2020 Volume 250 Issue 3 Pages 137-152
2020 Volume 250 Issue 3 Pages 137-152
When patients present with persistent bodily complaints that cannot be explained by a symptom-linked organic pathology (medically unexplained symptoms), they are diagnosed with ‘functional’ somatic syndromes (FSS). Despite their prevalence, the management of FSS is notoriously challenging in clinical practice. This may be because FSS are heterogeneous disorders in terms of etiopathogenesis. They include patients with primarily peripheral dysfunction, primarily centrally driven somatic symptoms, and a mix of both. Brain-imaging studies, particularly data-driven pattern recognition methods using machine learning algorithms, could provide brain-based biomarkers for these clinical conditions. In this review, we provide an overview of our brain imaging data on brain-body interactions in one of the most well-known FSS, irritable bowel syndrome (IBS), and discuss the possible development of a brain-based biomarker for FSS. Anticipation of unpredictable pain, which commonly elicits fear in FSS patients, induced increased activity in brain areas associated with hypervigilance during rectal distention and non-distention conditions in IBS. This was coupled with dysfunctional inhibitory influence of the medial prefrontal cortex (mPFC) and pregenual anterior cingulate cortex (pACC) on stress regulation systems, resulting in the activated autonomic nervous system (ANS) and neuroendocrine system stimulated by corticotropin-releasing hormone (CRH). IBS subjects with higher alexithymia, a risk factor for FSS, showed stronger activity in the insula during rectal distention but reduced subjective sensitivity. Reduced top-down regulation of the ANS and CRH system by mPFC and pACC, discordance between the insula response to stimulation and subjective sensation of pain, and stronger threat responses in hypervigilance-related areas may be a candidate brain-based biomarker.
When patients present with persistent bodily complaints that cannot be explained by a symptom-linked organic pathology (medically unexplained symptoms), they are diagnosed with a ‘functional’ somatic syndrome (FSS) (Henningsen et al. 2007, 2018). FSS are common in all areas of medicine, with patients assigned to different types of specialists depending on the symptoms. These include pain at various locations (abdomen, chest, head, back, muscles or joints), diarrhea or constipation, dizziness, palpitations, fatigue, exhaustion, movement disorders, pseudo-seizures, and more (Henningsen et al. 2007, 2018). The most prevalent FSS include irritable bowel syndrome (IBS), functional dyspepsia, fibromyalgia, chronic fatigue syndrome, and chronic low-back pain, and these share clinical features (Henningsen et al. 2007, 2018; Crabtree and Ganty 2016). In one study, FSS were diagnosed in 40-49 % of all primary care patients (Haller et al. 2015). Despite this high prevalence, assessment and management of the FSS are challenging and often suboptimal in clinical practice (Henningsen et al. 2007, 2018; Graver 2017). The traditional biomedical approach, which often involves repeated, invasive procedures to find organic causes in bodily organs, is inconclusive (by definition, in FSS), costly, risky, and can increase patients’ anxiety about the unknown causes of disease (Henningsen et al. 2007, 2018; Graver 2017).
The standard approach of focusing on individual organs to which the symptoms are attributed treats each symptom as though it occurs in isolation. However, in reality, many patients have multiple symptoms and fulfill the criteria for more than FSS (Henningsen et al. 2007, 2018; Kim and Chang 2012). When this is the case, a central cause outside of individual organs is more likely. In addition, FSS have common psychological characteristics that make brain dysfunction a likely contributing cause: female predominance, high psychiatric comorbidity, particularly anxiety and depression; catastrophizing, i.e., believing symptoms reflect a (life-)threatening condition; hypersensitivity to somatic and/or visceral stimulation across body sites (Henningsen et al. 2007, 2018; Kim and Chang 2012); dysregulation of the neuroendocrine system (hypothalamic-pituitary-adrenal (HPA) axis) (Tak et al. 2011; Kim and Chang 2012) and autonomic nervous system (ANS) (Tak and Rosmalen 2010; Martinez-Martinez et al. 2014); peripheral immune activation (Kim and Chang 2012), and similar responsiveness to anti-depressant and psychological therapies (Henningsen et al. 2007, 2018; Wortman et al. 2018).
For example, “interoception” is the sense of one own’s physiological condition including pain, temperature, itch, sensual touch, muscular and visceral sensations, vasomotor activity, and hunger (Craig 2002, 2003). The interoceptive system can be the foundation of mood and is highly connected with the autonomic nervous system and play an important role in controlling appropriate homeostasis of the physiological bodily condition (Craig 2002). The change of the interoceptive system is suggested as a common cause of FSS. Similarly, central sensitization, which is hyper-excitability of central neurons to noxious and non-noxious stimuli, is supposed to be common in the etiology of FSS such as fibromyalgia, chronic fatigue syndrome, IBS, temporomandibular joint disorder, and tension headache (Neblett et al. 2013). Early life adversity may epigenetically change the central sensitivity (Liu et al. 2017), HPA system (Vaiserman and Koliada 2017), and immune system (Elwenspoek et al. 2017) and is associated with a higher risk for FSS (Afari et al. 2014). FSS not only share a common etiology but also have an influence on disease severity each other. Co-existing fibromyalgia increased somatic and visceral perception in IBS patients (Tremolaterra et al. 2014). Psychotherapies such as cognitive behavioral therapy, hypnotherapy, and mindfulness-based therapy have demonstrated evidence for efficacy for IBS as well as fibromyalgia, chronic fatigue syndrome, and chronic low-back pain (Henningsen et al. 2018). Because of these common (psychological) characteristics, FSS are collectively classified in the current psychiatric classification system [Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)] as somatic symptom disorder with a variety of background pathology of somatization, conversion (replacing unconscious mental conflict with physical symptoms), and/or illness anxiety disorder (Henningsen et al. 2007, 2018; Wortman et al. 2018).
FSS may also arise from interactions between “organic” organ pathology and psychological vulnerabilities. Despite high co-morbidity with background anxiety and mood disorders, FSS can develop in the absence of prior disorders, but be potentiated by anxiety and mood problems that develop after illness or injury (Henningsen et al. 2007, 2018). For example, in some cases in IBS, symptoms may originate in the gut, for example primarily driven by an increase in intestinal permeability after infection or by food antigens. Associated psychological distress may develop only later, which in turn further aggravates symptoms and may potentiate further organ pathophysiology (Ford et al. 2017).
Like other FSS, an IBS diagnosis is made according to symptom-based diagnostic criteria, as proposed by the Rome IV expert committee (Lacy et al. 2016). The current Rome IV criteria for IBS are “recurrent abdominal pain at least once per week, on average, in the previous 3 months, with a duration of at least 6 months, associated with two or more of the following criteria; related to defecation, change in the frequency of stool and/or change in the form of stool”. IBS patients are heterogeneous in terms of etiopathogenesis, which implies that FSS can contain patients with purely peripheral dysfunction, CNS-oriented somatic symptoms, and both pathologies mixed in most cases (Ford et al. 2017). This is because of the nature that function of the peripheral organs is mainly dominated by the autonomic nervous system, which is tightly connected to the brain and is easily influenced by brain conditions such as mood, personality, and stress responses. Conversely, peripheral dysfunction sends signals that can profoundly impact the brain, reducing subjective feelings of well-being and increasing negative mood (Craig 2002). Some patients with uncomplicated FSS may benefit from symptom-focused approaches, such as pharmacotherapy targeting peripheral functioning, while others with more complicated features such as multi-organ bodily and mental health symptoms may need brain-oriented approaches such as antidepressants or psychotherapy (Ford et al. 2017; Wortman et al. 2018). It is an ongoing debate whether FSS should be classified as a physical or mental disorder or as a psychosomatic disorder interposed between biomedical, organ-oriented and CNS-oriented cognitive interpersonal aspects (Ford et al. 2017).
Traditionally, the field of psychosomatic medicine has dealt with the mechanisms by which emotion, cognition, behavior, or social factors may impact physical disease or, conversely, how physical disease may result in altered emotion, cognition, or behavior (Lane et al. 2009a, b). Early in the 20th century, Walter Cannon proposed that subcortically generated emotions could generate downstream signals to the hypothalamus to influence peripheral physiological processes, or upstream signals to the neocortex for symbolic representation and expression (Cannon 1928). Papez proposed an interconnected circuit of cortical and subcortical structures that processed information from the environment and the body to generate emotions (Papez 1937). MacLean hypothesized that psychosomatic disorders resulted from impaired communication between a similar system, which he termed the “limbic system” (after limbus, or border) and neocortex (Maclean 1949). These early studies hypothesized a particular role of the brain in the association between psychological/cognitive conditions and functioning of peripheral organs.
It is only recently that developments in brain imaging have made it possible to study the human brain and its functioning in-vivo. Much of the functional brain imaging literature to date has focused on understanding the neural basis of cognitive, emotional, and social functions and their interrelations, in addition to brain dysfunction in neurological and psychiatric disorders (Petzschner et al. 2017). Psychosomatic research, and related branches of psychophysiology and psychoneuroimmunology, focus on how these brain functions are integrated with peripheral organ functioning via critical information transfer from systems such as the autonomic, neuroendocrine, and immune systems (Petzschner et al. 2017).
In this review, we provide an overview of our recent brain imaging studies on how the brain mediates the influence of psychological/cognitive processes on peripheral organ systems and how it regulates the autonomic and neuroendocrine system in health and IBS (as a prototypical FSS). All our studies were approved by the Ethics Committee of Tohoku University Graduate School of Medicine and conducted in accordance with the principles of the Declaration of Helsinki. Based on the results of these studies, we discuss whether integration of measurements of brain function, peripheral organ function and brain-body interfaces can be used as a new biological diagnostic criterion for FSS. Further, the recent paradigm shift in brain imaging analysis, driven by the advent of machine learning/statistical learning algorithms developed in the fields of artificial intelligence and statistics, have begun to try to decode mental states from brain activity (Sundermann et al. 2014; Wager 2015; Woo et al. 2017; Kragel et al. 2018b; Lotsch and Ultsch 2018). By integrating neuroimaging with measures of peripheral physiology and cognitive, psychiatric, neurological, and clinical assessments, it may be possible to predict clinical state and functional symptoms in cases where no organic peripheral cause can be found. We also discuss the challenges inherent in adopting these methods to assess individual patients with FSS, particularly surrounding diagnosis, choice of the appropriate therapy, and evaluation of therapeutic efficacy.
Hypersensitivity to peripheral stimulation is one of the common features of FSS. Visceral hypersensitivity can be observed in 30-40% of IBS patients (Naliboff et al. 1997; Zhou et al. 2010; Simrén et al. 2018) and also in functional dyspepsia (Mertz et al. 1998; Simrén et al. 2018), fibromyalgia (Staud 2002; Arnold et al. 2016), and chronic fatigue syndrome (Vecchiet et al. 2003; Nijs et al. 2012). Lower pain threshold has previously been considered as a biomarker of IBS (Mertz et al. 1995).
Initial brain imaging studies mainly aimed to verify the hypothesis that visceral signal processing in the brain may be different between patients with IBS and healthy controls (Tillisch et al. 2011; Mayer et al. 2015a 2019), and whether such altered brain activation pattern could be the origin of the unexplained symptoms. Studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) increased our understanding of how the brain encodes noxious colorectal distention (Tillisch et al. 2011; Mayer et al. 2015a; Kano et al. 2018). Similarly to somatic noxious stimulation, visceral stimulation activates a set of brain areas involved in processing ascending nociceptive input and correlated with pain experience across studies, including the insula (posterior/middle/anterior), anterior cingulate cortex (ACC) (subgenual/pregenual), midcingulate cortex (MCC) (anterior/posterior) thalamus, primary somatosensory cortex (see Fig. 1) (Kano et al. 2018). Other areas that are not known to encode nociceptive input are also activated, including prefrontal cortex and posterior partial cortex.
At a given stimulation level, such as rectal stimulation at 40mmHg pressure, healthy people may feel discomfort, but patients with IBS often feel abdominal pain, i.e., show visceral hypersensitivity (Dorn et al. 2007). When comparing brain activity between healthy controls and IBS patients at similar levels of colorectal distention, the intensity-coding areas discussed above – particularly the insula, thalamus, somatosensory cortex, and cingulate cortex (See Fig. 1 for reference) – are hyperactivated in patients with IBS (Naliboff et al. 2001; Ringel et al. 2003; Verne et al. 2003; Berman et al. 2008). Larsson et al. divided IBS patients into two groups: normosensitive (similar sensitivity to healthy controls) and hypersensitive patients (Larsson et al. 2012). Brain responses to distention were similar for normosensitive patients and healthy controls, but hypersensitive patients showed greater insula response to visceral stimulation than healthy controls and normosensitive IBS patients (Larsson et al. 2012). In our fMRI study, which matched patients and controls on subjective discomfort, brain responses were similar between healthy controls and patients with IBS (Kano et al. 2017a).
Other studies have found differences between IBS patients and controls when comparing brain activity at subjectively similar level of colorectal distention (i.e., at the individually determined maximum tolerable volume of the distention balloon). In one study, healthy controls and IBS patients demonstrated activation in the different parts of the anterior cingulate cortex (ACC) and prefrontal cortex (PFC) (Andresen et al. 2005). Another study with a subjectively similar level of distention reported that patients with IBS showed stronger activation in the left anterior insula and PFC, but these differences in activation decreased after controlling for anxiety and depression, suggesting that they are at least partially accounted for by differences in levels of mental health symptoms (Elsenbruch et al. 2010).
Together, these studies indicate that the visceral hypersensitivity in IBS patients is reflected in stronger brain activity in pain-related areas. This could be due to visceral hypersensitivity in the noxious signaling pathway at the peripheral level from gut to brain (Larsson et al. 2012), or due to changes in circuits within the brain. Besides, brain activity during rectal distention can also be modified by psychological factors such as depression or anxiety (Mayer et al. 2015a, b; Kano et al. 2018). Hence, despite similar symptoms, brain activity in IBS patients can be different depending on a variety of factors, including visceral hypersensitivity or psychological factors. Thus, brain activity may be different in IBS patients not specifically because of gut pathology, but because of patients’ physiological or psychological characteristics (Kano et al. 2018).

Brain areas processing noxious signals.
Regions related to (1) the perception of the afferent noxious signals (PB, thalamus, posterior insula, aMCC, PCC, S1) and integrate perception with other sensory information (S2, PPC); (2) emotional reactions (amygdala, subgenual and pregenual ACC, and hippocampus); (3) motor planning and response (BG, M1, and SMA); (4) cognitive and descending modulation (PFC, ACC, PAG, and RVM).
aMCC, anterior midcingulate cortex; BG, basal ganglia; M1, primary motor cortex; PAG, periaqueductal gray; PB, parabrachial nucleus; PCC, posterior cingulate cortex; PFC, prefrontal cortex; pgACC, pregenual anterior cingulate cortex; PCC, posterior cingulate cortex; PPC, posterior parietal cortex; RVM, rostroventral medulla; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; sgACC, subgenual anterior cingulate cortex; SMA, supplementary motor area.
One of the common cognitive and emotional features among patients with IBS is fear towards the unpredictable worsening of symptoms such as diarrhea, bloating, or abdominal pain (Spiegel et al. 2011). Patients with IBS or other FSS have a tendency to overestimate the potential for physical harm, and imagine worsening trajectories of symptoms and dysfunction (i.e., catastrophizing) (Spiegel et al. 2011; Graver 2017). They do not know where and when particular symptoms will be triggered, thereby enhancing their feeling that they cannot control their life due to the disease (Spiegel et al. 2011). Some patients try to avoid any situation in which they cannot reach the bathroom freely, such as buses, trains, airplanes, or crowded places, and consequently reduce participation in daily activities such as social gatherings (Spiegel et al. 2011). They become hypervigilant about their food, eating at restaurants, or anything that might exacerbate symptoms (Spiegel et al. 2011). This anticipatory concern may itself trigger their symptoms (Spiegel et al. 2011). A similar mechanism, in which cognitive and emotional factors influence and maintain symptoms, is observed in other FSS (Henningsen et al. 2007; Graver 2017). For example, in chronic musculoskeletal pain, due to the anticipated threat of intense pain, some patients constantly monitor and become vigilant to pain sensations (Nijs et al. 2012; Meier et al. 2018). They try to avoid pain-related movement and activities that potentially exacerbate their pain (Meier et al. 2018). This avoidance impairs their physical functioning, restricts their daily activities, and reduces participation in social activities (Gatchel et al. 2016). In both IBS and chronic musculoskeletal pain, symptom-specific fear is the main target of psychological therapies; many therapists focus on gradual exposure to situations the patient is avoiding because of fear of symptoms (Vlaeyen and Linton 2000; Ljótsson et al. 2013; Broderick et al. 2016; Gatchel et al. 2016; Kinsinger 2017; Hesser et al. 2018).
To study such effects at the brain level, we conducted an fMRI study to examine the hypothesis that unpredictability about the occurrence of an aversive visceral stimulus can impact its brain processing in patients with IBS. In this study, three types of visual cues before rectal distention informed the participants whether they would receive visceral stimulation with 100% certainty (the ‘threat’ condition), no stimulation (the ‘safe’ condition), or stimulation with 50% certainty (the ‘uncertain’ condition). Rectal distention was performed at an individually titrated level (Kano et al. 2017a). In the uncertain condition, IBS patients demonstrated stronger brain responses in the posterior and mid-cingulate cortices and the precuneus than in the certain visceral stimulation condition, even though the stimulation level for each participant was the same between both conditions. These areas may be associated with the orientation of the body and self-reference and may indicate that unpredictability induces increased attention to visceral sensation (Vogt 2005; Goffaux et al. 2014). Furthermore, on no-distention trials, healthy controls showed stronger brain activity in the bilateral insula after uncertain vs. safe cues. However, patients showed insular hyperactivity even in the ‘safe’ condition. The insula is considered a key component of the salience network, which is thought to be active in hypervigilant states (Mayer et al. 2015b; Kano et al. 2018). Thus, the brain of IBS patients may be hypervigilant even in relatively safe conditions.
Other studies also revealed the influence of cognitive and emotional aspects of expectation on brain responses in IBS patients and healthy controls (Berman et al. 2008; Icenhour et al. 2015). Using a classical Pavlovian conditioning paradigm in which neutral visual cues and painful rectal distentions were paired, Icenhour et al. made participants learn that one of the visual cues predicts rectal distention (the ‘threat’ condition), whereas the other cue predicted absence of rectal distention, thereby acting as a safety signal (Icenhour et al. 2015). Patients with IBS compared to healthy controls demonstrated increased response to the threat cue in the posterior cingulate cortex (PCC) and ventrolateral PFC, which are considered to be associated with hypervigilance, and in the amygdala, which may be related with pain-related fear, before distension was delivered (Icenhour et al. 2015). This may represent a hypervigilant state of the brain corresponding to exaggerated fear in patients with IBS in a situation that may induce their symptoms. Berman et al. (2008) characterized abnormalities in preparatory brain response before noxious stimulation in IBS. In their study, healthy controls showed decreased activity in the dorsal brainstem (i.e., the locus ceruleus complex and laterally adjacent parabrachial nuclei), subgenual anterior cingulate cortex (sACC), and amygdala during a cued anticipation period (Berman et al. 2008). The amplitude of the anticipatory decrease in the dorsal brainstem was associated with greater activation during distention in the right orbitofrontal cortex and bilateral sACC (Berman et al. 2008). The experience of stress during scanning was correlated negatively with the dorsal brainstem activity during anticipation (Berman et al. 2008). Patients with IBS compared to healthy controls showed a higher feeling of stress and failed to preparatory inhibition of the dorsal brainstem and enhanced brain response in the dorsal ACC and the dorsal brainstem during distention period (Berman et al. 2008). One interpretation of these findings is that fear towards visceral sensations impairs functioning of endogenous pain regulation systems in the brain. Paralleling these findings, patients with low back pain compared to healthy controls exhibited decreased functional connectivity between amygdala and periaqueductal gray (PAG) when watching video clips showing potentially harmful situations for the back (Meier et al. 2017). The amygdala-PAG connectivity strength was negatively correlated with pain-related fear which was assessed by a scale for kinesiophobia (phobia for movement which may induce pain) (Meier et al. 2018). The amygdala-PAG is one of the key descending pain control pathways and the fear of pain may disrupt the pathway (Bushnell et al. 2013). Similarly, a placebo study indicated an impaired pain modulatory pathway in patients with IBS (Schmid et al. 2015). Cognitive and emotional factors, particularly fear towards the symptoms may change brain processing of nociceptive signals from the body, as well as their endogenous modulation.
Stress is thought to initiate, amplify, and/or perpetuate the symptoms of IBS (Drossman 2011, 2016; Van Oudenhove et al. 2016; Pellissier and Bonaz 2017), and other FSS including fibromyalgia (Borchers and Gershwin 2015), chronic fatigue syndrome (Luyten et al. 2011), and chronic low-back pain (Abdallah and Geha 2017). In an early study, giving a false diagnosis of cancer to medical students who underwent a voluntary sigmoidoscopy, increased rectal contractility (Drossman 2016). Stress has been reported to change gastrointestinal motility, including findings of delayed gastric emptying, impaired gastric accommodation (Geeraerts et al. 2005; Ly et al. 2015), prolonged small bowel motility, and increased colonic motility and secretion (Fukudo and Suzuki 1987; Fukudo 2013; Van Oudenhove et al. 2016; Vanner et al. 2016; Pellissier and Bonaz 2017). Stress is also known to increase visceral perception, intestinal permeability, and emotional responses to abdominal events, and influence gut microbiota in healthy people and patients with IBS (Vanner et al. 2016; Pellissier and Bonaz 2017). The main mediators in the brain regulating peripheral function under these stress responses are the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis, as well as aspects of the immune system (Vanner et al. 2016; Pellissier and Bonaz 2017). These systems mediate the bidirectional communication between the brain and peripheral organs including the gut (Vanner et al. 2016; Pellissier and Bonaz 2017).
Studies on FSS have been focusing mainly on altered brain processing and modulation of afferent signals from the body as potential mechanisms underlying symptom generation in general, and the impact of psychological processes in particular. However, the central mechanisms driving changes in peripheral functions under stress, and the nature of the psychological influences that most strongly impact these mechanisms, remain understudied. In the case of the gut, the afferent branches of the ANS transfer mechanical, chemical, and thermal sensory signals, whether physiological or nociceptive, as well as microbiota-related, immune, and endocrine signals from the GI tract to the brain. At the same time, the sympathetic and the parasympathetic efferent branches of the ANS modulate the enteric nervous system and, thereby, gut function (Vanner et al. 2016). The central autonomic network in the brain is responsible for generating such ANS output in response to the afferent input (Pellissier and Bonaz 2017) and it consists of the frontal cortex, the insula, ACC, the amygdala, the paraventricular nucleus of the hypothalamus, the PAG, the parabrachial nucleus, the nucleus of the solitary tract and the reticular formation in the brainstem (Benarroch 2001).
The other main mediator is the corticotropin-releasing factor (CRH) system. CRH is in part a hypothalamic hypophysiotropic hormone that releases adrenocorticotropic hormone (ACTH) from the anterior pituitary gland and regulates cortisol secretion from the adrenal gland along the HPA-axis. However, it is also a neurotransmitter that stimulates the neurons in medial prefrontal areas, including pACC, in the hippocampus, and in the hypothalamic nuclei (Stengel and Tache 2010; Fukudo 2013; Pellissier and Bonaz 2017). CRH binds with high and moderate affinity to CRH receptors CRH1 and CRH2, respectively (Taché and Million 2015). Dense CRH1 receptor expression is found in the forebrain, subcortical limbic structures and amygdala, whereas the expression in the hypothalamus is low under basal conditions but markedly up-regulated by stress. Moreover, CRH1 receptors are prominently expressed in the anterior pituitary gland (Taché and Million 2015). Stress induces activation of the sacral parasympathetic nucleus through projections from Barrington’s nucleus in the pons, which is activated by brain CRH (Stengel and Tache 2010). There are CRH1 and CRH2 (less prominently) receptors in the myenteric and submucosal nervous plexus of the distal gut by which CRH impact gut functions such as motility, permeability, and sensitivity (Stengel and Tache 2010). The CRH1 and CRH2 receptors interact in the myenteric neurons (Taché and Million 2015). Acute stress-related colonic stimulation engages mainly the CRH1-mediated colonic stimulatory pathway and CRH2 also to dampen the colonic response to stress (Taché and Million 2015). To reveal the mechanisms underlying stress effects on GI function and symptoms in patients with IBS, researchers have examined changes in ACTH or cortisol responses to a variety of stressors, including rectal distention, psychological stress, or CRH injection (Fukudo et al. 1998; Dinan et al. 2006; Tanaka et al. 2016; Videlock et al. 2016). In addition, ANS dysfunction in patients with IBS has been demonstrated at rest and in response to stressors such as visceral stimulation, meal intake, or psychological stressors, though the results have not always been consistent (Chang 2011).
To date, most studies have measured ANS and neuroendocrine activation in isolation, without concomitant measures of brain and gut function (Gianaros and Sheu 2009; Gianaros and Wager 2015; Kraynak et al. 2018). Connecting these is crucial to improve our understanding of the pathophysiology of IBS. Few studies have investigated brain regulation of stress mediators in disease conditions such as IBS (Pellissier et al. 2010; Mayer et al. 2015b; Pellissier and Bonaz 2017). We addressed these issues using functional brain imaging in IBS patients and controls. For this purpose, ACTH, cortisol and ANS responses to intravenous CRH administration, as well as the resulting change in gut motility, were investigated. The same subjects also underwent an examination of brain responses to colorectal distention (Fig. 2a-c) (Kano et al. 2017b). Patients with IBS, compared to healthy controls, demonstrated an increased ACTH response over 120 minutes. Male IBS patients, compared to male healthy controls, had increased colonic motility responses (Fig. 2a). Female IBS patients, compared to female healthy controls, on the contrary, showed an altered sympathovagal balance indicated by the ratio of the low frequency (LF) component over the high frequency (HF) component of heart rate variability (HRV) after CRH injection, and lower basal parasympathetic tone indicated by the HF component. Furthermore, we explored the association between brain activity during colorectal distention and individual ACTH responses to CRH injection as an index of physiological stress reactivity. Healthy controls with stronger brain activation in the pregenual anterior cingulate (pACC) demonstrated a lower ACTH response, but this association was not observed in patients with IBS (Fig. 2b, c). The medial prefrontal cortex (mPFC) and pACC have shown effects consistent with inhibitory regulation of the HPA axis response (Herman et al. 2003; Urry et al. 2006). Therefore, mPFC/pACC activity may represent individual differences in inhibitory control of the HPA axis in response to stress conditions. The increased CRH release in IBS, possibly due to chronic stress-induced dysfunction of the mPFC/pACC inhibitory system, may induce upregulation and/or increased activation of CRH1 receptors in the pituitary gland (Kano et al. 2017b). The colorectum motility response to CRH administration was exaggerated in IBS patients (Kano et al. 2017b), which indicates upregulation of CRH-CRH1 signaling in the colon (Taché and Million 2015) in the same IBS population with an altered brain CRH system. Systemic changes of the CRH system not only in the brain but also at the peripheral colonic level may occur in IBS.
In addition to the CRH regulation system in the brain, we studied brain regulation of ANS function in IBS patients and healthy controls (Fig. 2d-f) (Kano et al. 2019). ANS activity during rest, before and during colorectal distention were measured. Healthy controls demonstrated an increased sympathovagal balance (LF/HF) during colorectal distention, while that response was blunted in patients with IBS. In healthy participants, but not in IBS patients, basal parasympathetic tone, indicated by the HF component, was inversely correlated with the perception threshold of colorectal distention (Fig. 2d), indicating that subjects with higher parasympathetic tone had less visceral sensitivity. Similarly, inter-individual differences in basal parasympathetic tone were positively correlated with the brain response to colorectal distention in the right caudate, and the pregenual (p)ACC and anterior midcingulate (aMCC) in controls but not in patients with IBS (Fig. 2e, f). The pACC and aMCC are part of the central autonomic network (CAN) that regulates ANS output (Benarroch 2001), as well as primary sources of top-down modulation of (visceral) nociception (Russo and Sheth 2015). These brain areas are thought to play an important role in mediating the functional interaction between parasympathetic function and visceral nociception. Thus, this coupling may be dysfunctional in patients with IBS.
Most of previous reports have found alterations in peripheral measures with IBS, such as those related to the ANS, CRH, visceral nociceptive perception and gut motility, but often assumed that brain networks regulating these functions work similarly in healthy controls and patients. However, our studies indicate that regulatory brain circuits themselves may be dysfunctional in patients with pathological conditions such as IBS. These abnormalities may drive peripheral dysfunctions, although caution is warranted in making causal inferences based on such cross-sectional associations. In IBS, which is conceptualized as a disorder of brain-gut interaction, complex, hierarchical changes with multiple bidirectional feedback loops between the different levels of the gut-brain axis may indeed be generating and perpetuating symptoms. It is therefore suggested that rather than being the result of a single common abnormality, several types of underlying pathology can produce the same pattern of symptoms that define FSS, but for different biological reasons. It is therefore unlikely that the pathophysiology of FSS including IBS can be explained by a single abnormality in either peripheral function, brain function, or the brain-periphery interface. Instead, we may need a different view from the traditional linear, monocausal biomedical one to capture the complexity of bidirectional brain-body interactions, taking into account individual differences in the alterations of these systems.
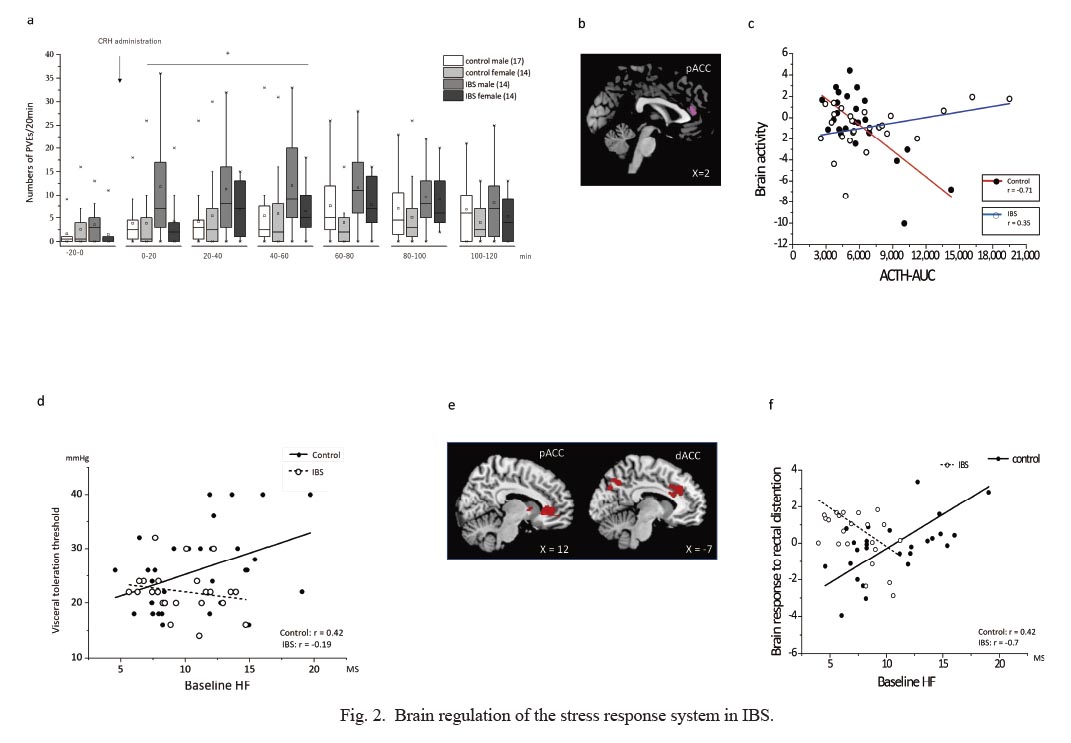
Brain regulation of the stress response system in IBS.
a. Effect of CRH administration on colorectum motility in patients with IBS and healthy control subjects. A phasic contraction was defined a 10 % reduction from baseline colorectum pressure tone (phasic volume event; PVE). Male IBS patients, compared to male healthy controls, had increased colonic motility responses after CRH administration.
b. Brain activity (first eigenvariate) in the pregenual anterior cingulate cortex (pACC) during rectal distention was associated with individual area under the curve of the ACTH responses (ACTH-AUC) to CRH administration differently between patients with IBS and control subjects.
c. Scatter plot and regression of brain activity during rectal distention and ACTH response in patients with IBS versus control subjects for the right anterior cingulate cortex. Healthy controls with stronger brain activation in the pACC demonstrated a lower ACTH response, but this association was not observed in patients with IBS.A scatter plot of the correlation between the baseline parasympathetic vagal tone (HF) and visceral perception threshold. In healthy participants, basal parasympathetic tone was inversely correlated with the perception threshold of colorectal distention, but not in IBS patients.
d. Brain activity in the dorsal anterior cingulate (dACC) and pACC during colorectal distention associated with the baseline parasympathetic vagal (HF) tone.
e. A scatter plot and regression of the brain activity during colorectal distention and baseline HF values in patients with IBS and healthy controls. Inter-individual differences in basal parasympathetic tone were positively correlated with the brain response to colorectal distention in the pregenual (p)ACC and anterior midcingulate (aMCC) in controls but not in patients with IBS.
Reproduced from (Kano et al. 2017b, 2019)
MS, meter second.
Alexithymia is a personality construct characterized by difficulty identifying and describing feelings, and externally oriented thinking (Taylor et al. 1999; Luminet et al. 2018). The construct was originally developed based on clinical observations on patients with classical psychosomatic diseases. Follow-up studies found high levels of alexithymia in patients with FSS including IBS, non-cardiac chest pain, and other chronic pain disorders, and also in eating disorders, diabetes, and others (Taylor et al. 1999; Lumley et al. 2007; Luminet et al. 2018). Alexithymia is considered to be a risk factor for physiological, mental, or behavioral health problems that are influenced by affect regulation (Taylor et al. 1999; Lumley et al. 2007; Luminet et al. 2018).
We have conducted several brain imaging studies to investigate how alexithymia contributes to FSS (Kano and Fukudo 2013). As did many other studies, we found alterations in emotion-related brain responses in alexithymics, i.e. reduced activity in the right middle insula and aMCC compared to non-alexithymic participants when looking at pictures depicting angry faces (Kano et al. 2003). In addition, the alexithymic participants showed reduced activity in the ventromedial prefrontal cortex during an emotion-guided decision-making task (Kano et al. 2011). During colorectal distention, healthy alexithymic participants showed increased activity in the right posterior insula, orbitofrontal cortex and midbrain even after regressing out the influence of anxiety (Fig. 3a) (Kano et al. 2007). Simultaneously, the alexithymic participants had higher adrenaline secretion and stronger pain ratings to colonic distention (Kano et al. 2007). More recently, we analyzed the influence of alexithymia on brain processing and perception of visceral noxious stimulation in healthy controls and patients with IBS (Kano et al. 2020). Although alexithymia scores were not different between healthy controls and IBS patients, patients with higher alexithymia scores demonstrated higher brain activity in the right insula during colorectal distention compared to alexithymic controls (Fig. 3b). This activity was survived after controlling negative emotion. However, patients with higher alexithymia scores reported less fear before colorectal distention and were hyposensitive to colorectal stimulation (Fig. 3c). These patients also demonstrated stronger ACTH responses to CRH administration compared to healthy controls with similar alexithymia scores.
This result in IBS patients is somewhat contradictory with previous findings in healthy participants with alexithymia (Kano et al. 2007, 2020). Healthy participants with alexithymia showed hypersensitivity but IBS patients reported alexithymia-associated hyposensitivity. Recent meta-analyses have pointed out a similar pattern for alexithymia effects in chronic pain disorders (Aaron et al. 2019). Enhanced alexithymia was correlated with disease severity across a variety of pain disorders, but was not consistently correlated with pain intensity (Di Tella and Castelli 2016; Aaron et al. 2019). Alexithymia may be a risk factor for subsequent pain, as reduced ability to label and describe emotions may lead to misperceptions of emotion-related physiological signals as signs of illness (Di Tella and Castelli 2016; Aaron et al. 2019).
Our brain study may provide novel insights to help interpret the complex pattern observed with alexithymia. Enhanced activity in the visceral sensory cortex (posterior and anterior insula) may indicate that the brains of alexithymic IBS patients are hypersensitive to noxious visceral stimulation, despite reduced conscious perception. This decoupling of objective and subjective responses to pain is a phenomenon that has also been described for subjective and neuroendocrine responses to psychosocial stress in fibromyalgia (Coppens et al. 2018). Although hypersensitivity to noxious bodily stimulation has been considered as a hallmark of chronic pain disorders, including FSS, a recent consensus statement pointed out that the pathophysiology of chronic pain cannot solely be explained by sensitivity to acute noxious stimulation (Davis et al. 2017). Another mechanism, for example, related to emotional and cognitive factors may facilitate the development of the chronic condition. With regard to alexithymia, decoupling of subjective sensations and physiological brain responses may be one of the possible pathophysiologies underlying chronification.

Brain imaging studies on the role of alexithymia contributing to IBS.
a. In healthy participants, alexithymic score was corelated with the brain activity in the right posterior insula, orbitofrontal cortex and midbrain during colorectal distention (Kano et al. 2007).
b. IBS patients with higher alexithymia scores demonstrated higher brain activity in the right insula during colorectal distention.
c. Scatter plot and robust regression analysis of the individual discomfort thresholds and alexithymia scores in patients with IBS and healthy controls. IBS patients with higher alexithymia scores were hyposensitive to colorectal distention.
Reproduced from (Kano et al. 2007, 2020) with permission.
Functional brain imaging studies, including in health and FSS, started in the early 1990s. The traditional analysis approach, which was used in the studies described in the previous sections, focused on brain activity during an event such as cognitive tasks or colorectal stimulation compared to a control condition to determine which brain regions were representing clinical features of in health and FSS (“brain mapping”) (Lane et al. 2009a, b; Wager 2015). Because FSS are characterized by physical symptoms without objective organic cause on general medical examination, neuroimaging has played a particularly important role in identifying changes in brain activity and structure with FSS that would otherwise be invisible. This has facilitated the acceptance of FSS in the medical community. It has also facilitated our understanding of the role of the brain in the pathophysiology of FSS.
Recent progress in analyses techniques in brain imaging, adopting e.g. machine learning, has advanced the direction of the approach from identifying brain regions activated by an event such as rectal distention (brain mapping) to deciphering brain activity patterns which discriminate one type of event from others (decoding) and/or predict the perceptual response to it (Wager et al. 2013; Wager 2015; Davis et al. 2017; Price et al. 2018). Machine learning, developed from computational science, is a family of methods for detecting patterns in data based on exemplar data (learning) and using the identified patterns to predict or classify future data (Dhar 2012; Murphy 2012). Such multivariate pattern analysis (MVPA) makes it possible to distinguish mental condition A from B or to identify the commonality between conditions C and B based on brain imaging data (Wager 2015; Woo and Wager 2015; Reddan and Wager 2018).
In a recent collaborative study, we sought to identify brain activity patterns in the mPFC that specifically represent cognitive control, negative emotion, and pain, but generalizes across different modalities within each of these three psychological domains (Fig. 4a-c) (Kragel et al. 2018a). The mPFC, including anterior midcingulate cortex, has been reported to be activated in multiple tasks associated with cognition, emotion and pain. However, generalizable representations across the tasks had not been identified. Functional MRI data from 18 studies included 270 participants in a balanced, hierarchical structure of 6 cognitive control studies (two working memory, two response selection, and two response conflict), 6 negative emotion induction studies {two visual, two social (e.g., social rejection or perception of others in pain) and two auditory}, and 6 pain studies (two thermal, two visceral, and two mechanical) (Fig. 4a). Using a machine learning approach, a generalizable pain representation was found in the aMCC across the 6 pain studies (Fig. 4b, c). This representation was specific to pain versus cognitive control and negative emotion domains. Similarly, representations of negative emotions were localized in the ventromedial PFC, and specific subtypes of cognitive control were represented in portions of the dorsal midcingulate (Fig. 4b, c).
In a second study, we used a similar approach to investigate brain pattern differences between somatic and visceral noxious sensations (in submission). Seven fMRI studies during two rectal distentions (Rubio et al. 2015; Kano et al. 2017a), a gastric distention, an esophageal distention (Kano et al. 2013), two thermal somatic stimulations, and a vestibular stimulation (Pazmany et al. 2017) were included. The study found representation of somatic stimulation in the somatomotor, dorsal attention, and ventral attention networks, whereas visceral stimulation induced a distinct profile of activity in frontoparietal and default mode networks.
Such a data-driven pattern recognition approach has the potential to identify a brain-based biomarker of a clinical condition, which could advance brain imaging studies from a research tool to investigate the mechanism of disease to a useful tool in clinical practice to diagnose a disease or to predict its evolution and/or treatment outcome (Davis et al. 2017). Recent studies have started to take this approach towards understanding brain contributions to chronic pain and multiple brain disorders, with promising results (Woo et al. 2017). For example, Labus et al. (2015) aimed to develop a structural MRI-based biomarker for IBS in which a classification model identified the regions that make the most important contribution to distinguish IBS from healthy controls. It has been pointed out that the accuracy level may not be high enough for useful diagnosis of IBS, and the data set was obtained from only one laboratory, thereby making its generalization potential unclear. However, this study is an important starting point for biomarker discovery and validation for IBS and FSS in general (Woo and Wager 2015). López-Solà et al. (2017) identified a brain fibromyalgia signature based on fMRI responses to pressure pain and no-painful multisensory stimulation. The brain patterns discriminate fibromyalgia from healthy controls with high sensitivity and specificity and correlated with clinical symptoms in fibromyalgia patients, although this is a single cohort study and needs replication in an independent sample (López-Solà et al. 2017). Drysdale et al. (2017) used resting state fMRI data in a large multisite sample and developed a diagnostic classifier based on distinct patterns of dysfunctional connectivity in limbic and frontostriatal networks to subdivide patients with depression into four neurophysiological subtypes with high sensitivity and specificity and predict the treatment effect by repetitive transcranial magnetic stimulation (rTMS). Vachon-Presseau et al. developed a brain biomarker (subcortical limbic volume asymmetry, sensorimotor cortical thickness, and functional coupling of prefrontal regions, anterior cingulate, and periaqueductal gray) and psychological disposition (interoceptive awareness and openness) that could predict responsiveness to placebo in patients with chronic back pain (Vachon-Presseau et al. 2018). The same group proposed a prospective, longitudinal study to develop a brain-based biomarker predicting the translation from subacute back pain to chronic pain based on the hypothesis that the circuit of mPFC and nucleus accumbens (NAc) influences pain chronification (Reckziegel et al. 2019).
A brain-based biomarker approach could be used for FSS. This requires a paradigm shift from searching brain activity during some tasks or conditions towards deciphering brain activity. This represents a great step to a new clinical application of neuroimaging and it has just begun. At this point, it is still uncertain whether it will be useful in clinical practice. A good brain biomarker, in general, should have a high enough sensitivity and specificity for diagnostic classification or prediction and it should be generalizable across diverse groups of individuals and be tested prospectively in new patients (Wager 2015; Davis et al. 2017; Reddan and Wager 2018). We are proceeding with collaborative, international multi-site studies and using diverse noxious stimulation data. A useful brain marker may not only be able to distinguish a disease from a healthy condition, but it should catch useful clinical features to treat or to prevent chronification of the disease. For extracting such targeted neuroanatomical or neurofunctional features, empirical brain imaging studies investigating mechanisms of diseases may be helpful. In the case of FSS including IBS, many studies including ours, provide not only a brain-based concept but also a systemic framework to comprehend the brain-body interactions. Recently, Petzschner et al. have proposed a comprehensive framework of this brain-body interaction in health and disease from a new viewpoint of computational psychosomatics (Petzschner et al. 2017). The proposal is still conceptual, but the comprehensive model may be suitable for FSS. For example, maladaptive beliefs such as fear or exaggerated threat could cause persistent sympathetic activation mediated via projections from mPFC/ACC and/or insula, considered as higher homeostatic regulation regions, on sympathetic effector regions including hypothalamus, amygdala, or PAG. They then induce somatic symptoms such as diarrhea. In contrast, chronic change of physical conditions could probably result in dissociation of physical sensation and ability to perceive it correctly, that then may decrease one’s belief of mastery over bodily states and increase the loss of control of the bodily state. This may lead further to an anxiety state and helplessness. Our previous studies have shown that the circuit from mPFC/ACC and insula to hypothalamus, amygdala, and PAG is associated with ANS activity which is also connected to the CRH system and this could be a possible target of a brain-based biomarker in the top-down regulation on stress system for FSS (Kano et al. 2017b). Similarly, body sensation which is not properly founded by the physiological body condition leading to symptoms formed by strong cognitive and emotional influence, might be another target for a brain-based biomarker in the bottom-up miscoding of FSS (Fig. 5) (Kano et al. 2020). Considering a clinical useful brain biomarker, if a data-driven approach focusing on these circuits can predict a therapeutic target of the FSS, e.g., strong influence of cognitive or emotional modification, dysfunction of the physiological stress response system, or at a relatively peripheral dysfunctional stage, it will be a great advance of clinical practice of FSS.

Brain activity patterns in the mPFC representing cognitive control, negative emotion, and pain.
a. Hierarchical structure of studies. Functional MRI data from 18 studies included 270 participants in a balanced, hierarchical structure of 6 cognitive control studies (two working memory (WM), two response selection (RS), and two response conflict (RC)), 6 negative emotion induction studies {two visual, two social (e.g., social rejection or perception of others in pain) and two auditory}, and 6 pain studies (two thermal, two visceral, and two mechanical).
b. Inset brain rendering represent the anatomical parcellation of medial frontal cortex.
c. Latent patterns of activity that generalize across studies and subdomains, but are specific for the domains of pain, cognitive control, and negative emotion.
Reproduced from (Kragel et al. 2018b).
dmPFC, dorsal medial frontal cortex; pACC, perigenual anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; sgACC, subgenual anterior cingulate cortex; aMCC, anterior midcingulate cortex; pMCC, posterior cingulate cortex.

Possible target for a brain biomarker of FSS.
(1) Top-down dysregulation from mPFC/ACC to amygdala, hypothalamus and PAG, which are effectors of the ANS connected with the CRH system, which regulate the peripheral organ function and may produce or maintain somatic symptoms. Hypervigilance may worsen the dysregulation. (2) A chronic change of physical condition may produce discordance between physiological perception in the (posterior) insula and the subjective feeling of the bodily condition in the PFC, which may cause further hypervigilance and maladaptive belief.
ACC, anterior cingulate cortex; Amy, amygdala; ANS, autonomic nervous system; CRH, corticotropin-releasing hormone; Hypo, hypothalamus; PAG, periaqueductal gray.
Chronic somatic symptoms without clear organic cause proven by medical examination, called FSS, have not been always managed well in clinical practice despite the high prevalence. The reason is that its pathophysiology is conceptualized as a dysfunction in brain-body interaction which does not originate from a single organ. Moreover, symptoms-based criteria can be adapted to patients with a heterogeneous pathological background, which can be peripheral oriented, brain oriented, and most often mixed. Brain imaging studies over the past 20 years have facilitated our understanding of the contribution of the brain to FSS etiopathogenesis. Our brain imaging studies in patients with IBS provided information on the mechanism of FSS: the influence of an unpredictable situation on brain processing of noxious visceral perception, dysfunction of brain regulation of the stress response system including the ANS and CRH related system, and the stronger activity in the visceral perception cortex with discordant subjective sensations associated with alexithymia. Recent advances in analysis of brain imaging data, such as machine learning begun the challenge to make a brain-based biomarker to diagnose or to predict clinical outcome. From our brain imaging data, the mPFC/ACC and insula projection to hypothalamus, amygdala, and PAG associated with the ANS and CRH related system as top-down regulation, and cognitive and emotional modulation or discrepancy between subjective sensation and physiological perception can be a possible candidate brain-based biomarker. There is still a huge gap between what brain imaging studies have provided so far and a real tool for clinical practice. Therefore, it is desirable that a brain-based biomarker will be developed from a variety of large data, to be tested in individual patients and to be optimized so that it can predict a target of clinical therapy for each patient.
The authors declare no conflict of interest.