Abstract
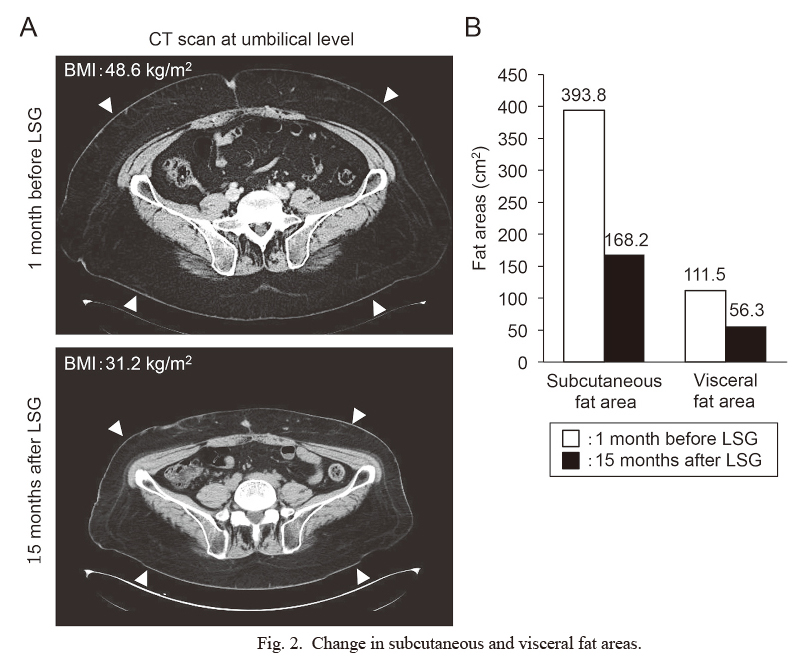
Hypothalamic obesity is a clinical syndrome characterized by severe and refractory obesity that is caused by hypothalamic function impairment. Recently, bariatric surgery has been attempted for patients with hypothalamic obesity after craniopharyngioma, but experiences have not yet been accumulated in other hypothalamic disorders. Here, we report the case of a 39-year-old male patient with panhypopituitarism who received laparoscopic sleeve gastrectomy (LSG) after intracranial germinoma treatment. The patient was diagnosed with intracranial germinoma at age 15 and achieved complete remission after radiotherapy (total 50 Gy). He was obese during diagnosis [body mass index (BMI), 29.2 kg/m2], and his obesity gradually worsened after the intracranial germinoma treatment, and LSG was considered when his BMI was 48.6 kg/m2. After 1 month of hospitalized diet-exercise program, LSG was performed. After LSG, his BMI gradually decreased and reached 38.8 kg/m2 on the day of discharge (6 weeks after the surgery). Five months after LSG, his insulin resistance improved, but insulin hypersecretion remained. Fifteen months after the surgery, his BMI was 31.2 kg/m2, with marked decrease in visceral and subcutaneous fat areas (from 393.8 cm2 and 168.2 cm2 before the surgery to 111.5 cm2 and 56.3 cm2, respectively.). To our knowledge, this is the first case of LSG for hypothalamic obesity after intracranial germinoma treatment. Although the pathophysiology of hypothalamic obesity is different from that of primary obesity, LSG could be a successful therapeutic choice for patients with hypothalamic obesity after the intracranial germinoma treatment.
Introduction
The hypothalamus is regarded as the center of appetite, satiety, and energy homeostasis (Müller 2016). Functional disorders in the hypothalamus cause the dysregulation of energy homeostasis, which results in severe weight gain termed as hypothalamic obesity (Müller 2016). Hypothalamic disorders are induced by various causes such as intracranial tumor infiltration, trauma, inflammatory diseases, or as a result of surgical and/or radiation therapy for intracranial tumors represented by craniopharyngioma (Bingham et al. 2012; Rosenfeld et al. 2014). Although hypothalamic obesity threatens long-term quality of life (Müller 2010; Erfurth 2015), lifestyle intervention or pharmacotherapy has not yet proven effective (Müller 2016).
Recently, compared with medical treatment, bariatric surgery has shown beneficial effects on weight reduction, the improvement of metabolic disorders, the prevention of cardiovascular diseases and long-term mortality in patients with severe primary obesity (Sjöström et al. 2007, 2012; Courcoulas et al. 2013, 2014). Based on its results with primary obesity, there are also reports of bariatric surgery in patients with hypothalamic obesity after craniopharyngioma; however, the weight reduction effect of bariatric surgery is currently controversial (Bretault et al. 2013; Weismann et al. 2013; Wijnen et al. 2017), and those experiences have not been well described in patients after intracranial diseases other than craniopharyngioma.
Here, we describe the case report of a patient after intracranial germinoma treatment who achieved remarkable weight reduction via laparoscopic sleeve gastrectomy (LSG). To our knowledge, this is a first case report of LSG for hypothalamic obesity after the intracranial germinoma treatment.
Case Presentation
A 39-year-old Japanese male with panhypopituitarism and hypertension was introduced for LSG because of severe obesity (height, 169 cm; weight, 138.8 kg; BMI, 48.6 kg/m2).
The patient first displayed polyuria at age 10 and was diagnosed with panhypopituitarism because of the lack of secondary sexual characteristics at age 15. He also complained of bitemporal hemianopsia, and brain MRI revealed a 15-mm suprasellar tumor and a 15-mm pineal tumor. Intracranial germinoma was the most likely because the levels of CEA and AFP in the cerebrospinal fluid (CSF) were low and HCGβ level in the CSF was higher than the reference range of plasma HCGβ (Table 1). After initiating hormone replacement [0.5 mg/day of dexamethasone, 50 µg/day of levothyroxine, and 20 µg/day of intranasal desmopressin acetate hydrate (nasal drop)], he underwent radiotherapy (20 Gy for local irradiation and 30 Gy for whole-brain irradiation) at age 16 and achieved complete remission. He had been obese (BMI of 29.2 kg/m2) at age 16, which had been considered as hypothalamic obesity owing to his medical history. The obesity had been refractory, and it worsened despite long-term medical intervention, such as nutrition instructions and hospitalized diet-exercise program.
At age 38, he was hospitalized due to the exacerbation of panhypopituitarism after the self-discontinuation of hormone replacement. His laboratory data just before re-initiating the hormone replacement is shown in Table 1. In spite of the loss of appetite, his BMI was 42.2 kg/m2, and soon after re-initiating the hormone replacement, his appetite recovered and BMI gradually increased to 47.0 kg/m2 over the period of 8 months following his discharge despite continuous nutrition instructions to him and his family. Fortunately, the various complications associated with obesity including diabetes mellitus, cardiovascular disease, and osteoarthropathy (Jensen et al. 2014) had not been developped in the patient. Decreasing the risk of developing those complications via weight reduction was important for maintaining his health in the future even after his elderly parents who took care of him would be no more. Therefore, we considered bariatric surgery as an effective intervention to improve his severe obesity. He received 5 mg/day of carvedilol as an antihypertensive drug; his hormone replacement medications comprised 15 mg/day of hydrocortisone, 100 µg/day of levothyroxine, 0.4 mg/day of somatotropin, 125 mg/week of testosterone enanthate, and 5 µg/day of desmopressin acetate hydrate (nasal spray).
Additionally, he had a borderline intelligence or mild intellectual disability as assessed by the Wechsler Adult Intelligence Scale-III (Table 1), but he could understand the importance of bariatric surgery. According to the guidelines of the Japanese Society for Treatment of Obesity (Guidelines Committee, Japanese Society for Treatment of Obesity 2013), bariatric surgery is indicated only for primary obesity. Although his obesity was classified as secondary obesity, his hypothalamic damage was irreversible and the effect of medical treatment was not expected. Through discussion among the members of the surgical unit, we confirmed that bariatric surgery could be performed according to previous reports on hypothalamic obesity. Then, we consulted the psychiatric units and doctors who had been evaluating his higher order function and confirmed that his intelligence is sufficient for undergoing bariatric surgery. Therefore, we concluded that he was a candidate for LSG, the only bariatric surgery covered by Japanese medical insurance. After obtaining informed consent from him and his parents, the surgical unit, the rehabilitation unit and we planned LSG with preceded 1-month hospitalized diet-exercise program for weight reduction to prevent perioperative complications due to obesity. The hospitalized care succeeded in achieving our weight reduction goal before the surgery (BMI decreased from 48.6 to 43.3 kg/m2) (Fig. 1A), and LSG was performed. The perioperative hormone replacement combined a stress dose of glucocorticoid and regular doses of levothyroxine, somatotropin, and testosterone. Because strict water restriction would be unavoidable after LSG and his thirst was intact, we adjusted the dose of desmopressin before the surgery to easily compensate his water intake with infusion fluid after LSG. Beginning 21 days after the surgery, moderate fasting hypoglycemia was observed but recovered until 3 weeks after his discharge. Because his secretional capacity of counter-regulatory hormones for hypoglycemia, including cortisol and GH, were insufficient due to hypopituitarism, we increased the dose of hydrocortisone (20 mg/day) and somatotropin (0.7 mg/day) until his fasting glucose level normalized.
Soon after the surgery, diet-exercise program was re-initiated, and his BMI decreased until his discharge 6 weeks after LSG (BMI decreased from 43.3 to 38.8 kg/m2) (Fig. 1A). After his discharge, although his hospitalized diet-exercise program was over, his BMI was continuously decreased to 31.2 kg/m2 until 15 months after LSG (Fig. 1B). An abdominal computed tomography scan showed the marked decrease in his visceral and subcutaneous fat areas. The imaging analyses with ImageJ software (https://imagej.nih.gov/ij/) demonstrated that 1 month before LSG, his visceral and subcutaneous fat areas were 393.8 cm2 and 168.2 cm2 in size, respectively, which decreased to 111.5 cm2 and 56.3 cm2, respectively, 15 months after LSG (Fig. 2).
His 7-day average systolic blood pressure was 125.1 ± 4.6 mmHg 1 week before the surgery (he was receiving 5 mg/day of carvedilol during this time); 5 weeks after the surgery, the blood pressure decreased to 120.4 ± 9.8 mmHg without any antihypertensive drugs. Additionally, 75-g oral glucose tolerance tests were performed before and 5 months after LSG when his BMI was 34.0 kg/m2. As shown in Table 2, the levels of fasting plasma glucose and insulin as well as C-peptide immunoreactivity (CPR) before and 5 months after LSG seemed almost comparable. However, 5 months after LSG, the peak values of plasma insulin and CPR were observed at 60 min after glucose loading, in contrast to the findings of 75-g oral glucose tolerance test before LSG, where the highest values of plasma insulin and CPR were observed at 120 min after glucose loading. Plasma glucose after glucose loading seemed to normalize more rapidly at 5 months after LSG than before. His homeostasis model assessment for insulin resistance (HOMA-R) level, HOMA for beta cell function (HOMA-β) level, and insulinogenic index before LSG were 2.4, 360.0, and 2.5 pmol/mmol, respectively, and 5 months after the surgery, the values were 1.5, 306.0, and 3.5 pmol/mmol, respectively (Table 3).

Discussion
We have described the effect of LSG on weight reduction for a male patient with severe hypothalamic obesity and panhypopituitarism due to intracranial germinoma in his childhood. In terms of hypothalamic obesity, a small systematic review and an international registry revealed that any type of bariatric surgery, including LSG, successfully caused weight reduction in patients after craniopharyngioma (Bretault et al. 2013; Ni and Shi 2018) and in those with multiple hypothalamic diseases, except for intracranial germinoma (Rose et al. 2018). These results are similar to those obtained in this case report. A recent systematic review showed that LSG decreased BMI by 5-10 kg/m2 after 12 weeks in patients (Ni and Shi 2018). However, other clinical studies suggested that the beneficial effects of LSG in patients with craniopharyngioma were lesser than the effects of Roux-en-Y gastric bypass, the other procedure of bariatric surgery (Weismann et al. 2013; Wijnen et al. 2017). In our case, because preoperative hospitalized diet-exercise program succeeded in decreasing the BMI by 5.3 kg/m2 after 1 month, decrease in BMI by 4.5 kg/m2 during hospitalization and 6 weeks after LSG could also be affected by hospitalized diet-exercise program to a certain extent. However, because BMI was gradually decreased by 12.1 kg/m2 for nearly 13 months even after discharge, LSG would also have a significant effect on his sustainable weight reduction. Although long-term observation and evaluation are necessary, our case suggests that LSG can be a treatment for hypothalamic obesity after intracranial germinoma treatment, similar to that after craniopharyngioma treatment. Here, we discuss the weight reductive mechanisms and therapeutic indications of LSG in hypothalamic obesity after intracranial germinoma treatment.
First, hypothalamic obesity has a complex pathology. In such patients, a degree of hyperphagia, impaired satiety, and obsessive food-seeking behavior has been observed (Skorzewska et al. 1989). Increased vagal and decreased sympathetic tones induce insulin hypersecretion with insulin and leptin resistance (Lustig et al. 2003; Abuzzahab et al. 2019) and impair energy expenditure and basal metabolic rate (Holmer et al. 2010). These factors are important causes of hypothalamic obesity. LSG promotes weight reduction via two main mechanisms. One is the mechanical mechanism that induces the restriction of both volume and stomach distensibility (Yehoshua et al. 2008); the other is the endocrinological mechanism that induces appetite restriction by altering the levels and interactions of the diet, gut, and brain hormones (Korner and Leibel 2003; Ionut et al. 2013). Ghrelin is a major hormone secreted by the oxyntic glands in the fundus of the stomach (Abdemur et al. 2014); it increases food intake and induces weight gain (Nakazato et al. 2001) by stimulating the hypothalamus. LSG significantly decreases the postprandial levels of ghrelin and promotes weight reduction by inducing satiety in patients with primary obesity (Ionut et al. 2013). Glucagon-like peptide-1 (GLP-1) is other major hormone primarily secreted by small intestine L cells after food contact (Koliaki and Doupis 2011). GLP-1 not only increases insulin secretion but also slows gastric emptying and intestinal motility and reduces appetite via sensory vagal nerves and direct gut-specific mechanisms (Koliaki and Doupis 2011). LSG increases GLP-1 and promotes improved glucose metabolism, which reduces hunger and increases satiety in patients with primary obesity (Ionut et al. 2013). Because the hypothalamic regulation of satiety is impaired in patients with hypothalamic obesity (Skorzewska et al. 1989), the effects of ghrelin or GLP-1 on the regulation of the hypothalamic appetite are limited, whereas the effects on gastrointestinal tract are preserved. Therefore, in the present study, the mechanical mechanisms and gastrointestinal effects via GLP-1 mainly contributed to weight reduction, especially after the patient’s discharge.
Second, in hypothalamic obesity, enhanced insulin secretion is also thought to compensate for insulin resistance, which further increases visceral fat accumulation, resulting in severe obesity (Adachi et al. 2007). Before LSG, our patient showed higher levels of HOMA-R, the marker of insulin resistance, and higher levels of fast plasma insulin, CPR, and HOMA-β, indicating enhanced insulin secretion that is consistent with the clinical features of hypothalamic obesity. 5 months after LSG, HOMA-R improved with a marked decrease in both visceral and subcutaneous fat areas and in plasma glucose levels after the glucose load, but fast plasma insulin, CPR, and HOMA-β remained at high levels. Additionally, the insulinogenic index that indicates early insulin secretory capacity maintained high levels before and 5 months after LSG. Therefore, in our case, although LSG improved insulin resistance along with decreasing fat areas and improving glycemic metabolism, insulin secretion remained higher, especially in the early phase after the glucose load. This clinical feature is different from that of the patients with primary obesity in whom insulin hypersecretion is ameliorated with weight reduction after LSG (Nannipieri et al. 2013; Thomas et al. 2016). Because LSG increased GLP-1 (Ionut et al. 2013) that enhances insulin secretion after diet (Koliaki and Doupis 2011), it is possible that, in our case, LSG could further enhance insulin secretion, although insulin hypersecretion state already existed. Therefore, normalizing excessive insulin secretion would not be a major mechanism of weight reduction in our case. These physiological differences and limitations may be why the weight reductive effects of LSG on hypothalamic obesity are thought to be lesser than those on primary obesity (Weismann et al. 2013; Wijnen et al. 2017).
Third, treatment with cranial radiation therapy is a risk factor for intellectual disability in pediatric brain tumor survivors (Tonning Olsson et al. 2014). Our patient had a mild intellectual disability, but this disability did not prevent him from successfully following the 1-month diet exercise before and after LSG. This is an important reason why the patient achieved his remarkable weight reduction after LSG. If such patients have severe intellectual disabilities, bariatric surgery could be dangerous because this surgery involves many restrictions. Our case indicates the importance of cross-departmental evaluation for higher brain dysfunction in these patients.
In summary, we describe the first case report of a patient with panhypopituitarism who was suffering from severe hypothalamic obesity due to radiotherapy for intracranial germinoma who underwent LSG. LSG resulted in marked weight reduction, and the effects sustained for at least 15 months. As long as the treatment of panhypopituitarism and the evaluation of intellectual disability are adequate, LSG will remain as a beneficial therapeutic option in patients with refractory hypothalamic obesity with panhypopituitarism after intracranial germinoma treatment.
Acknowledgments
We thank Ms. Yasuko Tsukada and Ms. Akane Sugawara (Tohoku University) for technical support and secretarial assistance. We would like to thank Enago (https://www.enago.jp) for the English language review.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Abdemur,
A.,
Slone,
J.,
Berho,
M.,
Gianos,
M.,
Szomstein,
S. &
Rosenthal,
R.J.
(2014) Morphology, localization, and patterns of ghrelin-producing cells in stomachs of a morbidly obese population. Surg. Laparosc. Endosc. Percutan. Tech., 24, 122-126.
-
Abuzzahab,
M.J.,
Roth,
C.L. &
Shoemaker,
A.H.
(2019) Hypothalamic obesity: prologue and promise. Horm. Res. Paediatr., 91, 128-136.
-
Adachi,
M.,
Tsuchiya,
T.,
Muroya,
K.,
Asakura,
Y.,
Sekido,
K. &
Sato,
H.
(2007) Prevalence of obesity, hyperlipemia and insulin resistance in children with suprasellar brain tumors. Clin. Pediatr. Endocrinol., 16, 1-9.
-
Bingham,
N.C.,
Rose,
S.R. &
Inge,
T.H.
(2012) Bariatric surgery in hypothalamic obesity. Front. Endocrinol. (Lausanne), 3, 23.
-
Bretault,
M.,
Boillot,
A.,
Muzard,
L.,
Poitou,
C.,
Oppert,
J.M.,
Barsamian,
C.,
Gatta,
B.,
Müller,
H.,
Weismann,
D.,
Rottembourg,
D.,
Inge,
T.,
Veyrie,
N.,
Carette,
C. &
Czernichow,
S.
(2013) Clinical review: bariatric surgery following treatment for craniopharyngioma: a systematic review and individual-level data meta-analysis. J. Clin. Endocrinol. Metab., 98, 2239-2246.
-
Courcoulas,
A.P.,
Christian,
N.J.,
Belle,
S.H.,
Berk,
P.D.,
Flum,
D.R.,
Garcia,
L.,
Horlick,
M.,
Kalarchian,
M.A.,
King,
W.C.,
Mitchell,
J.E.,
Patterson,
E.J.,
Pender,
J.R.,
Pomp,
A.,
Pories,
W.J.,
Thirlby,
R.C.,
et al. (2013) Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA, 310, 2416-2425.
-
Courcoulas,
A.P.,
Goodpaster,
B.H.,
Eagleton,
J.K.,
Belle,
S.H.,
Kalarchian,
M.A.,
Lang,
W.,
Toledo,
F.G. &
Jakicic,
J.M.
(2014) Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg, 149, 707-715.
-
Erfurth,
E.M.
(2015) Endocrine aspects and sequel in patients with craniopharyngioma. J. Pediatr. Endocrinol. Metab., 28, 19-26.
-
Guidelines Committee, Japanese Society for Treatment of Obesity
(2013) JSTO guidelines for safety, quality, and excellence in bariatric surgery (2013 edition). http://plaza.umin.ne.jp/~jsto/gakujyutsu/updata/surgery_guideline_2013.pdf [Accessed: August 1, 2018] (in Japanese).
-
Holmer,
H.,
Pozarek,
G.,
Wirfalt,
E.,
Popovic,
V.,
Ekman,
B.,
Björk,
J. &
Erfurth,
E.M.
(2010) Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J. Clin. Endocrinol. Metab., 95, 5395-5402.
-
Ionut,
V.,
Burch,
M.,
Youdim,
A. &
Bergman,
R.N.
(2013) Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity (Silver Spring), 21, 1093-1103.
-
Jensen,
M.D.,
Ryan,
D.H.,
Apovian,
C.M.,
Ard,
J.D.,
Comuzzie,
A.G.,
Donato,
K.A.,
Hu,
F.B.,
Hubbard,
V.S.,
Jakicic,
J.M.,
Kushner,
R.F.,
Loria,
C.M.,
Millen,
B.E.,
Nonas,
C.A.,
Pi-Sunyer,
F.X.,
Stevens,
J.,
et al. (2014) 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation, 129, S102-138.
-
Koliaki,
C. &
Doupis,
J.
(2011) Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther., 2, 101-121.
-
Korner,
J. &
Leibel,
R.L.
(2003) To eat or not to eat: how the gut talks to the brain. N. Engl. J. Med., 349, 926-928.
-
Lustig,
R.H.,
Hinds,
P.S.,
Ringwald-Smith,
K.,
Christensen,
R.K.,
Kaste,
S.C.,
Schreiber,
R.E.,
Rai,
S.N.,
Lensing,
S.Y.,
Wu,
S. &
Xiong,
X.
(2003) Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab., 88, 2586-2592.
-
Müller,
H.L.
(2016) Craniopharyngioma and hypothalamic injury: latest insights into consequent eating disorders and obesity. Curr. Opin. Endocrinol. Diabetes Obes., 23, 81-89.
-
Müller,
H.L.
(2010) Increased daytime sleepiness in patients with childhood craniopharyngioma and hypothalamic tumor involvement: review of the literature and perspectives. Int. J. Endocrinol., 2010, 519607.
-
Nakazato,
M.,
Murakami,
N.,
Date,
Y.,
Kojima,
M.,
Matsuo,
H.,
Kangawa,
K. &
Matsukura,
S.
(2001) A role for ghrelin in the central regulation of feeding. Nature, 409, 194-198.
-
Nannipieri,
M.,
Baldi,
S.,
Mari,
A.,
Colligiani,
D.,
Guarino,
D.,
Camastra,
S.,
Barsotti,
E.,
Berta,
R.,
Moriconi,
D.,
Bellini,
R.,
Anselmino,
M. &
Ferrannini,
E.
(2013) Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J. Clin. Endocrinol. Metab., 98, 4391-4399.
-
Ni,
W. &
Shi,
X.
(2018) Interventions for the treatment of craniopharyngioma-related hypothalamic obesity: a systematic review. World Neurosurg., 118, e59-e71.
-
Rose,
S.R.,
Horne,
V.E.,
Bingham,
N.,
Jenkins,
T.,
Black,
J. &
Inge,
T.
(2018) Hypothalamic obesity: 4 years of the international registry of hypothalamic obesity disorders. Obesity (Silver Spring), 26, 1727-1732.
-
Rosenfeld,
A.,
Arrington,
D.,
Miller,
J.,
Olson,
M.,
Gieseking,
A.,
Etzl,
M.,
Harel,
B.,
Schembri,
A. &
Kaplan,
A.
(2014) A review of childhood and adolescent craniopharyngiomas with particular attention to hypothalamic obesity. Pediatr. Neurol., 50, 4-10.
-
Sjöström,
L.,
Narbro,
K.,
Sjöström,
C.D.,
Karason,
K.,
Larsson,
B.,
Wedel,
H.,
Lystig,
T.,
Sullivan,
M.,
Bouchard,
C.,
Carlsson,
B.,
Bengtsson,
C.,
Dahlgren,
S.,
Gummesson,
A.,
Jacobson,
P.,
Karlsson,
J.,
et al. (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med., 357, 741-752.
-
Sjöström,
L.,
Peltonen,
M.,
Jacobson,
P.,
Sjöström,
C.D.,
Karason,
K.,
Wedel,
H.,
Ahlin,
S.,
Anveden,
A.,
Bengtsson,
C.,
Bergmark,
G.,
Bouchard,
C.,
Carlsson,
B.,
Dahlgren,
S.,
Karlsson,
J.,
Lindroos,
A.K.,
et al. (2012) Bariatric surgery and long-term cardiovascular events. JAMA, 307, 56-65.
-
Skorzewska,
A.,
Lal,
S.,
Waserman,
J. &
Guyda,
H.
(1989) Abnormal food-seeking behavior after surgery for craniopharyngioma. Neuropsychobiology, 21, 17-20.
-
Thomas,
F.,
Smith,
G.C.,
Lu,
J.,
Babor,
R.,
Booth,
M.,
Beban,
G.,
Chase,
J.G. &
Murphy,
R.
(2016) Differential acute impacts of sleeve gastrectomy, Roux-en-Y gastric bypass surgery and matched caloric restriction diet on insulin secretion, insulin effectiveness and non-esterified fatty acid levels among patients with type 2 diabetes. Obes. Surg., 26, 1924-1931.
-
Tonning Olsson,
I.,
Perrin,
S.,
Lundgren,
J.,
Hjorth,
L. &
Johanson,
A.
(2014) Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr. Neurol., 51, 515-521.
-
Weismann,
D.,
Pelka,
T.,
Bender,
G.,
Jurowich,
C.,
Fassnacht,
M.,
Thalheimer,
A. &
Allolio,
B.
(2013) Bariatric surgery for morbid obesity in craniopharyngioma. Clin. Endocrinol. (Oxf.), 78, 385-390.
-
Wijnen,
M.,
Olsson,
D.S.,
van den Heuvel-Eibrink,
M.M.,
Wallenius,
V.,
Janssen,
J.A.,
Delhanty,
P.J.,
van der Lely,
A.J.,
Johannsson,
G. &
Neggers,
S.J.
(2017) Efficacy and safety of bariatric surgery for craniopharyngioma-related hypothalamic obesity: a matched case-control study with 2 years of follow-up. Int. J. Obes. (Lond.), 41, 210-216.
-
Yehoshua,
R.T.,
Eidelman,
L.A.,
Stein,
M.,
Fichman,
S.,
Mazor,
A.,
Chen,
J.,
Bernstine,
H.,
Singer,
P.,
Dickman,
R.,
Beglaibter,
N.,
Shikora,
S.A.,
Rosenthal,
R.J. &
Rubin,
M.
(2008) Laparoscopic sleeve gastrectomy: volume and pressure assessment. Obes. Surg., 18, 1083-1088.