2020 Volume 250 Issue 3 Pages 153-159
2020 Volume 250 Issue 3 Pages 153-159
Recently, biological disease-modifying antirheumatic drugs (bDMARDs) have revolutionized the treatment of rheumatoid arthritis (RA) and provided patients with a higher chance of achieving clinical remission. Among them, abatacept (ABT), which selectively inhibits T cell activation through blocking costimulation signal, has been reported efficacious in controlling disease activity. Previous studies have shown that ABT has a high retention rate of up to three years with tolerable adverse events; however, it remains unclear whether this is maintained in the longer term. Here we conducted a retrospective five-year follow-up study to explore prognostic factors concerning better retention. In total, 98 patients who were treated with ABT from May 2011 to July 2019 in Osaki Citizen Hospital were enrolled, including 73 female patients (74.5%). The Kaplan-Meier method was used to estimate the retention rate of ABT. The mean age of ABT initiation was 72.1 years. Concomitant methotrexate was prescribed for 39 patients, and ABT was used as the first-line bDMARD for 65 patients. Rheumatoid factor (RF) was positive in 79 patients. One-, three-, and five-year retention rates of ABT were 83.3%, 66.2%, and 62.7%, respectively. Approximately two-thirds of discontinuation resulted from an inadequate response. Multivariate logistic regression analysis revealed that positive RF was associated with better drug retention. Receiver operating characteristics analysis showed that patients with high RF (≥ 45 IU/mL) had better retention rate of ABT. In conclusion, ABT shows high retention rate among patients with positive RF. The present study may provide better insights when selecting bDMARDs.
Rheumatoid arthritis (RA) is a chronic inflammatory disease and causes joint destruction (Smolen et al. 2016). When patients are diagnosed, the current treatment guideline recommends the immediate initiation of methotrexate (MTX) because joint damage advances quickly, particularly in the first two years, which is known as the window of opportunity (Smolen et al. 2017).
The recent paradigm shift in the treatment of RA has introduced the use of biological disease-modifying antirheumatic drugs (bDMARDs) for patients who have an inadequate response to MTX (Smolen et al. 2017). These agents provide patients with a higher chance of achieving clinical remission. Abatacept (ABT) is one of the bDMARDs; it is also a fusion protein comprising the extracellular domain of human cytotoxic T-lymphocyte antigen 4 (CTLA4) and a fragment of the Fc domain of human IgG1 (Linsley et al. 1991). ABT selectively modulates T cell activation, thus reducing disease activity and inhibiting joint damage as efficacious as tumor necrosis factor alpha inhibitor (Genovese et al. 2005).
Previous studies have shown that ABT has a high retention rate of up to three years with tolerable adverse events (Ebina et al. 2019). However, it remains unclear whether this is maintained in the longer term. Moreover, there are few reports regarding predictive factors suitable for ABT treatment. We, therefore, conducted a retrospective five-year follow-up study to identify prognostic factors that related to better retention.
From May 2011 to July 2019, 102 patients who fulfilled the 2010 classification criteria of RA (Aletaha et al. 2010) were treated with ABT intravenously or subcutaneously. Among them, four patients died during the follow-up. ABT was effective for them; however, they died from cerebellar hemorrhage, cardiac failure, other malignancies or an unknown cause. The remaining 98 patients were enrolled in the subsequent analysis (Fig. 1). All patients were followed up to five years until July 2019. The study protocol was approved by the Ethics Committee of the Osaki Citizen Hospital (No. 20190822-25) and performed in accordance with the Declaration of Helsinki.

Patients enrolled in this study.
One hundred and two patients with rheumatoid arthritis (RA) were treated with abatacept from May 2011 to July 2019 in Osaki Citizen Hospital. Four patients died during the clinical course, and the remaining ninety-eight patients were enrolled in the subsequent analysis.
Medical records were retrospectively reviewed. We obtained data about the age at the time of ABT initiation, sex, concomitant use of MTX, rheumatoid factor (RF), and anti-citrullinated peptide antibody (ACPA). We also checked whether or not ABT was used as the first-line therapy of bDMARDs. The primary inadequate response was defined as no or little response after the ABT initiation, whereas the secondary inadequate response was defined as the loss of efficacy once after the patients were in remission.
Statistical analysisGraph Pad Prism 8 and EZR software wer used to perform the statistical analysis (Kanda 2013). The Fisher’s exact test was used for binary data, and the Mann-Whitney U-test was used for continuous data, as previously described (Tomiyama et al. 2016). The Kaplan-Meier method was used to estimate the retention rate of ABT, and the log-rank test was used to calculate p values (Ishizuka et al. 2016; Yoshida et al. 2016). Multivariate logistic regression analysis was used to identify factors that were associated with discontinuation of ABT. Receiver operating characteristics (ROC) analysis was performed to determine the most suitable cut-off value that maximized the are under the curve (AUC). P values less than 0.05 were considered statistically significant.
Among 102 patients treated with ABT, 4 patients in remission died. The remaining 98 patients were thus enrolled in this analysis (Table 1, Fig. 1). The mean age at the time of ABT initiation was 72.1 years, and the predominant patient population was female (73 patients, 74.5%). The mean disease duration was 76.2 months, the mean swollen joint counts (SJC) were 5.0, and the mean tender joint counts (TJC) were 4.9. RF was positive in 79 patients (80.6%), whereas positive ACPA was detected in 55 of the 70 tested patients (55/70, 78.6%). The mean serum C-reactive protein (CRP) level was 2.65 mg/dL. Concomitant MTX was prescribed for 39 patients (39.8%) and ABT was used as the first-line bDMARD for 65 patients (66.3%). The mean dose of prednisolone was 3.41 mg/day.
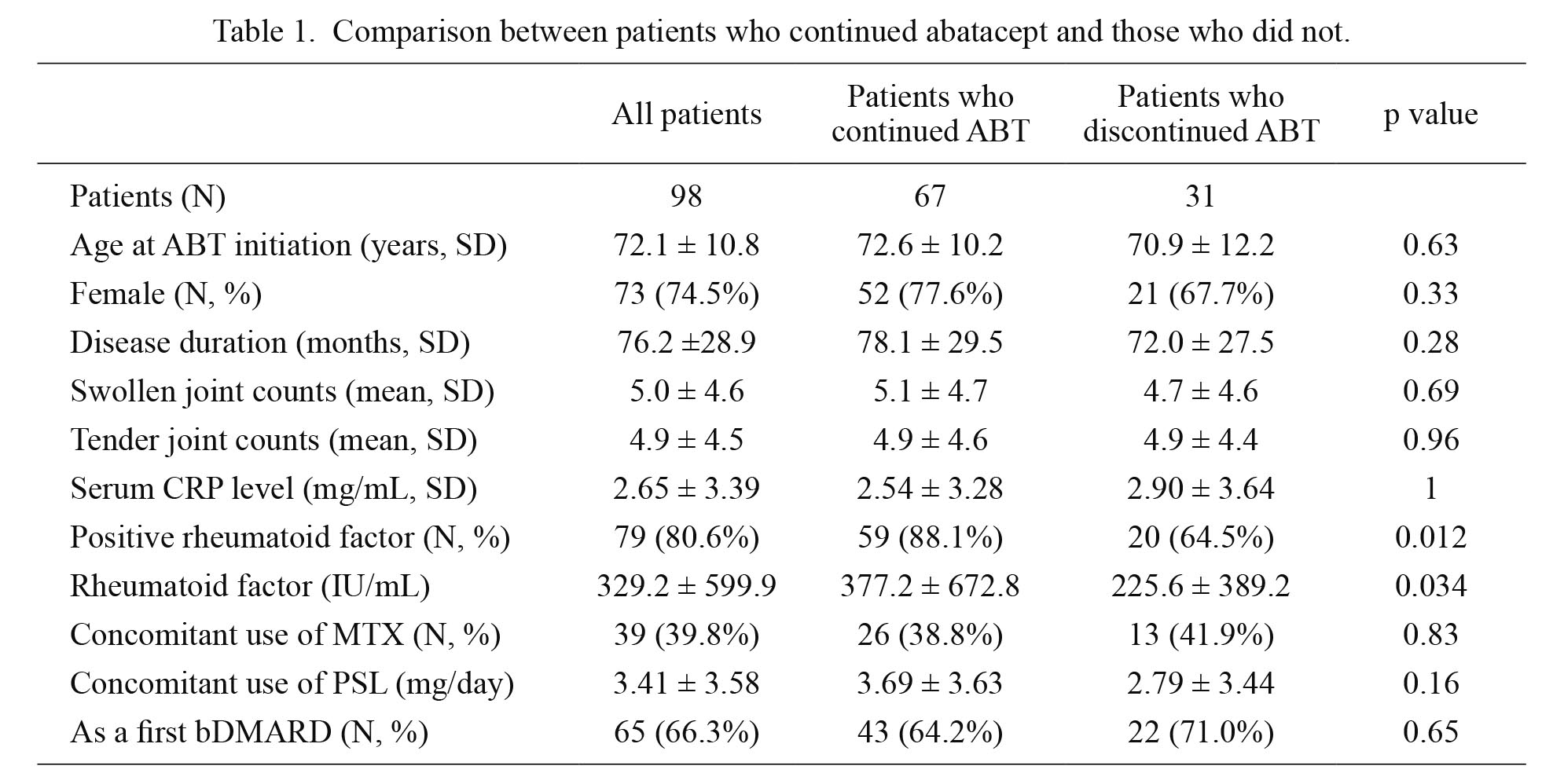
Comparison between patients who continued abatacept and those who did not.
ABT, abatacept; bDMARD, biological disease-modifying antirheumatic drugs; CRP, C-reactive protein; MTX, methotrexate; PSL, prednisolone.
One-, three-, and five-year retention rates of ABT in 98 patients were 83.3%, 66.2%, and 62.7%, respectively (Fig. 2A). Retention rate was particularly high after three years. Reasons for discontinuation included primary inadequate response (N = 11), secondary inadequate response (N = 10), infection (N = 3), remission (N = 1), malignancy (N = 1), and other reasons (N = 5) (Fig. 2B). Overall, approximately two-thirds of discontinuation resulted from inadequate response, and no new safety signals were detected.

Retention rate of abatacept and reasons for discontinuation of abatacept in patients with rheumatoid arthritis.
(A) The retention rate for abatacept was plotted by the Kaplan-Meier method up to five years. (B) Reasons for discontinuation of abatacept during the clinical course were listed.
Primary and secondary inadequate response accounted for two-thrids of discontinuation.
We plotted the retention curve based on the abovementioned clinical characteristics (Fig. 3). Among them, age at the time of ABT initiation, sex, concomitant use of MTX, ACPA, and whether ABT was used as the first-line bDMARDs did not affect the retention rate (Fig. 3A-E); however, RF was positively associated with better drug retention (p = 0.004, Fig. 3F). In patients with positive RF, one-, three-, and five-year retention rates of ABT were 87.2%, 73.5%, and 73.5%, respectively. On the other hand, the retention rate in patients with negative RF considerably declined within a year. Of the 6 RF-negative patients who discontinued ABT during the first year, 5 patients had primary inadequate response causing the discontinuation.

Retention rate of abatacept plotted according to different factors.
The retention rate for abatacept was determined according to (A) age at the time of drug initiation, (B) sex, (C) concomitant methotrexate (MTX) use, (D) first-line therapy as biological disease-modifying antirheumatic drug (bDMARD) or second-line or above bDMARD therapy, (E) anti-citrullinated protein antibody (ACPA), and (F) rheumatoid factor (RF). p values were calculated using the log-rank test.
We compared the clinical characteristics of the patients who had discontinued ABT and those who had not discontinued it during the follow-up period (Table 1). ACPA is not included in this table because not all patients were tested. Thirty-one patients who had discontinued ABT tested significantly less positive for RF (20/31, 64.5%) than the sixty-seven patients who had continued the therapy (59/67, 88.1%, p = 0.012). Titer of RF was also significantly higher in patients who had continued ABT than in those who had discontinued the therapy (377.2 and 225.6 IU/mL, respectively, p = 0.034). Other clinical characteristics were not different between the two groups.
Multivariate analysis for identifying factors associated with ABT discontinuationThen, we applied multivariate logistic regression analysis to identify prognostic factors for ABT discontinuation (Table 2). Age at ABT initiation, sex, disease duration, SJC, TJC, RF, serum CRP level, concomitant use of MTX, and whether ABT was used as the first-line bDMARDs were included as variables. Both univariate and multivariate analyses revealed that positive RF was negatively associated with ABT discontinuation, indicating that patients with positive RF had higher retention rate of ABT than those with negative RF (Table 2).
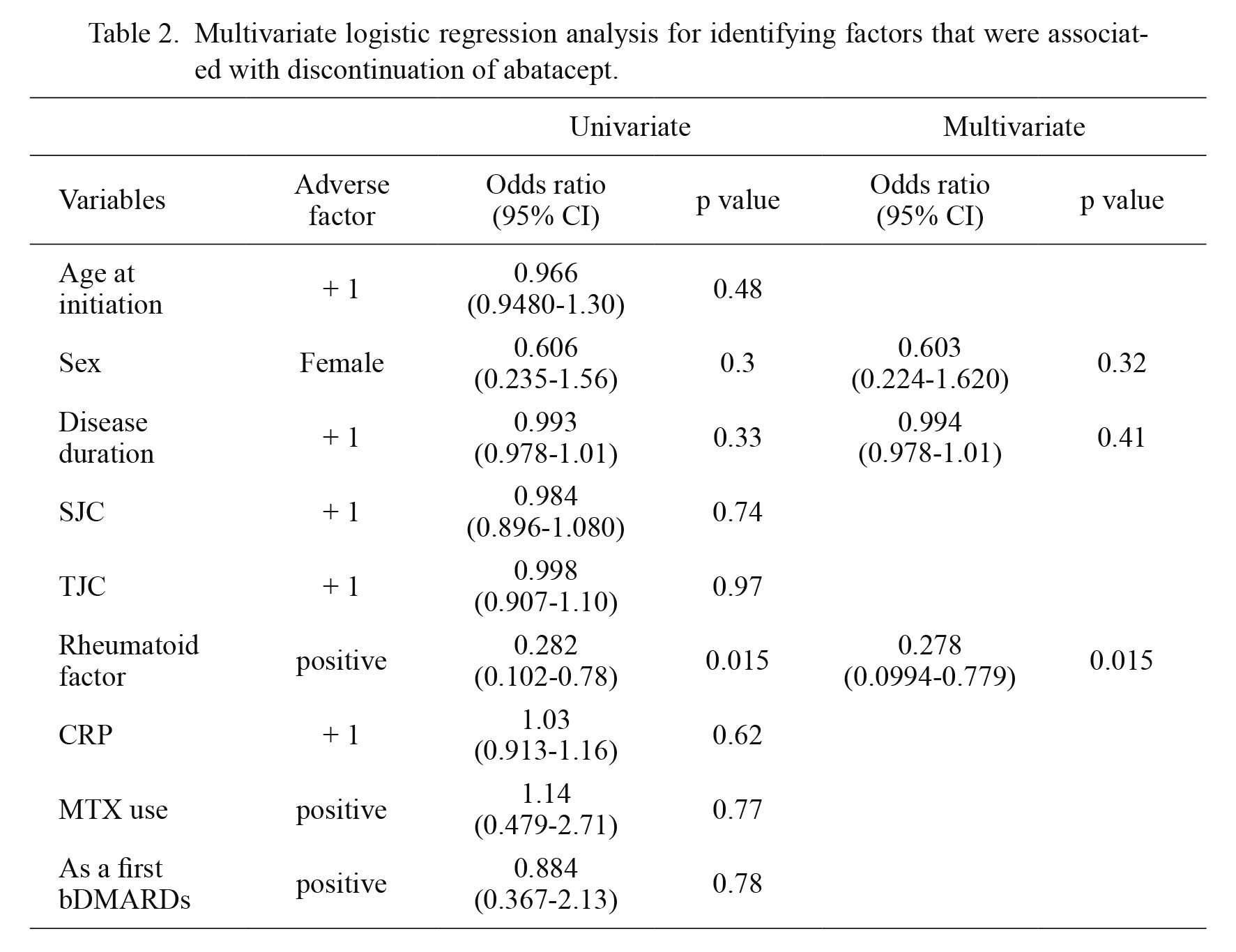
Multivariate logistic regression analysis for identifying factors that were associated with discontinuation of abatacept.
bDMARD, biological disease-modifying antirheumatic drugs; CI, confidence interval; CRP, C-reactive protein; MTX, methotrexate; PSL, prednisolone; SJC, swollen joint counts; TJC, tender joint counts.
Finally, we performed ROC analysis to determine the most optimal cut-off value of RF (Fig. 4). The cut-off value of RF was determined to be 45 IU/mL (Fig. 4A), and the drug retention rate was significantly higher in the group with RF ≥ 45 IU/mL than in the group with RF < 45 IU/mL (Fig. 4B).
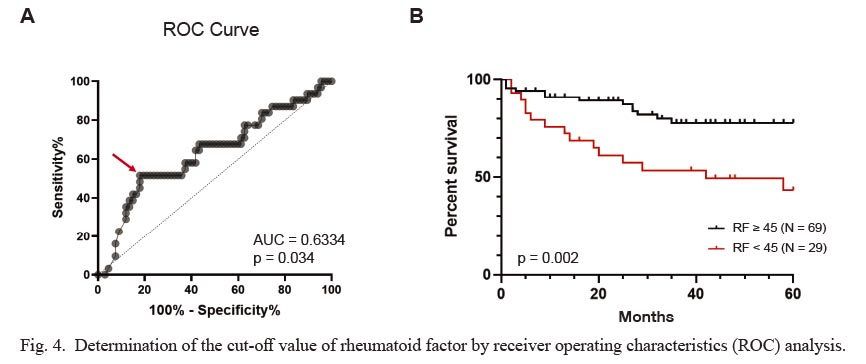
Determination of the cut-off value of rheumatoid factor by receiver operating characteristics (ROC) analysis.
(A) The cut-off value of RF was determined by ROC analysis. Red arrow (RF 45 IU/mL) shows the highest sensitivity and specificity. (B) The retention rate of abatacept was compared between the group with RF ≥ 45 IU/mL and the group with RF < 45 IU/mL. p value was calculated using the log-rank test.
In this study, we retrospectively analyzed the clinical characteristics of 98 patients with RA who were treated with ABT. The results demonstrated that positive RF is associated with better retention of ABT. This study provides real-world evidence for routine clinical practice. This study also followed the patients up to five years, which, for the first time, showed that the retention rate of ABT is particularly high after three years. In other words, after three years of ABT administration, few patients wished to stop it regardless of its economic burden.
Demographics of the patients enrolled in this study showed that the mean age at the time of ABT initiation in 98 patients with RA was 72.1 years. Given the fact that the mean age of 883 patients who visited our hospital from April 2018 to March 2019 was 64.9 years (Kondo et al. 2019), relatively elderly patients were treated with ABT in our hospital. This may come from post-marketing surveillance in Japan showing that the incidence of serious infections is not statistically increased in elderly patients (≥ 65 years) compared with younger patients (< 65 years), rendering ABT a preferred drug for elderly patients, particularly in Japan (Harigai et al. 2019). Only 3 out of 98 patients discontinued ABT because of infection. Therefore, this study reproduced the surveillance and reconfirmed that ABT is relatively safe for elderly patients with RA.
One-, three-, and five-year retention rates of ABT in our cohort were 83.3%, 66.2%, and 62.7%, respectively (Fig. 2A). Ebina et al. (2019) reported a three-year follow-up study with ABT showing that the lowest discontinuation rate out of seven bDMARDs in Japanese elderly patients with RA. Adjusted ABT retention rate at three years, excluding non-toxic reasons and remission, was 78.1% (Ebina et al. 2019). Kubo et al. (2016) also documented the retention rate of ABT at week 52 was 73.7% in 194 patients with RA. Retention rate in our hospital was similar to what has been reported in previous studies up to three years. When stuck between three and five years, only two patients chose to discontinue ABT because of secondary inadequate response and pneumonia (Fig. 2A). Hirabayashi et al. (2016) have shown that clinical and structural remission rates increase annually during a three-year administration of tocilizumab, which blocks the interleukin-6 receptor. Although tocilizumab and ABT have different modes of action, we speculate that patients who are under ABT therapy between three and five years have high clinical and structural remission rates, which lead to a high retention rate of ABT between three and five years.
With regard to predictive factors for better ABT persistence, recent studies have shown that patients with positive ACPA or high baseline concentration of ACPA have a better retention rate of ABT (Sokolove et al. 2016; Endo et al. 2020). In our hospital, unfortunately, not all patients were examined for ACPA because some patients were diagnosed before 2007 when ACPA testing became commercially available. Because ACPA is known to be the strongest predictive factor for joint damage (Koga et al. 2017), we should have measured ACPA in all patients before initiating ABT. Another study has indicated that ABT appears to be benefit patients with a high RF concentration (Kubo et al. 2018), which is consistent with our findings (Fig. 4). The retention rate in patients with negative RF considerably declined within a year due to primary inadequate response in our study. If ABT is initiated in patients with negative RF and if the effect is poor in a few months, it may be better to stop it rather than wait for the effect. The reason for the observed different patterns of response between RF-positive and RF-negative patients remains unknown; however, RF-positive RA may be more dependent on acquired immunity centered on T cell immunity than RF-negative RA, which is the target of ABT.
Limitations of this study included the fact that it was done retrospectively, ACPA was not tested in all patients, and we used the retention rate as a surrogate marker for drug efficacy. Despite these limitations, this study shows that ABT has a high retention rate in RF-positive patients with RA and may provide better insights when selecting bDMARDs for patients with RA.
We would like to thank Ms. Kaori Sasaki for the help of ethical approval, Ms. Saori Hanashima for the data collection, and Enago (https://www.enago.jp) for the language editing.
S.O. and R.W. conceived the study design. S.O. collected the data. R.W. analyzed the data, wrote the manuscript. H.H. and H.F. finalized and approved the manuscript.
All authors (S.O., R.W., H.H., H.F.) have received speaker honoraria from Bristol-Myers Squibb outside of this work. All authors declared no conflict of interest.