2020 Volume 250 Issue 1 Pages 13-23
2020 Volume 250 Issue 1 Pages 13-23
The efficacy and safety of targeted treatment for elderly patients with rheumatoid arthritis (RA) was considered. Patients with RA who met the ACR/EULAR 2010 classification criteria and were treated consecutively for > 3 years, were recruited and classified into three age groups with 10-year increments from 65 years. Treatment protocol that aims to achieve clinical remission within 6 months was commonly adopted. The salient features are the rapid increase in dosages of conventional synthetic anti-rheumatic drugs (csDMARDs) and the administration of need-based concomitant biologic/targeted synthetic drugs and/or glucocorticoid steroid, and immediate tapering of glucocorticoid steroid and csDMARDs is required on attaining clinical remission. Disease activity score and other clinical indices specific for RA treatment, and the prevalence of adverse events were compared between the groups. The numbers of patients in the groups of the < 65 years, 65-74 years, and ≥ 75 years were 269, 155, and 152. No significant difference was observed between any pairs of groups with respect to disease activity; stable course after achievement of minimum disease activity was observed in all groups. However, the prevalence of adverse events, especially serious infection, in the oldest group was higher than that in the younger groups, which was likely attributable to the higher frequency of administration of glucocorticoid steroid after minimum disease activity obtained and higher prevalence of cardiovascular comorbidities. Targeted treatment is feasible even for patients aged ≥ 75. However, glucocorticoid steroid administration is considered as a risk of adverse events and should be tapered immediately.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that is characterized by joint destruction and the consequent impaired ability to perform activities of daily living (ADL). About three decades ago, RA typically affected individuals in their late middle age (Rasch et al. 2003); however, due to advances in treatment of RA and increase in life expectancy, individuals in their 6th or 7th decade of life account for a large proportion of patients affected by RA (Kato et al. 2017; Ruffing and Bingham 2017; Peckel 2018; Kobak and Bes 2018). In the recent decade, hitherto unconsidered issues related to aging in the context of RA have evoked increasing interest; these include treatment considerations in relation to the functional decline and age-related increase in various risks (Deal et al. 1985; Kavanaugh 1997; Tutuncu et al. 2006; Koeller et al. 2009). Age-related decline in function of various organ systems, especially immune, metabolic, and neuromotor system, may influence the outcomes of RA therapy. Therefore, a major concern is whether the therapy indicated for younger generations is also suitable for elderly patients with RA. In this context, determination of any special considerations for elderly patients with RA is a key imperative.
Age-related decline in immune system mainly pertains to the acquired immunity wherein the characteristic changes are alteration in T-cell function, impaired function of memory T-cells, and thymus involution; subsequently, compensatory hyperactivation of innate immunity, as well as hyperexpression of osteopontin, occurs due to increase in the PD-1 positive T-cell population (Sakamoto et al. 2016). The persistent compensatory activation of innate immunity increases the chances of hyperreaction to both external and internal stimuli. These sequential immune system changes (also referred to as immunosenescence) typically begin between the 5th and 6th decades of life (Hamazaki and Minato 2018). Therefore, elderly onset RA often presents with violent inflammation (Villa-Blanco and Calvo-Alen 2009; Ruban et al. 2016; Tan et al. 2017). A secondary problem in elderly patients is their vulnerability to infection. Elderly patients with RA are at a higher risk of serious infection or hospitalization (Wolfe et al. 2006).
Metabolic problems include decline in renal function and impaired digestion and absorption (Kinirons and O’Mahony 2004; Wynne 2005; Klotz 2009). Consequently, the half-life of drugs is prolonged, and the serum concentrations of drugs are maintained at a higher level as compared with that in younger patients; this leads to accumulation of drugs in the target cells (Castleden and George 1979). Therefore, the dose-dependent side effects of methotrexate (MTX) in elderly patients with RA tend to be more frequent and more severe as compared with that in younger patients (McKendry and Dale 1993; Hirshberg et al. 2000; Wynne 2005; Innala et al. 2014). Thus, elderly patients with RA require more diligent care including more frequent follow-up and close monitoring.
Age-related decline in neuromotor function is another key challenge in the treatment of elderly patients with RA. Cognitive impairment and frailty are particularly vexed issues in this patient population (Andrews et al. 2017). Exercise program is necessary for elderly patients with RA in order to maintain ADL function (Cooney et al. 2011).
Based on these considerations, we have adopted a “Touch Down Strategy” for treatment of elderly patients with RA. The strategy is so named because of its objective to achieve rapid clinical remission, followed immediately by progressive decrease in dose. “Touch Down Strategy” has a tactic that when an elderly RA patient “touch” on clinical remission, initiate dose “down” of drugs as soon as possible. The Touch Down Strategy is described in detail in the Methods section.
This study aimed to evaluate the efficacy and safety of Touch Down Strategy for elderly RA patients, especially for those aged ≥ 75 years. The primary endpoint of this study was the disease activity of elderly patients with RA after achievement of target; secondary endpoints were comorbidities and adverse events experienced during treatment relative to those in younger patients with RA.
Between August 2010 and July 2015, a total of 893 patients who qualified the 2010 American College of Rheumatology/the European League Against Rheumatism (ACR/EULAR) classification criteria for RA (Aletaha et al. 2010) were referred to the institute. Patients who had dropped out or died before 3-year follow-up were excluded. Patients who had been treated continuously for > 3 years were recruited. These patients had been treated according to the treat-to-target treatment protocol (Smolen et al. 2010, 2016) regardless of the patient’s age; the key aspects of the protocol were as follows: (1) Administer MTX as an anchor drug. (2) Monitor disease activity and evaluate the disease activity index every 3-6 months. (3) Primary treatment target is to attain clinical remission. (4) Shared decision-making for treatment modification such as increase in dose of MTX or other disease-modifying anti-rheumatic drugs (DMARDs) including biologic DMARDs (bDMARDs) with full participation of the patients. (5) If primary target is impossible to attain, then the target is changed to low disease activity as a secondary target. (6) After attainment of target, maintenance of the target status is the next target. In addition, we implement the “Touch Down Strategy” for elderly patients with RA aged ≥ 65 years.
Touch down strategyThe Touch Down Strategy basically succeeds to treat-to-target overarching philosophy (Smolen et al. 2010) and then makes arrangement consideration for elderly RA clinical characteristics. This strategy entails three broad components: 1) After informed consent, initiate RA treatment with MTX 6 mg/week, or tacrolimus 1.0 mg/day if the patient has chronic lung disease and follow-up at least once a month. 2) Consider increasing the dosage of MTX by 1 mg/week or that of tacrolimus by 0.5 mg/day or maintain the drug dosage and start concomitant (bDMARDs) or other conventional synthetic DMARDs (csDMARDs) unless clinical remission is attained. If required, consider concomitant glucocorticoid (GCS) administration every other month. The target is to achieve clinical remission within 6 months. 3) Immediately on achievement of clinical remission, tapering of GCS therapy is initiated, and concurrently tapering of csDMARDs is considered when clinical remission is sustained for 3 months. Tapering of bDMARDs is considered last of all. If clinical remission is not achieved in 6 months, the same therapy protocol continued, and tapering is considered every 3 months (Fig. 1).
Our hypothesis is that elderly patients with RA tend to exhibit unnecessary inflammatory response over and above that induced by RA itself. Therefore, if additional inflammation is ameliorated, fewer essential DMARDs will be required as compared with those in younger patients with RA; this is because owing to impaired metabolic function, elderly patients with RA tend to accumulate higher serum levels of drugs for longer periods. Therefore, as soon as clinical remission is achieved, the risk of unnecessary adverse events and comorbidities should be minimized as soon as possible.

Treatment protocol of “Touch Down Strategy.”
Patients with RA were treated according to the protocol. When RA was diagnosed, treatment was initiated immediately with 6 mg per week methotrexate (MTX) or 1.0 mg per day tacrolimus (TAC), and/or 2-5 mg per day glucocorticoid steroid (GCS), and/or biological or targeted synthetic disease-modifying anti-rheumatic drugs (bDMARD or tsDMARD) during the same period to aim for clinical remission in 6 months. When clinical remission was achieved or disease activity reached a plateau, MTX tapering was initiated from 3 months thereafter. GCS tapering was initiated immediately, whereas b/tsDMARD tapering was initiated as a last order to avoid unnecessary adverse events and sustain clinical remission.
Patients were classified according to age at baseline: < 65 years (G-Y); 65-74 years (G-YO); and ≥ 75 years (G-OO). The 28-joints disease activity score with C-reactive protein (DAS28) as the disease activity index, Health Assessment Questionnaire Disability Index (HAQ) as a functional index, and pain score with visual analog scale (PS-VAS) as a pain index were monitored every other month. Sharp/van der Heijde Score (SHS) as a joint structural index was evaluated every other year. Drug administration history was also recorded. Data pertaining to comorbidities and adverse events were collected from the medical records.
ParametersPatient’s mean value of DAS28 at baseline and from baseline to the lowest DAS28 (minDAS) during treatment were calculated for each group. Time span from the baseline to minDAS was also calculated. Each group was further classified into three subgroups based on the mean DAS28 at minDAS: deep remission, DAS28 < 2.0; remission, DAS28 ≥ 2.0 and < 2.3; non-remission, DAS28 ≥ 2.3. Mean DAS28 from the time of attainment of minDAS to the last observation was also calculated. Mean HAQ, PS-VAS was calculated in a similar manner. MTX administration ratio and mean dosage during each time period (baseline to minDAS and minDAS to the last observation) were calculated for each group. Other administered drugs, such as tacrolimus and GCS, during each period were also calculated. The recorded adverse events were also collected.
Risk factors of serious infectionThe risk of serious infection was evaluated according to factors such as sex, age at onset, age at baseline, disease duration at baseline, age at minDAS, time span from baseline to minDAS, HAQ score at minDAS, PS-VAS at minDAS, SHS at minDAS, the administration and dosage of MTX, tacrolimus, GCS, and bDMARDs at minDAS and after minDAS, comorbidities in the cardiovascular system, osteoporosis, type2 diabetes mellitus, and interstitial lung disease at baseline.
Statistical analysisMean values of these parameters in each group were compared using analysis of variance (ANOVA) with Bonferroni correction. Between-group differences with respect to the prevalence of comorbidities and adverse events were also analyzed. As an additional test, the influence of GCS on parameters including DAS28 at baseline, minimum DAS28, time to DAS28 remission, time to DAS28 deep remission, number of cases with minimum DAS28, and prevalence of comorbidities and adverse events was evaluated using ANOVA with Bonferroni correction or M × N Chi-squared test. The risk factors of serious infection were evaluated using binary logistic regression analysis. First, the univariate model was evaluated followed by reevaluation of statistically significant factors using the multivariate model. All statistical significance was set below 1%. All statistical analyses were performed using StatPlus:mac® (AnalystSoft Inc., Walnut, CA, USA).
Ethical considerationsThe study was conducted in compliance with the Japanese Ethical Guidelines for medical and health research involving human subjects and according to the principles of the Declaration of Helsinki. The study protocol and consent forms were approved by the ethics committee of the institution (Yoshii Hospital Ethical Committee; approval number Y-Rheum-004). Patients and their families were informed that personal information would remain anonymous and would only be used for study. Data were included in this study after obtaining written informed consent of the patient and his/her family.
Of 893 patients, 576 were recruited for this study. The Kaplan-Meier survival ratio for following up of each age group is shown in Fig. 2. There is no statistical difference between any pair of the groups regarding survival ratio at any term, and final survival ratio for G-Y, G-YO, and G-OO was 64.5 (269 per 417), 66.8 (155 per 232), and 62.3% (152 per 244), respectively. Thus, the patients who were lost to follow-up within three years were 148 (35.5%), 77 (33.2%), and 92 (37.7%) for G-Y, G-YO, and G-OO, respectively. Major population in the lost patients had dropped out within six months in every groups. Almost of these patients were unable to search reason for dropping out because they had no contact to us. In the lost patients after six months, malignant tumor for reason was 3, 8, and 6 in G-Y, G-YO, and G-OO, respectively. Heart failure counted 12, 10, and 8, and severe infection counted 15, 10, and 12 in G-Y, G-YO, and G-OO, respectively. In the other reasons, 10 of G-OO patients moved to nursing home, whereas zero in G-Y, and three in G-YO group.
Clinical characteristics of patients disaggregated by study group are summarized in Table 1. Out of 576 patients, the number of patients in the G-Y, G-YO, and G-OO groups was 269, 155, and 152, respectively. There was no significant difference between any of the groups except for mean age at onset and at baseline and HAQ-DI at baseline. These parameters demonstrated significantly greater difference with increase in age. Mean disease duration at baseline in G-OO group was significantly longer than that in the G-YO and G-Y groups, whereas mean follow-up duration in the G-OO group was significantly shorter than that in the G-YO and G-Y groups.

Kaplan-Meier survival ratio curve until 36 months for each age group.
G-Y; double line: G-YO; broken line: G-OO; full line. Plotted points show mean value.
There is no significant difference of survival ratio between any pair of the groups at any term.
Overall survival ratios at 36 months for G-Y, G-YO, and G-OO, were 64.5%, 66.8%, and 62.3%, respectively. There were no significant differences between any pairs of groups at every other six months period.
G-Y, a patient group who are younger than 65 years old ; G-YO, a patient group who are between 65 to 74 years old ;G-OO, a patient group who are older than 75 years; BL, Baseline ; M, months.

Clinical background of the three age groups.
In columns except case represent, mean value and standard deviation separated by comma are shown.
In p value, comparison between the G-Y and G-YO group, G-Y and G-OO group, and G-YO and G-OO group separated with comma were shown, respectively.
G-Y, a patient group whose age at baseline is less than 65; G-YO, a patient group whose age at baseline is no less than 65 and less than 75; G-OO, a patient group whose age at baseline is no less than 75; YORA, a rheumatoid arthritis patient group whose age at onset was less than 65; yEORA, a rheumatoid arthritis patient group whose age at onset was no less than 65 and less than 75; oEORA, a rheumatoid arthritis patient group whose age at baseline is no less than 75; ACPA, anti-citrullinated cyclic polypeptide antibodies; DAS28, 28-joints disease activity score with C-reactive protein; SHS, Sharp/van der Heijde Score; HAQ-DI, Health Assessment Questionnaire Disability Index; PS-VAS, pain score with visual analog score.
No significant between-group differences were observed with respect to the mean DAS28 and PS-VAS at baseline, at minDAS, and from minDAS to the last observation; however, HAQ-DI in the G-OO group was significantly greater at minDAS and from minDAS to the last observation as compared with that in the G-YO and G-Y groups. No significant between-group differences were observed with respect to the mean time span from baseline to clinical remission or deep clinical remission as well as with respect to the mean annual change in SHS from baseline to the last observation (Table 2).
On subgroup analysis disaggregated by status of disease activity at minDAS, significant differences in DAS28 score were observed at minDAS and thereafter between subgroups of patients in deep clinical remission (dREM), clinical remission (REM), and non-remission (nREM) in all three age groups; however, no significant differences were observed with respect to the HAQ-DI and PS-VAS. Moreover, patient group that achieved dREM tended to exhibit a more stable course and had lower values relative to groups that did not attain dREM in all three age groups (Fig. 3).
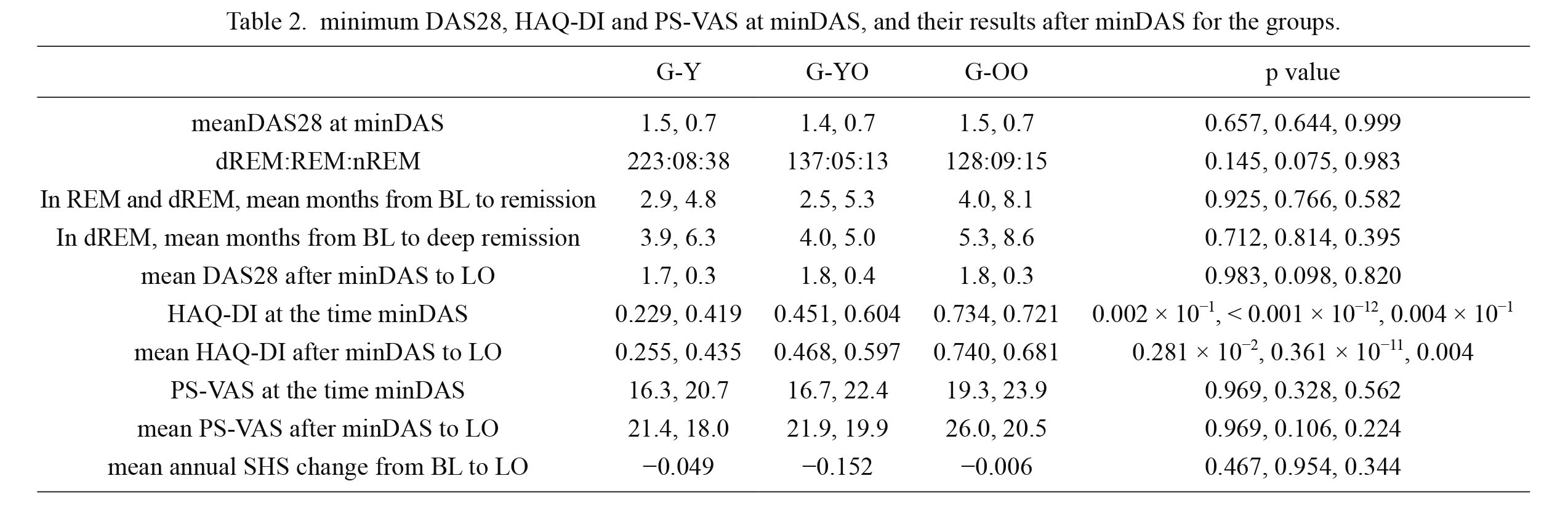
minimum DAS28, HAQ-DI and PS-VAS at minDAS, and their results after minDAS for the groups.
Except case representation, numbers in all columns are mean value and standard deviation separated with comma.
In p value, comparison between the G-Y and G-YO group, G-Y and G-OO group, and G-YO and G-OO group separated with comma were shown, respectively.
DAS28, 28-joints disease activity score with C-reactive protein; HAQ-DI, Health Assessment Questionnaire Disability Index; PS-VAS, pain score with visual analog scale; minDAS, minimum DAS28 in the treatment course; dREM, patients who attained deep clinical remission represented with DAS28 less than 2.0; REM, patients who attained clinical remission represented with DAS28 less than 2.3; nREM, patients who attained non clinical remission represented with DAS28 no less than 2.3; LO, last observation in treatment course; SHS, Sharp/van der Heijde Score; S.S., statistical significance.

Change of HAQ-DI, DAS28, and PS-VAS for subgroups separated by level of minimum DAS28 for each disease activity control group.
dREM; thick full line: REM; thin full line: nREM; dotted line. Plotted points show mean value.
Only dREM (a patient subgroup of whom minimum DAS28 was below 2.0) had been stable course at LO (last observation) in every groups, while REM had very unstable movement from minDAS to LO in the G-YO and G-Y group, and nREM had relatively higher increase for PS-VAS in every groups.
No significant differences were observed between the G-OO, G-YO, and G-Y groups with respect to MTX administration ratio at minDAS and after minDAS, while initial average dose, and mean dosage from baseline to minDAS and from minDAS to the last observation was significantly greater in the G-Y group than in the G-OO group. With respect to tacrolimus, there was no significant difference between any pair of groups. Mean dosage of GCS administration from baseline to minDAS and from minDAS to the last observation was not significantly different between any of the groups. The GCS administration ratio from baseline to minDAS in the G-OO group was significantly higher than that in the G-YO and G-Y groups; however, it showed a significant decrease after minDAS to the last observation. At the last observation, no significant difference in this respect was observed between any of the groups (Table 3). There was no GCS administration after minDAS in all the groups. Therefore, cumulative GCS administration ratio is equal to that until minDAS.
The administration ratio of biologic DMARD (bDMARD) or targeted synthetic DMARD (tsDMARD) in the G-Y, G-YO, and G-OO group were 28.4%, 18.1%, and 13.8%, respectively. The G-OO group showed lower administration ratio relative to the other two groups, and G-YO showed lower administration ratio relative to the G-Y group; however, the between-group differences were not statistically significant. No significant differences were observed with respect to the results of bDMARD and tsDMARD therapy. The details of the therapy (names, number of patients, and results) in each group are shown in Table 4.

Oral drug administration ratio and dosage in phase for each group.
In mean value columns, mean value and standard deviation separated with comma are shown.
GCS administration after minDAS counted zero in every groups. Therefore, Cumulative GCS administration ratio is equals to that until minDAS. In p value, comparison between the G-Y and G-YO group, G-Y and G-OO group, and G-YO and G-OO group separated with comma were shown, respectively.
G-OO, a patient group whose age at baseline is no less than 75 years old; G-YO, a patient group whose age at baseline is no less than 65 and less than 75 years old; G-Y, a patient group whose age at baseline is less than 65 at baseline; MTX, methotrexate; TAC, tacrolimus; GCS, glucocorticoid steroid; minDAS, a moment of minimum DAS28 attained; LO, at last observation in treatment course.

bDMARD or tsDMARD drugs thrown patients and results for each group.
bDMARD, biologic disease modifying anti-rheumatic drug; tsDMARD, targeted synthetic disease modifying anti-rheumatic drug; nREM, disease activity status was non-remission at minimum; REM, disease activity status was remission at minimum; dREM, disease activity status was deep remission at minimum; AE, adverse event.
The frequency and prevalence of comorbidities and main adverse events are shown in Table 5. The G-OO group showed a significantly higher prevalence of serious infection, hospitalization, dementia, and cardiovascular diseases as compared with that in the G-YO and G-Y groups; in addition, the prevalence of these comorbidities and adverse events in the G-YO group was significantly greater than that in the G-Y group.
The influence of GCS was more evident. Interstitial lung disease, pneumocystis pneumonia, acute infection, serious infection, hospitalization, and cardiovascular diseases were significantly more prevalent in the group of patients who received GCS than in the group that did not receive GCS. The mean DAS28 at baseline in the group that received GCS (4.5) was significantly higher than that in the group that did not receive GCS (3.8); however, the mean DAS28 at minDAS in the group that received GCS (1.3) was significantly lower than that in the group that did not receive GCS (1.6). The distribution of dREM, REM, and nREM in the group that received GCS was 191, 10, and 6, respectively, whereas that in the group that did not receive GCS was 297, 12, and 60, respectively. The number of patients in nREM in the GCS group was significantly lower than that in the non-GCS group (Table 6).
We performed binary logistic regression analysis of the incidence of adverse events (such as acute infection, serious infection, and hospitalization) disaggregated by presence or absence of GCS treatment as well as by age groups. The odds ratio for adverse events according to GCS administration and age groups were 2.86 and 0.71, 4.31 and 2.03, and 3.27 and 1.88, respectively (p values: 1.4 × 10−7 and 5.6 × 10−3, 6.6 × 10−3 and 2.0 × 10−2, and 6.9 × 10−8 and 1.4 × 10−6 p value, respectively). GCS administration was associated with higher odds ratios and stronger statistical significance than the age groups for all adverse events (Table 7).

Frequency and prevalence of comorbidities for each group.
In columns, prevalence and ratio in parenthesis are shown.
In p value, comparison between the G-Y and G-YO group, G-Y and G-OO group, and G-YO and G-OO group separated with comma were shown, respectively.
G-OO, a patient group whose age at baseline is no less than 75 years old; G-YO, a patient group whose age at baseline is no less than 65 and less than 75 years old; G-Y, a patient group whose age at baseline is less than 65 at baseline; ILD, interstitial lung disease; PCP, pneumocystis pneumonia.

Efficacy and Safety of GCS administration.
In columns, prevalence and ratio in parenthesis are shown.
GCS+, a patient group who had been administered glucocorticoid steroid; GCS−, a patient group who had not been administered glucocorticoid steroid; DAS28, 28-joints disease activity score with C-reactive protein; minDAS, time when minimum DAS28 was attained; dREM, deep remission while DAS28 < 2.0; REM, remission while DAS28 ≥ 2.0 and DAS28 < 2.3; nREM, non-remission while DAS28 ≥ 2.3; ILD, interstitial lung disease; PCP, pneumocystis pneumonia.

Results of binary logistic regression analysis for each adverse event with age group and GCS.
GCS, glucocorticoid steroid administration.
Age at onset, baseline, and minDAS, the HAQ score at minDAS, dose of GCS at minDAS, administration of GCS after minDAS, comorbidities in the cardiovascular system; osteoporosis, and interstitial lung disease were regarded as significant factor using the univariate model. Furthermore, comorbidities in the cardiovascular system was the only factor demonstrating significant correlation with the occurrence of serious infection (p = 0.00281). Administration of GCS after minDAS showed tendency to correlate, however, no significant correlation was demonstrated (p = 0.0248).
Our institute is the only institute certified as institute specialized in rheumatology by the Japanese College of Rheumatology in a community-based setting. The population in the catchment area is 90,000, and the estimated patients with RA in the community are approximately 650-900; thus, 90%-100% of patients with RA in the community consult our institute. Although our community is small, areal cohort data collection is possible at one institute.
There is no clear consensus on whether aggressive treatment for elderly patients with RA is as effective as that for younger patients with RA (Pease et al. 1999; Wolfe et al. 2006; Tutuncu et al. 2006; Schmajuk et al. 2007; Schneeweiss et al. 2007; Koeller et al. 2009; Martin et al. 2014). Treatment to target is the consensus therapeutic strategy for patients with RA (Solomon et al. 2014). Even for elderly patients with RA, targeting low disease activity has been shown to be a realistic treatment strategy that can predict radiological deformity progression at 52 weeks in 12 weeks (Sugihara et al. 2015). However, aggressive treatment is still avoided by general rheumatologists owing to the high risk for comorbidities (Tutuncu et al. 2006; Wolfe et al. 2006; Schneeweiss et al. 2007). Our study revealed that aggressive treatment with a target to achieve clinical remission in 6 months is a feasible strategy for elderly patients with RA and yields good results. Our Touch Down Strategy entails rapid escalation of MTX or tacrolimus with small doses of GCS or bDMARD to alleviate excessive inflammation. This strategy was successful in that the time elapsed from baseline to achievement of minimum disease activity in the G-OO and G-YO groups was similar to that in the G-Y group; in addition, the mean disease activity at minDAS also showed no significant difference between the three groups. These results support our hypothesis.
Mean MTX dosage is rather small than in the other countries. In general, Japanese optimal dose is thought to be low than in the other countries, that is shown in the C-OPERA clinical trial (Atsumi et al. 2016). Tacrolimus is often used in the study despite this drug is not used in the other countries than in Japan. The reason is this drug has some advantage in patients with RA who are suffered by chronic interstitial lung diseases, such as lung fibrosis, connective tissue disease derived interstitial lung disease (Witt et al. 2016). In elderly patients who have lung disease have higher risk for severe infection with the use of MTX. Kawai and Yamamoto (2006) investigated the efficacy and safety of tacrolimus after DMARDs failure for treatment of elderly patient with RA prospectively, and suggested that administration of 1.5-3.0 mg/day tacrolimus is well-tolerated and provides clinical benefit. Therefore, tacrolimus is used preferable.
Mean disease duration in the G-OO was significantly longer than the other two groups. We have worried that windows of opportunities were lost in these patients, however, the results revealed no statistical difference. This may cause because relatively higher sensitivity by the drugs is demonstrated in the elderly patients. However, this delay may cause higher administration rate in the G-OO group than in the other groups at the time of minDAS.
Not only at minDAS, but also after minDAS, the mean disease activity in the G-OO and G-YO groups showed no significant difference from that in the G-Y group. This tendency was also observed with respect to PS-VAS, although significant between-group differences were observed with respect to HAQ-DI. HAQ-DI is strongly influenced by aging, which is the reason why mean HAQ-DI scores in the G-OO group demonstrated the highest value, followed by those in the G-YO and G-Y groups at every phase.
On classification of the three age groups into three subgroups each according to the minimum DAS28 value, the DAS28, HAQ-DI, and PS-VAS scores after minDAS were more stable in the G-OO group than in the G-YO and G-Y groups. In the G-YO and G-Y groups, HAQ-DI in the subgroup that attained DAS28 ≥ 2.3 at minDAS remained stable after minDAS; however, subgroups with DAS28 ≥ 2.0 and DAS28 < 2.3 at minDAS showed an increase in HAQ-DI after minDAS. PS-VAS also remained unstable after minDAS in the G-YO and G-Y groups when minimum DAS28 was ≥ 2.0 or < 2.3 at minDAS. In the G-Y group, the PS-VAS in the subgroup with minimum DAS28 ≥ 2.3 also showed an unstable increase after minDAS. These results suggested that regardless of the patient’s age, achievement of deep clinical remission with aggressive treatment that aims at clinical remission is predictive of stable clinical results in 3 years. Especially in patients aged ≥ 75 years, treatment aimed at achievement of clinical remission can result in more stable disease not only with respect to disease activity but also with respect to pain control and maintenance of ADL.
However, as a reflection point, use of GCS administration should be judged as strategic error owing to the significantly higher prevalence of serious infection and hospitalization in the G-OO than in the G-Y group. One of the reasons must be the higher frequency of GCS administration in the G-OO group. The subgroup that received GCS exhibited higher prevalence of comorbidities such as ILD, PCP, acute and serious infection, hospitalization, and cardiovascular disease; however, at the same time, this subgroup showed significantly better results with respect to attainment of minimum DAS28 and avoidance of non-remission as compared with that in the subgroup that did not receive GCS. In the G-OO group, GCS was administered from a very early stage of treatment; thus, GCS administration ratio at minDAS was higher than that in the other two groups, although GCS tapering was frequently considered after minDAS. GCS administration exhibited stronger statistical power and higher odds ratio than aging. These results suggest that GCS should not be used to easily alleviate inflammation even in the short-term owing to the high risk of comorbidities. The results of the risk factors of serious infection in the present study also demonstrate that age, GCS administration, and comorbidities correlate with the occurrence of serious infection using the univariate model of binary logistic regression analysis. Although comorbidities in the cardiovascular system demonstrate the strongest risk factor, administration of GCS after minDAS also has been regarded as a significant factor within 5%. This is consistent with the findings of previous studies (Wolfe et al. 2006; Bakker et al. 2012; Dixon et al. 2012). Even though GCS administration was associated with a significantly better minimum value of DAS28, the increased risk of adverse events or comorbidities is a quid pro quo that must be considered.
Patients who had lost to follow-up until 3 years were approximately 20% in every groups. There is no significant difference in the rate, however, G-OO patient had tendency to move to nursing home. It is inevitable issue, although how they are coped with RA is unknown. There is no statistical difference among the three groups regarding the other reason.
Some limitations of this study need to be considered while interpreting our results. 1) This was not a prospective study, but a retrospective cohort study. 2) Patients who died were excluded from the analysis; therefore, causal association between cause of death and therapy protocol is unclear. 3) This was a single-center study. 4) The ethnic and gender differences were not evaluated. However, these factors do not affect the fundamental importance of this study. The results of this study suggest that aggressive treatment of elderly patients with RA aiming at clinical remission is a realistic strategy that can predict stable prognosis in 3 years not only with respect to disease activity but also ADL and pain control. This treatment strategy is likely to improve the quality of life of patients.
In conclusion, the suggested treatment protocol, named Touch Down Strategy, is a viable and realistic treatment strategy for elderly, especially for latter-stage elderly patients with RA. Administration of glucocorticoids alleviates disease activity; however, the increased risk of adverse events is a trade-off.
The authors would like to thank Kaoru Kuwabara, Sayori Masuoka, and Mariko Osaki for their dedicated data collection.
Ichiro Yoshii wrote this article. Tatsumi Chijiwa helped to give an idea that the RA group should be divided and compare the RA-nonrem group and the RA-rem group statistically. Naoya Sawada conducted listing of independent factors that should be selected for propensity score matching.
The authors declare no conflict of interest.