2019 Volume 249 Issue 3 Pages 231-236
2019 Volume 249 Issue 3 Pages 231-236
Thyroid dysfunction (TD) is caused by thyroid peroxidase (TPO) antibody, as seen in Hashimoto’s disease. TD is a common problem of reproductive age and may impair fetal development. Here, we determined the effect of TPO antibody on perinatal outcomes in Japanese women with TD before conception. A retrospective study involving cases of maternal TD with term singleton birth was conducted. The subjects with TD were divided into two groups according to the presence (n = 22) or absence (n = 20) of TPO antibody. The control groups matched for age, parity, and gestational weeks were selected for TPO antibody-positive (n = 44) and -negative TD subjects (n = 40), respectively. Using the standard curve of Japanese placental weight, the frequency of placental weight less than the 50th percentile (small placenta) was examined. Placental weight was lower among TPO antibody-positive TD subjects, compared with TPO antibody-negative TD subjects (p < 0.01). However, other outcomes were similar between the groups. Importantly, compared with control mothers, placental weight was significantly lower (p < 0.01), birth weight tended to be lower (p = 0.07), and the incidence of gestational diabetes mellitus was higher (p = 0.02) among TPO antibody-positive subjects. There was no significant difference in placental weight between TPO antibody-negative subjects and controls. The frequency of small placenta was significantly higher in TPO antibody-positive subjects (odds ratio: 16.7) even when considering diabetes and pregnancy induced hypertension. Thus, the presence of TPO antibody is associated with lower placental weight among Japanese women having TD.
The frequency of thyroid dysfunction among Japanese adults is about 10%, where subclinical hypothyroidism is most frequently seen (Kasagi et al. 2009). The frequency of overall thyroid dysfunction seems to be similar to those of other countries (Kasagi et al. 2009; American College of Obstetricians and Gynecologists 2015). Given its high frequency, there are many concerns regarding the impact of maternal thyroid dysfunction, and especially the impact of subclinical hypothyroidism, low maternal free thyroxine concentrations, or thyroid autoimmunity on pregnancy outcome. Consequently, there have been many reports regarding the association of maternal thyroid dysfunction and maternal-fetal prognosis. For example, subclinical hypothyroidism or low maternal free thyroxine concentrations in early pregnancy can affect infant intellectual development (Pop et al. 1999; Korevaar et al. 2016). For this reason, the question of intervention in the treatment of maternal thyroid deficiency in early pregnancy has been raised. At present, treatment interventions for subclinical hypothyroidism in early pregnancy do not appear to improve the intellectual development of the child (Thompson et al. 2018). There may be some reason why an intervention strategy that simply normalizes thyroid function is ineffective. Hence, it is necessary to consider the involvement of thyroid autoimmunity.
The causes of hypothyroidism comprise thyroid autoimmunity and inadequate iodine intake. The prevalence of thyroid antibody is comparable across ethnic groups (about 6%, Benhadi et al. 2007). Among Japanese women with low-risk pregnancies, the prevalence of thyroid peroxidase (TPO) antibody is 6.7% (Orito et al. 2009). However, the proportion of subclinical hypothyroidism by thyroid autoimmunity may be regional. According to a recent report in Japan, 16% of low-risk pregnant women with subclinical hypothyroidism possess thyroid antibodies, and subclinical hypothyroidism is not associated with adverse pregnancy outcomes (Furukawa et al. 2017). On the other hand, the prevalence of thyroid antibodies is higher among women in other countries with subclinical hypothyroidism (around 50%) compared with Japanese women, and is associated with adverse pregnancy outcomes (Blumenthal et al. 2016; Pop et al. 2018). Given the low prevalence of thyroid antibodies in Japanese women having subclinical hypothyroidism, the effects of antibodies may not be reflected in the overall pregnancy outcome. It is therefore necessary to evaluate the effect of thyroid antibodies on pregnancy outcome in the group comprising maternal thyroid dysfunction with antibodies.
To date, no reports have detailed the direct effect of thyroid antibodies on pregnancy outcomes in Japan. Even after therapeutic intervention comprising hormone replacement for women suffering from thyroid dysfunction with thyroid antibodies, pregnancy outcomes may be affected. We therefore conducted a study to determine the effect of maternal thyroid antibodies on adverse pregnancy events among women with known maternal thyroid dysfunction.
We undertook a retrospective study of pregnant women who were diagnosed with maternal thyroid dysfunction, such as Hashimoto’s disease, subclinical hypothyroidism, and others before conception from January 2013 to July 2019 at the University Hospital. The University of Miyazaki is a tertiary center. Our University Hospital dealt mainly with at-risk cases and the total number of deliveries was 1809 during the period investigated. Cases having the following criteria were selected: term (≥ 37 weeks of gestation), singleton pregnancy, no congenital anomalies, and presence of data for thyroid peroxidase (TPO) antibody. Cases that had not been tested for TPO antibody or whose test results were unknown were excluded from the study. We obtained approval for the study from the constituted Ethics Committee of the University of Miyazaki (#2019 O-0505).
Laboratory methods and management of thyroid conditionSerum levels of TPO antibody were assayed by electrochemiluminescence immunoassay (ECLIA, Roche-Diagnostics K.K., Tokyo, Japan) or by chemiluminescent enzyme immunoassay (CLEIA, FUJIREBIO Inc., Tokyo, Japan). A TPO antibody titer of 16 IU/ml or more by ECLIA and 5.2 IU/ml or more by CLEIA were the cut-off values, and regarded as positive for TPO antibody. Serum levels of thyroid stimulating hormone (TSH) were determined by CLEIA (ABBOTT JAPAN Co.,Ltd, Chiba, Japan). During pregnancy, TSH was measured every 1-3 months depending on the level of the TSH. Endocrinologists adjusted the level of TSH during pregnancy to 2.5~3.0 μU/mL since the current upper limit of serum TSH at the first and second trimester is considered to be 2.5~3.0 μU/mL (Stagnaro-Green et al. 2011).
Data collectionFrom the medical charts of the group under investigation, the following maternal and neonatal demographic data were collected: maternal age, parity (primipara), history of abortion, timing of final checking for the presence of TPO antibody, type of maternal thyroid dysfunction including thyroidectomy, insufficient control, having hormone replacement treatment, and having preexisting type 2 diabetes mellitus (DM). The time at which the presence of TPO antibody was checked was divided into five periods; pre-pregnancy, 1st trimester, 2nd trimester, 3rd trimester, and postpartum. Perinatal outcomes included evaluation of gestational diabetes mellitus (GDM), pregnancy-induced hypertension (PIH), gestational age at delivery (weeks), sex of infant (male), birth weight (g), placental weight (g), infant head circumference (cm), umbilical artery pH (UA pH), umbilical artery pO2 (UA pO2), an Apgar score of less than 7.0, incidence of neonatal thyroid dysfunction, and incidence of admission to a neonatal intensive care unit. GDM was diagnosed if one or more of these readings were elevated in the 75-g oral glucose tolerance test: plasma glucose level at fasting ≥ 92 mg/dl, at 1 hour ≥ 180-mg/dl, and at 2 hours ≥ 153 mg/dl (Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus et al. 2010). PIH included preeclampsia and gestational hypertension. Preeclampsia was diagnosed when hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) and proteinuria (dipstick ≥ 1+) developed after 20 weeks of gestation. Gestational hypertension was diagnosed when hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) without proteinuria developed after 20 weeks of gestation.
Data analysisWe initially divided the study into two groups according to positive or negative TPO antibody status, and then compared the demographic data and perinatal outcomes between the positive and negative TPO antibody groups. The control groups matched for age, parity, and gestational weeks were selected for comparison with TPO antibody-positive and -negative maternal thyroid dysfunction subjects, respectively, and then the study and control groups were compared. To determine sample size of the control group, we took the alpha error as 0.05, beta error as 0.2, power of study 0.8, and allocation ratio as 1:2 for a two-tailed test. Additional evaluation was made regarding a comparison of placental weight. For this purpose, we used the standard curve of placental weight in Japanese that had been categorized by gestational week, parity, and fetal sex (Ogawa et al. 2016). We defined a small placenta as a case where the placental weight corresponds to less than the 50th percentile of the placental weight. Then, the frequency of small placenta was compared between the TPO antibody-positive and control groups. To determine which parameter had a stronger association with small placenta, a multiple logistic regression analysis was also performed. Comparisons between groups were made using the χ2 test, Fisher’s exact probability test, or t-test. Data are expressed as number, incidence (%), or mean ± SD. Probability values < 0.05 were considered significant.
We identified 67 cases of maternal thyroid dysfunction during the study period, including 16 cases that were less than 37 weeks of gestation and 9 cases with the unknown status of TPO antibody. The remaining 42 cases were included and were divided into two groups according to the presence (n = 22) or absence (n = 20) of TPO antibody. Based on power analysis, 44 pregnant women were selected as control for the TPO Ab-positive subjects with maternal thyroid dysfunction and 40 pregnant women for the TPO Ab-negative subjects with maternal thyroid dysfunction (Table 1).
There were no significant differences between the TPO antibody-positive and TPO antibody-negative groups in terms of maternal age, parity, history of abortion, insufficient control, or having hormone replacement treatment (Table 1). The timing for final checking of the presence of TPO antibody was not significantly different from that of the TPO antibody-negative group (p = 0.43). The type of maternal dysfunction in the TPO antibody-positive group was not significantly different from that of the TPO antibody-negative group (p = 0.60); however, Hashimoto’s disease was more common in the TPO antibody-positive group (Table 1).
The percentage of male sex in the TPO antibody-positive group (37%) was lower than that in the TPO antibody-negative group (75%, p = 0.01). Placental weight in the TPO antibody-positive group (531 ± 87 g) was lower than that in the TPO antibody-negative group (608 ± 80 g, p = 0.01). There were no significant differences between the TPO antibody-positive and TPO antibody-negative groups in terms of the incidence of GDM/DM, the incidence of PIH, gestational age at delivery, birth weight, infant head circumference, UA pH, UA pO2, an Apgar score of less than 7.0, the incidence of neonatal thyroid dysfunction, or the incidence of admission to a neonatal intensive care unit (Table 2).
We then compared perinatal outcomes such as placental weight, birth weight, and infant head circumference between TPO antibody-positive and control groups. There were no significant differences in the demographic data between the TPO antibody-positive and control groups except for the incidence of GDM/DM (p = 0.02, Table 2). Placental weight in the TPO antibody-positive group was lower than that of the control group (621 ± 116 g, p < 0.01). Birth weight and infant head circumference did not differ between groups. We also compared perinatal outcomes between TPO antibody-negative and control groups. There were no significant differences in the incidence of GDM/DM (p = 1.00). There was no difference in birth weight (p = 0.73), placental weight (p = 0.27), and infant head circumference (p = 0.36) between TPO antibody negative and control groups. On the other hand, there was significant difference in the incidence of PIH (p = 0.04, Table 2).
Finally, we compared the frequency of small placenta between TPO antibody-positive and control groups. As described in the method section, a small placenta was defined as a case where the placental weight corresponds to less than the 50th percentile of the placental weight in Japan. As a result, there were 17 cases of small placenta in TPO antibody-positive group and 8 cases of small placenta in control group. The frequency of small placenta was significantly higher in the TPO antibody-positive group compared with the control group (17/22 vs. 8/44, respectively, Odds ratio: 5.1, p = 0.01). Because of the higher incidence of GDM and PIH in the TPO antibody-positive group (Table 2), we then performed a multiple logistic regression analysis to confirm the independence of TPO antibody-positive status for small placenta. Analysis showed that TPO antibody-positive status had a significantly positive effect on small placenta when considering the effects of the presence of DM/GDM and PIH (Odds ratio: 16.7, p < 0.01, Table 3).
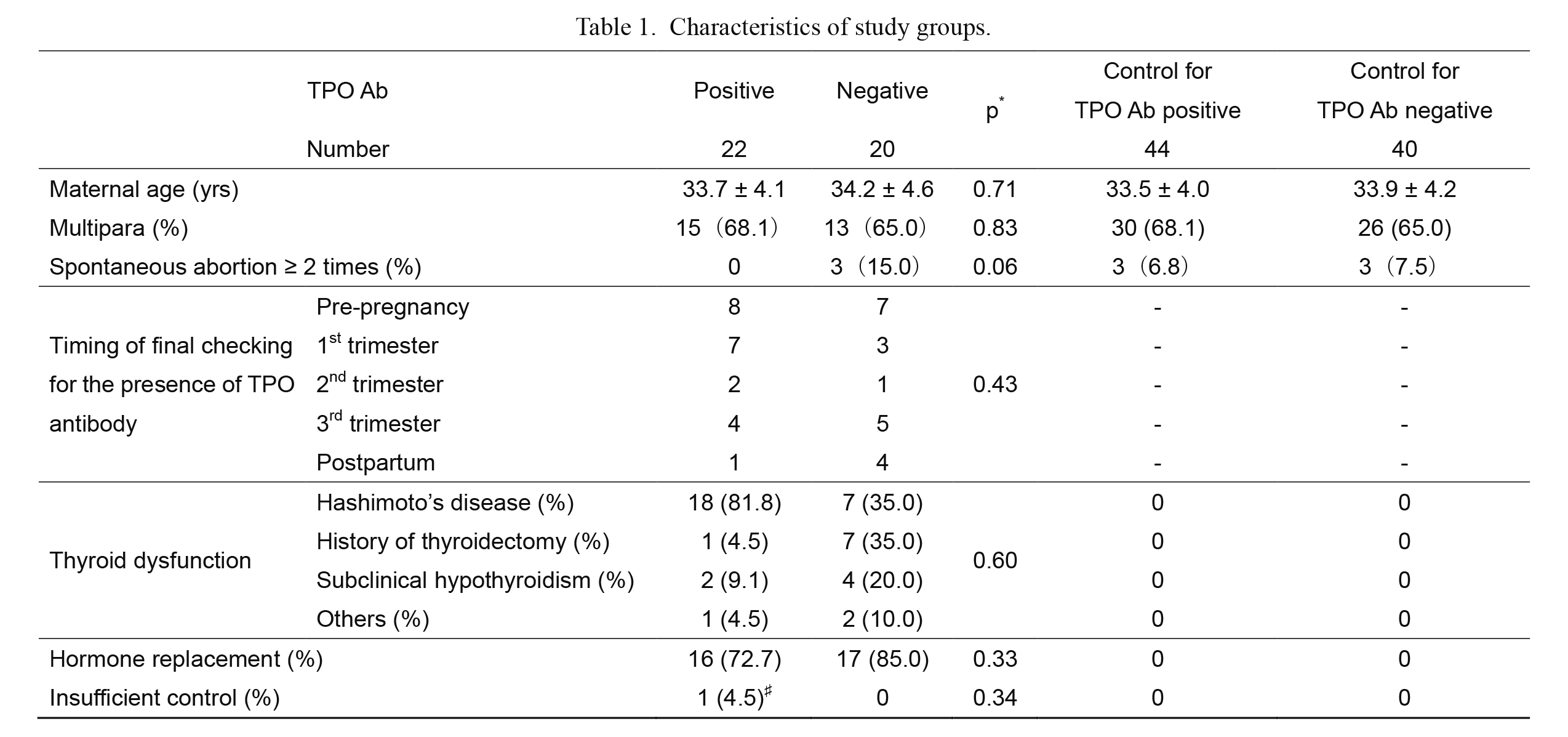
Characteristics of study groups.
Results are expressed as number, incidence (%), or mean ± SD.
TPO, thyroid peroxidase; TPO Ab, TPO antibody.
*TPO Ab positive vs. TPO Ab negative.
♯Thyroid function was normalized after 22 weeks.

Perinatal outcomes of study groups.
Results are expressed as number or incidence (%). Comparison between groups was made using the χ2 test, Fisher’s exact probability test, or t-test. Placental weight in the TPO antibody-positive group was lower than that in the TPO antibody-negative and control groups.
TPO, thyroid peroxidase; TPO Ab, TPO antibody; GDM, gestational diabetes mellitus; DM, type 2 diabetes mellitus; PIH, pregnancy-induced hypertension; NICU, neonatal intensive care unit.
*TPO antibody positive vs. TPO antibody negative.
**TPO antibody positive vs. control.
***TPO antibody negative vs. control.

The independence of TPO antibody-positive status for small placenta.
Comparison was made by a multiple logistic regression analysis.
GDM, gestational diabetes mellitus; DM, type 2 diabetes mellitus; PIH, pregnancy-induced hypertension.
Our study showed that Japanese women having known maternal thyroid dysfunction and being TPO antibody positive developed placenta with lower weight even with a euthyroid state. Conflicting reports have detailed the possibility that the presence of TPO antibody gives rise to increased adverse pregnancy outcomes other than miscarriage and premature birth (Männistö et al. 2009, 2010; Wilson et al. 2014; Plowden et al. 2017; Huang et al. 2019), and no consensus has been reached. We have shown for the first time that TPO antibody reduces placental weight. Although this result could have an indirect effect on fetal development such as body weight or infant head circumference, these effects were not observed within our study. However, the birth weight tended to be lower in TPO-positive women compared with TPO-negative women and control subjects. Since the placenta has sufficient reserve capacity, fetal development itself might have been secured as a result. To date, only one report has appeared detailing the relationship between TPO antibody and placental growth (Männistö et al. 2009), showing that TPO antibody promotes increased weight of the placenta, contrary to our results.
It has been suggested that TPO antibody may pass through the placenta and affect the fetal developmental environment. An animal study has shown that administration of TPO antibody increases the rate of miscarriages and reduces the weight of the fetus (Lee et al. 2009). The authors speculated that TPO antibody might affect “post-implantation” embryonic development. If so, it is also expected to affect placental development as indicated by our findings. Additionally, human transfer of TPO antibody to the fetus has been demonstrated (Seror et al. 2014). That report showed that there is a positive correlation between maternal anti-TPO antibody levels and cord blood anti-TPO antibody levels, and so TPO antibody may affect fetal thyroid function as well as fetal and placental development. Another report has indicated that TPO antibody affects fetal brain development, and not other body growth parameters such as birth weight, birth length, abdominal circumference, or chest circumference (Wilson et al. 2014). From these findings, it can be postulated that TPO antibody adversely affects growth, including that of the placenta. In fact, investigations that we are currently conducting also indicate that placental weight is reduced among women with autoimmune diseases (unpublished data). On the other hand, only one report has appeared regarding placental development in relation to TPO antibody; namely, placental weight was increased, contrary to the findings of our present study (Männistö et al. 2009). Even if TPO antibody affects placental growth, TPO antibody appears to be unrelated to miscarriages or fetal growth failures.
It is difficult to account for the reports concerning the different impact of TPO antibody on pregnancy outcomes except for miscarriage or premature birth. In the report on brain size in women having TPO antibody, the negative effect was only pronounced in non-Hispanic whites (Wilson et al. 2014). Although the prevalence of thyroid antibody is comparable across ethnic groups (Benhadi et al. 2007), there may be differences in sensitivity to TPO antibody depending on race. Additionally, recent reports have indicated that clinically euthyroid pregnant women with TPO antibody have relative hypothyroidism (Loh et al. 2016), and that there is a dose dependency of TPO antibody concentration in relation to thyroid function (Korevaar et al. 2018). Recently, it has been shown that both low and high TSH levels in early pregnancy affect fetal brain volume (Jansen et al. 2019). If thyroid function is mildly impaired by TPO antibody, clinically euthyroid pregnant women having TPO antibody might be managed without thyroid hormone treatment and subsequent disturbance in fetal and placental growth. According to our study, placental weights were affected even with hormone treatment. Consequently, being TPO antibody positive may be an independent risk factor. Furthermore, complications such as diabetes associated with autoimmune diseases may also be affected. Several reports have indicated that being TPO antibody positive is an independent risk factor when considering GDM, which may promote placental growth (Ortega-González et al. 2000; Huang et al. 2019). According to a report by Männistö et al. (2009), TPO antibody positive mothers had more large-for-gestational age (LGA) infants in addition to having higher placental weights. The increase in LGA infants and placental weight is reminiscent of GDM involvement. However, there were no GDM cases in the TPO antibody-positive group of their study (Männistö et al. 2009). In our study, multiple logistic regression analysis showed that DM/GDM had no effect on small placenta. GDM was also well controlled in our study groups, so it seems to have little impact, and the effect on placental weight may be related to the presence of TPO antibody. There is a consensus that TPO antibody is an independent factor involved in growth, although it is not clear why the results show the inverse in terms of fetal and placental growth. Lastly, the major difference between study background by Männistö et al. (2009) and the present study is the prevalence of known thyroid dysfunction. All our subjects have had thyroid dysfunction before pregnancy, compared to only 4.2% of their subjects (Männistö et al. 2009). The high prevalence of overt thyroid dysfunction may have an impact on fetal and placental weight.
This study has some limitations. Since the number of studied cases is small, investigation of a larger number of cases is necessary. Furthermore, since placental pathological examination was performed only with some of the cases in this study, placental evaluations could not be presented here. Future investigations should include detailed placental pathological examinations in an effort to determine whether a characteristic finding can be obtained in cases of known maternal thyroid dysfunction in the presence of TPO antibody. Finally, although this is a clinical study, it will be necessary to investigate the effects on placental development in vitro.
In conclusion, we demonstrate that the presence of TPO antibody is associated with reduced placental growth in Japanese women having thyroid dysfunction. Further studies of Japanese women are required to determine the effects of TPO antibody on pregnancy outcomes, to compare racial or regional differences, and to clarify the independent nature of TPO antibody with respect to pregnancy outcomes by a comparison of reports from a variety of countries.
This study was supported by a Grant-in-Aid for Clinical Research from the Miyazaki University Hospital.
The authors declare no conflict of interest.