
Exosomes, small vesicles with a diameter of approximately 30-150 nm, are surrounded by a double-layered lipid membrane (Raposo and Stoorvogel 2013). They carry proteins, mRNAs, or miRNAs and transport them to adjacent or distant cells (Henderson and Azorsa 2012). Exosomes are released by several cell types and detected in a number of body fluids, such as blood plasma, urine, and AH (Michael et al. 2010; Vlassov et al. 2012; Dismuke et al. 2015). The major function of exosomes is intercellular communication by transferring exosomal RNA and protein between cells. Thus, exosomes have been emerging as important biomarkers for several human diseases (Vlassov et al. 2012; Schneider and Simons 2013; Liu et al. 2016). This study aimed to characterize the size, concentration, and miRNA profile of exosomes between subjects with and without axial myopia. To further explore the role of exosomal miRNA in myopia, we predicted their potential target genes and functions in myopia using bioinformatics analysis.
Material and Methods
AH sample collection
The study protocol was approved by the Research Ethics Committee of Taipei City Hospital (TCHIRB-10505111) and conducted according to the tenets of the Declaration of Helsinki. All study participants provided written informed consent before enrollment. Human AH samples were collected from 16 patients undergoing cataract surgery at the Zhongxing Branch of Taipei City Hospital. The diagnostic criterion for myopia was axial length > 26 mm. The control eyes were collected from patients with senile cataract who had no myopia and other ocular or systemic diseases. We collected 100 µl of AH from each patient by anterior chamber paracentesis, using a needle inserted through the peripheral cornea at the beginning of the procedure. Undiluted AH samples were stored at −80°C until further studies.
Isolation of exosomes
Exosomes were isolated from AH using the ExoQuick precipitation solution (System Biosciences, Inc., Mountain View, CA, USA) according to its recommended procedure. AH was centrifuged at 3,000 × g for 15 min to remove cellular debris, after which the supernatants were collected. Phosphate-buffered saline (PBS) was added to the supernatants to a final volume of 250 µl. Then, 63 µl of precipitation solution for each 100 µl of AH were added to the supernatants and incubated overnight. The exosome pellets were obtained by centrifugation at 12,000 × g for 90 min and then suspended in 100 µl PBS. The numbers and sizes of exosomes were measured using NanoSight NS10 (Malvern Instruments, Rancho Cucamonga, CA, USA).
Total RNA isolation and miRNA analysis by OpenArray
The total RNA was extracted from the pooled human AH exosome samples in two groups (8 myopia patients and 8 control subjects) using the TRIsure RNA isolation kit (Bioline, London, UK) according to the recommended procedure. The RNA sample was quantified using NanoDrop Spectrophotometer ND-1000 (Wilmington, DE, USA).
MicroRNA profiling studies were analysed by TaqMan® OpenArray® MicroRNA Panels (Thermo Fisher, Waltham, MA, USA). This technology uses a microscope slide-sized plate with 3,072 through-holes to perform real-time PCR. The technical services provided by the Genomics Center for Clinical and Biotechnological Applications (National Yang-Ming University) according to the recommended procedure. Briefly, cDNA was synthesized with Megaplex Primer Pools A and B and MicroRNA Reverse Transcription Kit (Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s protocol. Then, cDNA was amplified using the primer pools Megaplex PreAmp Pools A and B and TaqMan PreAmp Master Mix Solution (Thermo Fisher, Waltham, MA, USA). Amplified cDNA was added to the TaqMan OpenArray Real‐Time PCR Master Mix (Thermo Fisher, Waltham, MA, USA) and distributed on the 384‐well sample loading plate. TaqMan® OpenArray® Human MicroRNA Panels are automatically loaded using the AccuFill™ System. Real-time PCR was conducted on the QuantStudio 12K Flex Real‐Time PCR System (Thermo Fisher, Waltham, MA, USA). The OpenArray output was analyzed using the Thermo Fisher Cloud software according to their recommended procedures (Pereira et al. 2018). MiRNA species with cycle threshold (Crt) value ≥ 35 were considered below the detection threshold. Because of the lack of a universally applicable endogenous control for miRNAs isolated from exosomes, we defined the miRNAs as myopia-specific or myopia-absent depending on the Crt values that could be detected only in myopia or control samples.
Prediction of miRNA target genes
All bioinformatics analyses for miRNA targeting gene prediction were performed by Ingenuity Pathway Analysis (IPA) knowledge base (Qiagen, Hilden, Germany). The IPA knowledge base contains a large amount of miRNA-mRNA interaction information from either particular algorithm prediction or experiments from previous published literature. By screening the IPA knowledge base, we decided to focus on 28 myopia-associated genes. Pairing the core sequences of miRNA and mRNA sequences of myopia-associated genes by the IPA knowledge base, we obtained the potential interaction of myopia-specific or myopia-absent miRNAs with myopia-associated genes.
Statistical analyses
Data were expressed as mean ± standard error of the mean. The measurements of axial lengths and exosome concentration were analyzed using a paired sample t-test. All data analyses were performed using SPSS version 24 (SPSS Inc., Chicago, IL, USA). A P value < 0.05 indicated a statistically significant difference.
Results
Collection of AH samples and exosome isolation
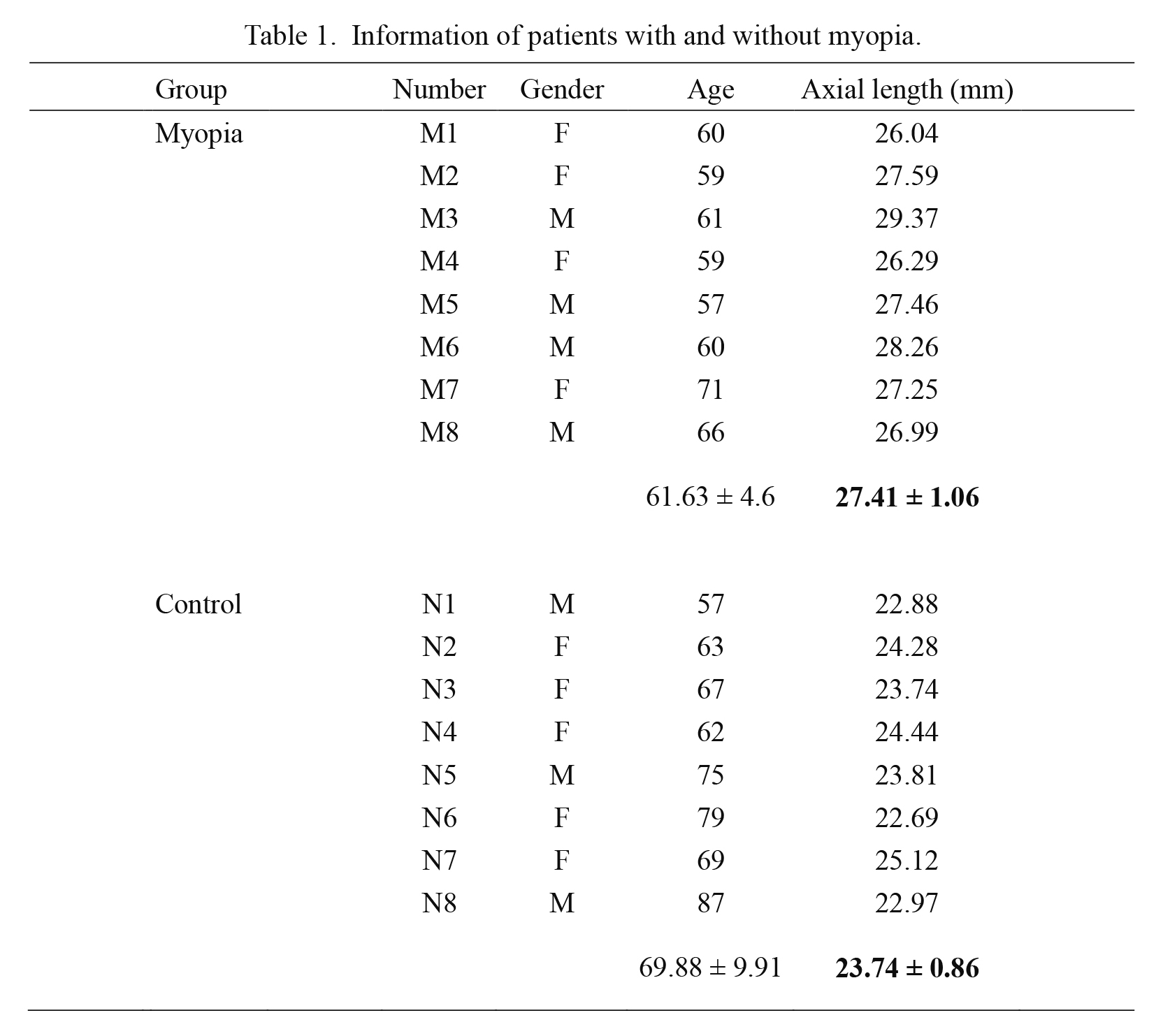
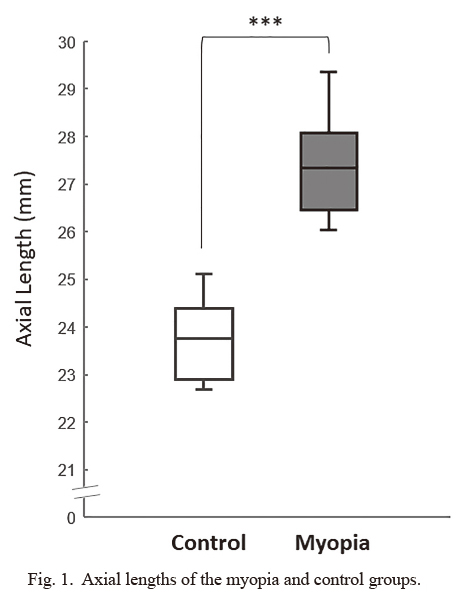
The AH samples were collected from patients undergoing cataract surgery at Taipei City Hospital. Myopia patients was defined as having an axial length > 26 mm. Between the myopia and control groups (8 myopia and 8 control), there were no differences in sex and age, except for axial length (P = 2.5136 × 10−6) (Table 1 and Fig. 1).
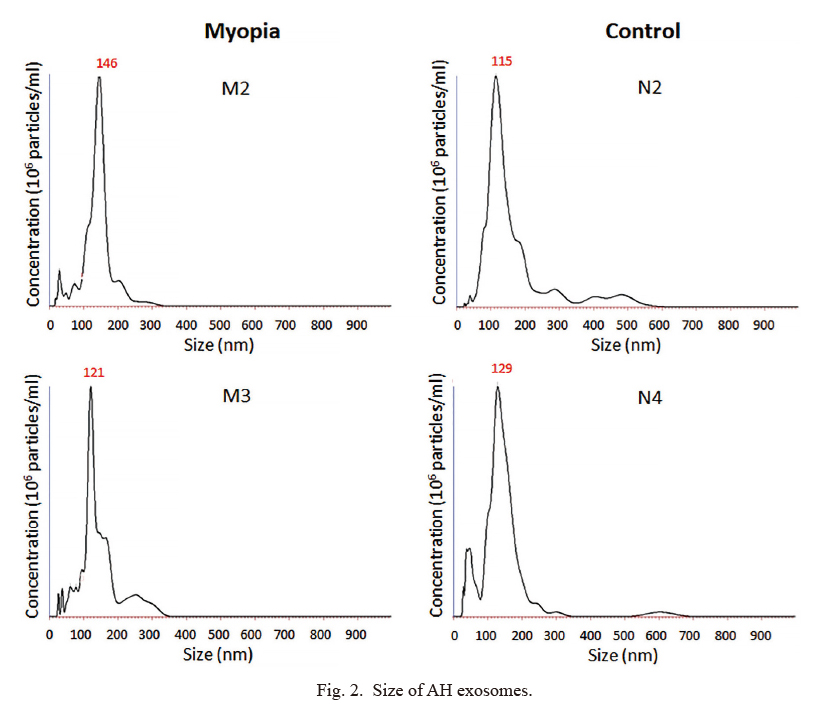
Exosomes were isolated from AH individually by using the ExoQuick precipitation solution. The numbers and sizes of exosomes were determined by nanoparticle tracking analysis system (Fig. 2). By calculating the exosomes for each sample, there was no significant difference in the numbers of exosomes obtained from the myopia and control groups (P = 0.773283) (Table 2).
Total RNA isolation and miRNA analysis by OpenArray
For the miRNA from each individual was insufficient for miRNA analysis (data not shown), we decided to mix equal amounts of individual exosomes of the same group (8 patients for myopia and 8 subjects for control) for total RNA isolation by TRIsure RNA isolation kit. Unexpectedly, we obtained 2.78-fold total RNA amount from the myopia group (4,007 ng) compared with that from control group (1,440 ng), although its implication remains to be elucidated. Then an equal amount of total RNA from each group was applied for high-throughput screening of 754 known human miRNAs by using TaqMan OpenArray Human MicroRNA Panel. Since the absence of internal control that could be used for miRNA QPCR quantitative in exosome, we selected the miRNAs that were only detected or not detected in the myopia group as myopia-specific or myopia-absent miRNAs. Finally, we obtained 15 myopia-specific miRNAs and four myopia-absent miRNAs (Table 3).

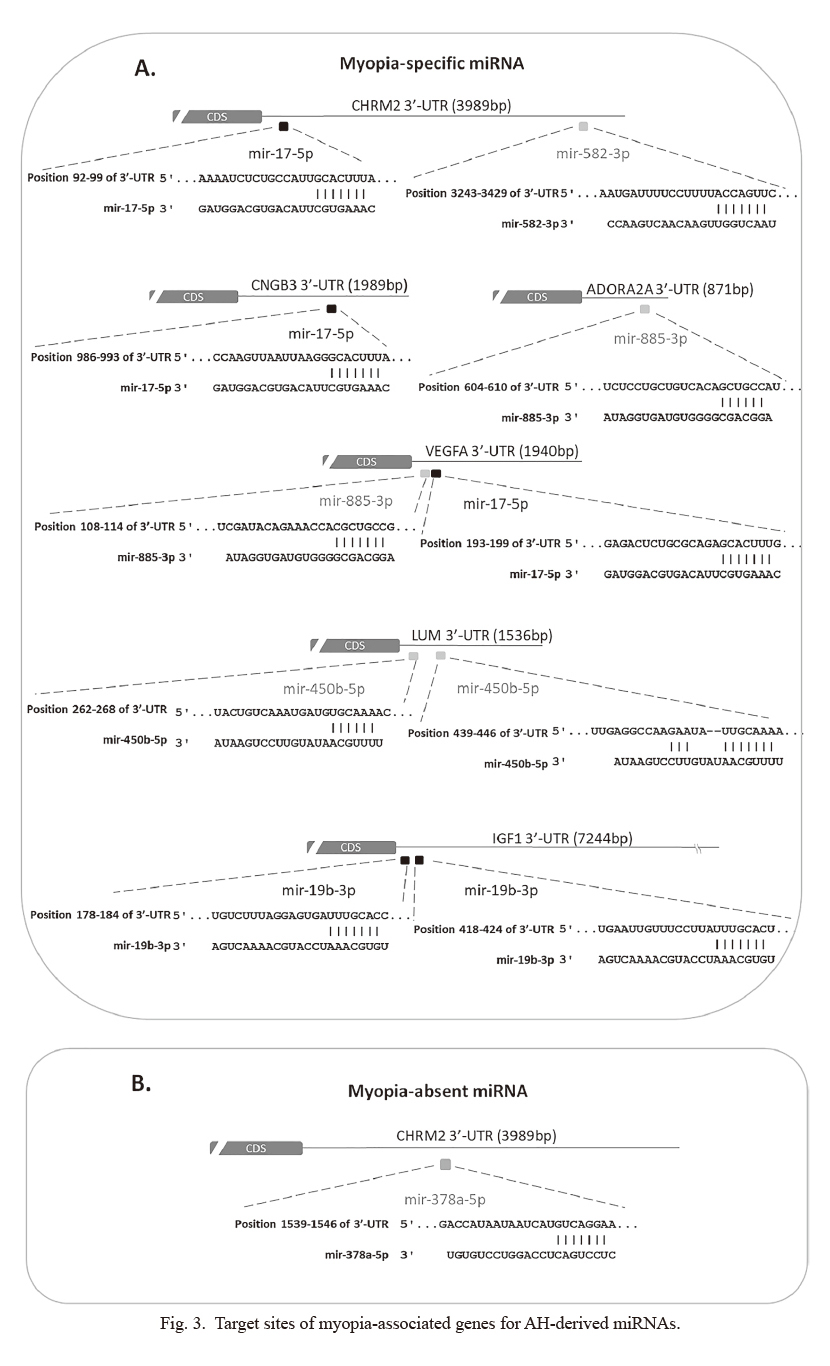
To explore the potential role of these miRNAs in myopia development, we attempted to search for their potential target genes. In most case, mature miRNAs interact with 3′-UTR (untranslated region) of target mRNAs by using 6~8 nucleotides (seed sequence) at their 5′end (O’Brien et al. 2018). Then the binding of miRNA on the 3′-UTR of mRNA will induce mRNA degradation or translation repression (O’Brien et al. 2018). In addition, the binding sites of miRNA on their target mRNA are usually evolutionarily conserved in mammals (Zhang and Wang 2017). Therefore, many sequence-based prediction software were used to predict target genes for miRNA.For the short length of miRNA binding site, prediction software usually product a huge number of target mRNAs, which makes it difficult to identify the exact disease associated miRNA-mRNA interaction. To minimize our miRNA target numbers, we decided to focus on 28 well-known myopia-associated genes. By using the miRNA-mRNA matchmaking tool of Ingenuity Pathway Analysis (IPA), we identified six genes (cholinergic receptor muscarinic 2 [CHRM2], cyclic nucleotide-gated channel beta 3 [CNGB3], vascular endothelial growth factor A [VEGFA], adenosine A2a receptor [ADORA2A], insulin-like growth factor 1 [IGF1], and lumican [LUM]) that may be regulated by 5 myopia-specific miRNAs (has-miR-582-3p, has-miR-17-5p, has-miR-885-3p, has-miR-19b-3p, and has-miR450b-5p). On the other hand, CHRM2 could be the target of a myopia-absent miRNA, has-miR-378a-5p (Fig. 3A, B). Four miRNA-mRNA binding site sequences were highly conserved in mammals (Fig. 3). In addition, has-miR-17-5p regulated the expression of VEGFA was indicated in previous studies (Ye et al. 2008). These results suggested that exosomal miRNAs might involve in myopia development.
Discussion
In this study, we demonstrate that exosomes are common in AH of patients with myopia. The sizes and numbers of exosomes in patients with myopia and control subjects showed no significant differences. However, exosomes collected from the myopia group contained more total RNA amounts than those in the control group. Using bioinformatics tools for miRNA targets prediction, we identified six myopia-specific miRNAs and one myopia-absent miRNA and their potential target genes associated with myopia. Further studies would be required in understanding their molecular mechanism and pathogenesis of myopia. To our knowledge, this is the first study to analyze the miRNA profiles in exosomes that were isolated from the AH of patients with myopia.
AH is not only a nutrient supplier and waste transporter but also a mediator for cell-cell communication (Ji et al. 2018). Proteomic and transcriptomic analyses have demonstrated that numbers of proteins and miRNAs present in AH (Kim et al. 2012; Dunmire et al. 2013; Tanaka et al. 2014; Soria et al. 2015). Moreover, alterations in protein or miRNA profiles have been associated with various eye diseases, such cataract, glaucoma, and corneal diseases (Dunmire et al. 2013; Tanaka et al. 2014; Drewry et al. 2018; Suzuki et al. 2019). Therefore, protein or miRNA profiles in AH may reflect specific functions in the eyes and are the best candidates of diagnostic biomarkers for ocular diseases. Recent studies suggest that these “cargoes” are packaged into small vesicles, i.e., exosomes, and delivered to specific target tissues (van der Merwe and Steketee 2017). However, since the limitation of only up to 100 μl of AH per eye can be safely collected through surgery (Wecker et al. 2016). Few AH samples make it more difficult to isolate and analyze miRNA or protein profiles in exosomes for individual patients.
The origin and target of these exosomes in AH is still unclear. Previous studies have shown that exosomes are secreted by trabecular meshwork cells containing a unique glaucoma-causing protein, myocilin (Hoffman et al. 2009). Dismuke et al. (2015) suggested that the primary source of exosomes is the non-pigmented ciliary epithelium, where AH is actively secreted. Theoretically, any cell in contact with AH may contribute to the release or uptake exosomes for cellular communication. To explore their origin and targets of AH exosomes will be helpful to understand the development of eye diseases.
Even though there is no significant difference in the number of exosomes, exosomes from the myopia group contained more RNA amounts than those from the control group. By OpenArray analysis, we identified 15 myopia-specific and 4 myopia-absent miRNAs (Table 3). Two myopia-specific miRNAs, has-miR-518d-3p and has-miR-518d-3p, have been shown to express in the retinal tissue in previous studies. Ayaz and Dinc (2018) suggested that has-miR-518d-3p was upregulated in human retinal pigment epithelial cell line against oxidative stress. Desjarlais et al. (2019) found has-miR-152-3p in the choroid was overexpressed in an oxygen-induced retinopathy mouse model. Since evidence suggested oxidative stress may help explain the altered regulatory pathways in myopia (Francisco et al. 2015), further studies are required to explore the function of has-miR-518d-3p and has-miR-152-3p in myopia development.
To explore the role of myopia-specific and myopia-absent miRNAs, miRNA-mRNA matchmaking tools of IPA was applied to predict their targets. We obtained six potential downstream genes, which have been shown to be associated with myopia (Fig. 3). ADORA2A is a type of adenosine receptor and expressed in the retina, choroid, and sclera. Its expression is increased in the deprivation myopia animal model and may play a role in the regulation of eye growth (Cui et al. 2010). Muscarinic antagonists, such as atropine and pirenzepine, have been therapeutically used to slow the progression of myopia in children (Ganesan and Wildsoet 2010). CHRM2 is one of the muscarinic receptor subtypes. Its mutant mice are resistant to lens-induced myopia (Barathi et al. 2013). Injection of IGF1 into the eyes of chicks increases the axial length (Feldkaemper et al. 2009). A genetic study also indicated the association of IGF1 polymorphisms and high-grade myopia in humans (Metlapally et al. 2010). VEGF is a dimeric glycoprotein that is responsible for neovascularization and fenestrations of the choriocapillaris (Kwak et al. 2000). Bevacizumab, an antibody against human VEGF, has been shown to inhibit choroidal thickening and suppress deprivation myopia in chicken (Mathis et al. 2014). CNGB3 is found to affect photoreceptors and cause eye diseases, especially achromatopsia (Kohl et al. 2005). Recent genome-wide scan of families with myopia reveal significant linkage to CNGB3 (Musolf et al. 2019). LUM, a keratan sulfate proteoglycan, is one of the major extracellular matrix components of the sclera. LUM mutation may induce the increase in axial lengths and abnormal structures and distribution of collagen fibrils in mouse sclera (Song et al. 2016). Genetic polymorphism of LUM has been suggested as a risk factor for the pathogenesis of high myopia (Wang et al. 2017). Gene knockout animal studies suggested that LUM was potential pathological myopia gene (Chakravarti et al. 2003; Yeh et al. 2010). Therefore, increasing the expression of has-miR-450b-5p (as observed in our studies) to inhibit the expression of LUM would be a reasonable explanation for the development of myopia. Further studies are required to confirm this hypothesis.
Our results are the pioneering findings to understand the potential role of exosomal miRNA in myopia development. Further analysis to identify the target tissues of these exosomes and understand the function of miRNAs may provide significant information for the diagnosis, clinical treatment, and potential prognosis of myopia.
Acknowledgments
We acknowledge the technical services provided by the Genomics Center for Clinical and Biotechnological Applications of National Yang-Ming University. The core facility is supported by the National Core Facility for Biopharmaceuticals (NCFB), Ministry of Science and Technology. This study was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 106-2314-B-532-007).
Conflict of Interest
The authors declare no conflict of interest.
References
-
Ayaz,
L. &
Dinc,
E.
(2018) Evaluation of microRNA responses in ARPE-19 cells against the oxidative stress. Cutan. Ocul. Toxicol., 37, 121-126.
-
Barathi,
V.A.,
Kwan,
J.L.,
Tan,
Q.S.,
Weon,
S.R.,
Seet,
L.F.,
Goh,
L.K.,
Vithana,
E.N. &
Beuerman,
R.W.
(2013) Muscarinic cholinergic receptor (M2) plays a crucial role in the development of myopia in mice. Dis. Model. Mech., 6, 1146-1158.
-
Carr,
B.J. &
Stell,
W.K.
(1995) The Science Behind Myopia. In Webvision: The Organization of the Retina and Visual System, edited by Kolb, H., Fernandez, E. & Nelson, R., Salt Lake City, UT.
-
Chakravarti,
S.,
Paul,
J.,
Roberts,
L.,
Chervoneva,
I.,
Oldberg,
A. &
Birk,
D.E.
(2003) Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Invest. Ophthalmol. Vis. Sci., 44, 2422-2432.
-
Cheng,
C.Y.,
Hsu,
W.M.,
Liu,
J.H.,
Tsai,
S.Y. &
Chou,
P.
(2003) Refractive errors in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest. Ophthalmol. Vis. Sci., 44, 4630-4638.
-
Coca-Prados,
M. &
Escribano,
J.
(2007) New perspectives in aqueous humor secretion and in glaucoma: the ciliary body as a multifunctional neuroendocrine gland. Prog. Retin. Eye Res., 26, 239-262.
-
Cui,
D.,
Trier,
K.,
Zeng,
J.,
Wu,
K.,
Yu,
M. &
Ge,
J.
(2010) Adenosine receptor protein changes in guinea pigs with form deprivation myopia. Acta Ophthalmol., 88, 759-765.
-
Desjarlais,
M.,
Rivera,
J.C.,
Lahaie,
I.,
Cagnone,
G.,
Wirt,
M.,
Omri,
S. &
Chemtob,
S.
(2019) MicroRNA expression profile in retina and choroid in oxygen-induced retinopathy model. PLoS One, 14, e0218282.
-
Dismuke,
W.M.,
Challa,
P.,
Navarro,
I.,
Stamer,
W.D. &
Liu,
Y.
(2015) Human aqueous humor exosomes. Exp. Eye Res., 132, 73-77.
-
Drewry,
M.D.,
Challa,
P.,
Kuchtey,
J.G.,
Navarro,
I.,
Helwa,
I.,
Hu,
Y.,
Mu,
H.,
Daniel Stamer,
W.,
Kuchtey,
R.W. &
Liu,
Y.
(2018) Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet., 27, 1263-1275.
-
Duan,
X.,
Lu,
Q.,
Xue,
P.,
Zhang,
H.,
Dong,
Z.,
Yang,
F. &
Wang,
N.
(2008) Proteomic analysis of aqueous humor from patients with myopia. Mol. Vis., 14, 370-377.
-
Dunmire,
J.J.,
Lagouros,
E.,
Bouhenni,
R.A.,
Jones,
M. &
Edward,
D.P.
(2013) MicroRNA in aqueous humor from patients with cataract. Exp. Eye Res., 108, 68-71.
-
Feldkaemper,
M.P.,
Neacsu,
I. &
Schaeffel,
F.
(2009) Insulin acts as a powerful stimulator of axial myopia in chicks. Invest. Ophthalmol. Vis. Sci., 50, 13-23.
-
Francisco,
B.M.,
Salvador,
M. &
Amparo,
N.
(2015) Oxidative stress in myopia. Oxid. Med. Cell. Longev., 2015, 750637.
-
Ganesan,
P. &
Wildsoet,
C.F.
(2010) Pharmaceutical intervention for myopia control. Expert Rev. Ophthalmol., 5, 759-787.
-
Goel,
M.,
Picciani,
R.G.,
Lee,
R.K. &
Bhattacharya,
S.K.
(2010) Aqueous humor dynamics: a review. Open Ophthalmol. J., 4, 52-59.
-
Henderson,
M.C. &
Azorsa,
D.O.
(2012) The genomic and proteomic content of cancer cell-derived exosomes. Front. Oncol., 2, 38.
-
Hoffman,
E.A.,
Perkumas,
K.M.,
Highstrom,
L.M. &
Stamer,
W.D.
(2009) Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci., 50, 1313-1318.
-
Holden,
B.,
Sankaridurg,
P.,
Smith,
E.,
Aller,
T.,
Jong,
M. &
He,
M.
(2014) Myopia, an underrated global challenge to vision: where the current data takes us on myopia control. Eye (Lond.), 28, 142-146.
-
Ji,
Y.,
Rong,
X. &
Lu,
Y.
(2018) Metabolic characterization of human aqueous humor in the cataract progression after pars plana vitrectomy. BMC Ophthalmol., 18, 63.
-
Jia,
Y.,
Hu,
D.N. &
Zhou,
J.
(2014) Human aqueous humor levels of TGF- beta2: relationship with axial length. Biomed. Res. Int., 2014, 258591.
-
Kim,
T.W.,
Kang,
J.W.,
Ahn,
J.,
Lee,
E.K.,
Cho,
K.C.,
Han,
B.N.,
Hong,
N.Y.,
Park,
J. &
Kim,
K.P.
(2012) Proteomic analysis of the aqueous humor in age-related macular degeneration (AMD) patients. J. Proteome Res., 11, 4034-4043.
-
Kohl,
S.,
Varsanyi,
B.,
Antunes,
G.A.,
Baumann,
B.,
Hoyng,
C.B.,
Jagle,
H.,
Rosenberg,
T.,
Kellner,
U.,
Lorenz,
B.,
Salati,
R.,
Jurklies,
B.,
Farkas,
A.,
Andreasson,
S.,
Weleber,
R.G.,
Jacobson,
S.G.,
et al. (2005) CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur. J. Hum. Genet., 13, 302-308.
-
Kwak,
N.,
Okamoto,
N.,
Wood,
J.M. &
Campochiaro,
P.A.
(2000) VEGF is major stimulator in model of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci., 41, 3158-3164.
-
Lee,
Y.S.,
Tresguerres,
M.,
Hess,
K.,
Marmorstein,
L.Y.,
Levin,
L.R.,
Buck,
J. &
Marmorstein,
A.D.
(2011) Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. J. Biol. Chem., 286, 41353-41358.
-
Lin,
L.L.,
Shih,
Y.F.,
Hsiao,
C.K. &
Chen,
C.J.
(2004) Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann. Acad. Med. Singapore, 33, 27-33.
-
Liu,
Y.,
Bailey,
J.C.,
Helwa,
I.,
Dismuke,
W.M.,
Cai,
J.,
Drewry,
M.,
Brilliant,
M.H.,
Budenz,
D.L.,
Christen,
W.G.,
Chasman,
D.I.,
Fingert,
J.H.,
Gaasterland,
D.,
Gaasterland,
T.,
Gordon,
M.O.,
Igo,
R.P. Jr.,
et al. (2016) A common variant in MIR182 is associated with primary open-angle glaucoma in the NEIGHBORHOOD consortium. Invest. Ophthalmol. Vis. Sci., 57, 3974-3981.
-
Mathis,
U.,
Ziemssen,
F. &
Schaeffel,
F.
(2014) Effects of a human VEGF antibody (Bevacizumab) on deprivation myopia and choroidal thickness in the chicken. Exp. Eye Res., 127, 161-169.
-
Meng,
W.,
Butterworth,
J.,
Malecaze,
F. &
Calvas,
P.
(2011) Axial length of myopia: a review of current research. Ophthalmologica, 225, 127-134.
-
Metlapally,
R.,
Ki,
C.S.,
Li,
Y.J.,
Tran-Viet,
K.N.,
Abbott,
D.,
Malecaze,
F.,
Calvas,
P.,
Mackey,
D.A.,
Rosenberg,
T.,
Paget,
S.,
Guggenheim,
J.A. &
Young,
T.L.
(2010) Genetic association of insulin-like growth factor-1 polymorphisms with high-grade myopia in an international family cohort. Invest. Ophthalmol. Vis. Sci., 51, 4476-4479.
-
Michael,
A.,
Bajracharya,
S.D.,
Yuen,
P.S.,
Zhou,
H.,
Star,
R.A.,
Illei,
G.G. &
Alevizos,
I.
(2010) Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis., 16, 34-38.
-
Morgan,
A.,
Young,
R.,
Narankhand,
B.,
Chen,
S.,
Cottriall,
C. &
Hosking,
S.
(2006) Prevalence rate of myopia in schoolchildren in rural Mongolia. Optom. Vis. Sci., 83, 53-56.
-
Morgan,
I. &
Rose,
K.
(2005) How genetic is school myopia? Prog. Retin. Eye Res., 24, 1-38.
-
Morgan,
I.G.,
Ohno-Matsui,
K. &
Saw,
S.M.
(2012) Myopia. Lancet, 379, 1739-1748.
-
Musolf,
A.M.,
Simpson,
C.L.,
Alexander,
T.A.,
Portas,
L.,
Murgia,
F.,
Ciner,
E.B.,
Stambolian,
D. &
Bailey-Wilson,
J.E.
(2019) Genome-wide scans of myopia in Pennsylvania Amish families reveal significant linkage to 12q15, 8q21.3 and 5p15.33. Hum. Genet., 138, 339-354.
-
O’Brien,
J.,
Hayder,
H.,
Zayed,
Y. &
Peng,
C.
(2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. (Lausanne), 9, 402.
-
Pan,
C.W.,
Ramamurthy,
D. &
Saw,
S.M.
(2012) Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol. Opt., 32, 3-16.
-
Pereira,
T.,
Brito,
J.A.R.,
Guimaraes,
A.L.S.,
Gomes,
C.C.,
de Lacerda,
J.C.T.,
de Castro,
W.H.,
Coimbra,
R.S.,
Diniz,
M.G. &
Gomez,
R.S.
(2018) microRNA profiling reveals dysregulated microRNAs and their target gene regulatory networks in cemento-ossifying fibroma. J. Oral Pathol. Med., 47, 78-85.
-
Raposo,
G. &
Stoorvogel,
W.
(2013) Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol., 200, 373-383.
-
Russo,
A.,
Semeraro,
F.,
Romano,
M.R.,
Mastropasqua,
R.,
Dell’Omo,
R. &
Costagliola,
C.
(2014) Myopia onset and progression: can it be prevented? Int. Ophthalmol., 34, 693-705.
-
Schneider,
A. &
Simons,
M.
(2013) Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res., 352, 33-47.
-
Smith,
M.J. &
Walline,
J.J.
(2015) Controlling myopia progression in children and adolescents. Adolesc. Health Med. Ther., 6, 133-140.
-
Song,
Y.,
Zhang,
F.,
Zhao,
Y.,
Sun,
M.,
Tao,
J.,
Liang,
Y.,
Ma,
L.,
Yu,
Y.,
Wang,
J. &
Hao,
J.
(2016) Enlargement of the axial Length and altered ultrastructural features of the sclera in a mutant lumican transgenic mouse model. PLoS One, 11, e0163165.
-
Soria,
J.,
Villarrubia,
A.,
Merayo-Lloves,
J.,
Elortza,
F.,
Azkargorta,
M.,
Alvarez de Toledo,
J.,
Rodriguez-Agirretxe,
I.,
Suarez,
T. &
Acera,
A.
(2015) Label-free LC-MS/MS quantitative analysis of aqueous humor from keratoconic and normal eyes. Mol. Vis., 21, 451-460.
-
Suzuki,
N.,
Yamaguchi,
T.,
Shibata,
S.,
Nagai,
T.,
Noma,
H.,
Tsubota,
K. &
Shimazaki,
J.
(2019) Cytokine levels in the aqueous humor are associated with corneal thickness in eyes with bullous keratopathy. Am. J. Ophthalmol., 198, 174-180.
-
Tanaka,
Y.,
Tsuda,
S.,
Kunikata,
H.,
Sato,
J.,
Kokubun,
T.,
Yasuda,
M.,
Nishiguchi,
K.M.,
Inada,
T. &
Nakazawa,
T.
(2014) Profiles of extracellular miRNAs in the aqueous humor of glaucoma patients assessed with a microarray system. Sci. Rep., 4, 5089.
-
van der Merwe,
Y. &
Steketee,
M.B.
(2017) Extracellular Vesicles: Biomarkers, Therapeutics, and Vehicles in the Visual System. Curr. Ophthalmol. Rep., 5, 276-282.
-
Vlassov,
A.V.,
Magdaleno,
S.,
Setterquist,
R. &
Conrad,
R.
(2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta, 1820, 940-948.
-
Wang,
G.F.,
Ji,
Q.S.,
Qi,
B.,
Yu,
G.C.,
Liu,
L. &
Zhong,
J.X.
(2017) The association of lumican polymorphisms and high myopia in a Southern Chinese population. Int. J. Ophthalmol., 10, 1516-1520.
-
Wecker,
T.,
Hoffmeier,
K.,
Plotner,
A.,
Gruning,
B.A.,
Horres,
R.,
Backofen,
R.,
Reinhard,
T. &
Schlunck,
G.
(2016) MicroRNA profiling in aqueous humor of individual human eyes by next-generation sequencing. Invest. Ophthalmol. Vis. Sci., 57, 1706-1713.
-
Wong,
T.Y.,
Foster,
P.J.,
Hee,
J.,
Ng,
T.P.,
Tielsch,
J.M.,
Chew,
S.J.,
Johnson,
G.J. &
Seah,
S.K.
(2000) Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest. Ophthalmol. Vis. Sci., 41, 2486-2494.
-
Wong,
T.Y.,
Foster,
P.J.,
Johnson,
G.J.,
Klein,
B.E. &
Seah,
S.K.
(2001) The relationship between ocular dimensions and refraction with adult stature: the Tanjong Pagar Survey. Invest. Ophthalmol. Vis. Sci., 42, 1237-1242.
-
Wu,
P.C.,
Huang,
H.M.,
Yu,
H.J.,
Fang,
P.C. &
Chen,
C.T.
(2016) Epidemiology of Myopia. Asia Pac. J. Ophthalmol. (Phila), 5, 386-393.
-
Ye,
W.,
Lv,
Q.,
Wong,
C.K.,
Hu,
S.,
Fu,
C.,
Hua,
Z.,
Cai,
G.,
Li,
G.,
Yang,
B.B. &
Zhang,
Y.
(2008) The effect of central loops in miRNA:MRE duplexes on the efficiency of miRNA-mediated gene regulation. PLoS One, 3, e1719.
-
Yeh,
L.K.,
Liu,
C.Y.,
Kao,
W.W.,
Huang,
C.J.,
Hu,
F.R.,
Chien,
C.L. &
Wang,
I.J.
(2010) Knockdown of zebrafish lumican gene (zlum) causes scleral thinning and increased size of scleral coats. J. Biol. Chem., 285, 28141-28155.
-
Zhang,
F. &
Wang,
D.
(2017) The Pattern of microRNA binding site distribution. Genes (Basel), 8.
-
Zhang,
K.,
Zhu,
X.,
Chen,
M.,
Sun,
X.,
Yang,
J.,
Zhou,
P. &
Lu,
Y.
(2016) Elevated transforming growth factor-beta2 in the aqueous humor: a possible explanation for high rate of capsular contraction syndrome in high myopia. J. Ophthalmol., 2016, 5438676.
-
Zhang,
Y.,
Davidson,
B.R.,
Stamer,
W.D.,
Barton,
J.K.,
Marmorstein,
L.Y. &
Marmorstein,
A.D.
(2009) Enhanced inflow and outflow rates despite lower IOP in bestrophin-2-deficient mice. Invest. Ophthalmol. Vis. Sci., 50, 765-770.