2020 Volume 250 Issue 4 Pages 263-270
2020 Volume 250 Issue 4 Pages 263-270
Lung cancer is the leading cause of cancer-related death, and adenocarcinoma is the most common histological type of lung cancer. Syntaxin-binding protein 1 (STXBP1) is essential for exocytosis of secretory vesicles. Since exocytosis is the basic cellular process of cells, we investigated STXBP1 expression and clinical significance in lung adenocarcinoma. We performed quantitative real-time polymerase chain reaction in 20 pairs of lung adenocarcinoma and paired normal tissues, and demonstrated that the relative expression levels of STXBP1 mRNA in lung adenocarcinoma was significantly higher than those in normal lung tissues. We then carried out immunohistochemistry (IHC) to determine the expression profile of STXBP1 in 276 lung adenocarcinoma specimens, and categorized patients into subgroups with low or high STXBP1 expression, based on the IHC score. Moreover, STXBP1 expression phenotypes were categorized as membrane, cytoplasm, and mixed expression (both membrane and cytoplasm) expression. High STXBP1 protein accounted for 58.0% of all the 276 cases (160/276), and membrane, cytoplasm or mixed STXBP1 accounted for 28.75%, 25.63% and 45.63% in the 160 cases of high STXBP1 expression. The clinical significances of these phenotypes were evaluated by analyzing their correlation with clinicopathological factors, as well as their prognostic values. Consequently, the whole STXBP1 expression or membranal STXBP1 expression were correlated with poor prognosis and were independent prognostic factors of lung adenocarcinoma. The whole and membranal STXBP1 expression are independent prognostic factors of lung adenocarcinoma. STXBP1 detection is capable to help screen patients who may have poor prognosis and strengthen the adjuvant therapy more precisely.
Lung cancer is the leading cause of cancer-related death globally (DeSantis et al. 2019). Since the end of the 20th century, the morbidity and mortality of lung cancer have been increasing rapidly, especially in developing countries like China and India because of the air pollution. The major histological types of lung cancer includes adenocarcinoma, squamous-cell carcinoma, small-cell carcinoma, large-cell neuroendocrine carcinoma, and pulmonary carcinoid tumors (Swanton and Govindan 2016). Among them, adenocarcinoma is the most common histotype, accounting for about 40% of all types of lung cancer (Swanton and Govindan 2016). The treatment to patients with lung cancer, especially in an advanced stage, had great improvements because of the application of many targeted drugs. However, though great efforts and significant progressions have been made, the overall 5-year survival rates for patients with lung cancer are still very low, ranging about 18.1% (Skoulidis and Heymach 2019). More targeted therapies and treatment options are mainly based on the discovery of new biomarkers. In this era of precise treatment, more biomarkers in lung cancer, especially adenocarcinoma, should be identified to develop more targeted drugs and improve the outcome.
Syntaxin-binding protein (STXBP) family, also known as Munc18 family, is consisted STXBP1-3. STXBP is a component of the Sec/Munc-proteins, and essential for the assembly and disassembly of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex (Smyth et al. 2010; Rizo and Xu 2015). The STXBP1 is widely involved in the process of exocytosis, including vesicle fusion, priming, docking and membrane fusion (Gulyas-Kovacs et al. 2007), by interacting with GTP-binding proteins (Korteweg et al. 2005). Although exocytosis is a common behavior of all cells including normal and tumor cells (Wu et al. 2014), the expression and functions of STXBP1 in tumor progression were rarely studied. Only several sporadic studies hinted the possible role of STXBP1 in tumorigenesis. For example, transcriptome sequencing revealed that STXBP1 was suggested to be specifically deregulated in FGFR3-non-mutated muscle-invasive bladder cancer (Ho et al. 2012). Since lung cancer is the most common tumor type worldwide and leads to the most cancer-related deaths, we investigated the expression of STXBP1 in lung adenocarcinoma and evaluated the clinical significance of its expression.
In this study, we carried out quantitative real-time polymerase chain reaction (qRT-PCR) in 20 pairs of lung adenocarcinomas and paired normal tissues to compare the expression and then performed immunohistochemistry (IHC) to describe the expression and location of STXBP1 in 276 specimens of lung adenocarcinoma. Moreover, we classified STXBP1 expression phenotypes as membrane, cytoplasm, and mixed expression (both membrane and cytoplasm) and evaluated the clinical significance of these phenotypes.
Our study collected a consecutive cohort consisting of 594 patients who underwent radical resection of lung adenocarcinoma in YIDU Central Hospital and Shandong Cancer Hospital from 2012 to 2017. A total of 276 patients were selected into the final cohort if there were available tissue samples for IHC and systemic follow-ups. The final cohort comprised of 124 female patients and 152 male patients, with an average follow-up as 41.9 months. The mean age of patients was 61.8 years old. All specimens were obtained with the written consent of patients, and our study was approved by Ethics Committee of YIDU Central Hospital and the Ethics aboard of Shandong Cancer Hospital.
RNA extraction and qRT-PCRA prospective cohort including 20 patients with lung adenocarcinoma was established since 2019, and the fresh tumor tissues and adjacent normal lung tissues were got from surgery without interfering the pathological diagnosis. The consecutive 20 patients concluded 14 male patients and 6 female patients (Table 1). The diagnoses were confirmed by routine pathology. For qRT-PCR, total RNAs were extracted with Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA), and then used for cDNA genesis with RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). SYBR Green method was used for the real time PCR with StepOnePlus real-time PCR system (Applied Biosystems, Waltham, MA, USA). The results were standardized with 2−ΔΔCt method and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) level was set as baseline. The primer sequence of STXBP1 was: forward: 5′-TGGAAGGTGCTGGTGGTGGA-3′, reverse: 5′- GCAGTCGGCGGGTCCTTAA-3′.

Basic information of the perspective cohort of the 20 patients.
*Fisher test.
The expression of STXBP1 was evaluated with IHC by a previously reported streptavidin peroxidase complex method (Sun et al. 2019). Formalin-fixed and paraffin-embedded specimens were first deparaffinized and rehydrated with ethanol and xylene. After that, the slides were incubated in 3% H2O2 for inactivation of endogenous peroxidase, and in 0.01M citrate buffer (pH = 6.0) to get optimal antigen retrieval. 5% bovine serum albumin in phosphate buffer saline (PBS) was used to eliminate the unspecific antigen binding. After rinsed with PBS, specimens were treated with primary antibody of STXBP1 (1:500, Synaptic Systems, Goettingen, Germany) in 4℃ overnight, and then in the corresponding secondary antibody (Beyotime Biotechnology, Beijing, China) and peroxidase complex reagent for 2 hours in room temperature. 3,3’-diaminobenzidine solution (Beyotime Biotechnology) was finally applied to visualize the antigen.
IHC score quantificationThe IHC results and intracellular location of STXBP1 were semi-quantified with IHC score by two independent pathologists. Two distinct parts comprised the IHC score, which were the score of staining intensity and the score of positive cell percentage. The staining intensity score was: score 0 for negative staining, 1 for weak staining, 2 for moderate staining, and 3 for strong staining. The positive cell percentage score was: 0 for less than 10% positive cells, 1 for 10%-30% positive cells, 2 for 30%-50% positive cells, and 3 for > 50% positive cells. Final IHC score was the multiplication of both score, which ranged from 0 to 9. The cut-off of IHC score was set by the receiver operating characteristic curve according to a previous study (Xu et al. 2019). The total cohort was divided into subgroups by the cut-off, which was 3.5 in our study. The intracellular location of STXBP1 was categorized as membrane, cytoplasm and mixed (both membrane and cytoplasm) expression by the two independent pathologists, and cases without consensus were re-evaluated with a third pathologist.
Statistical analysisThe χ2 method was used to analyze the correlations between STXBP1 and clinicopathological factors. Overall survival (OS) curves were described with the Kaplan-Meier test and the statistical differences of OS were calculated with the log-rank test. The Cox-regression hazard model was applied to confirm the independent prognostic biomarkers. Difference between lung adenocarcinoma and normal lung tissues was analyzed by the Student t-test. P value less than 0.05 was considered as statistically significant. SPSS 22.0 software (IBM, Chicago, IL, USA) was used to analyze all data without special illustration.
As an important regulator of exocytosis, the relative expression levels of STXBP1 mRNA in lung adenocarcinomas and their paired lung tissues were measured by qRT-PCR. The relative expression levels of STXBP1 mRNA were significantly higher in lung cancer tissues (Fig. 1A), indicating its potential role in tumorigenesis of lung cancer. These 20 patients were further classified into groups with high and low STXBP1 mRNA with the average mRNA as the cut-off, which comprised 9 and 11 patients respectively. Moreover, the correlations between STXBP1 mRNA and the clinicopathological factors were analyzed with the Fisher test, but no significant relavant factors with STXBP1 was detected probably due to the small sample size (Table 1).
Considering that STXBP1 functions in the vesicle fusion with membrane by interacting with the SNARE complex, we suspected that the intracellular location of STXBP1 may be related with their functions in different cell phases. Thus, STXBP1 expression in lung adenocarcinoma cells were analyzed by the IHC to evaluate its expression and intracellular location. The cut-off of IHC score categorized the patients into low- and high-STXBP1 subgroups, comprising of 116 (42%) and 160 patients (58.0%) of the whole cohort (Table 2). Interestingly, we demonstrated that different cases had different subcellular localization of STXBP1. According to the location of STXBP1, patients were mainly classified into three subgroups: expression in membrane, cytoplasm, or both membrane/cytoplasm (mixed expression) (Fig. 1B, C). The percentage of membrane, cytoplasm, and mixed expression of STXBP1 were 16.7%, 14.9% and 26.4% of all cases, and accounted for 28.75%, 25.63% and 45.63% of high-STXBP1 cases, respectively. Compared with lung adenocarcinoma, STXBP1 showed substantially lower expression in tumor adjacent lung tissues. In the normal lung tissues, STXBP1 had weak staining, which was mostly observed in the cytoplasm (Fig. 1D). If the cut-off of tumor was applied to tumor-adjacent lung tissues, the STXBP1 high expression ratio was just 4.3% (12/276), which was much lower than in lung adenocarcinoma (58.0%).

Higher expression levels of STXBP1 in lung adenocarcinomas compared with the normal lung tissues.
A. qRT-PCR analysis of STXBP1 mRNA. The relative expression levels of STXBP1 mRNA were significantly higher in lung adenocarcinoma compared with tumor-adjacent tissues.
B. Representative IHC image of low expression of STXBP1.
C. Representative IHC image of high expression of STXBP1 in membrane (left panel), cytoplasm (middle panel) and both membrane and cytoplasm (right panel).
D. Representative IHC image of STXBP1 expression in normal lung tissue.
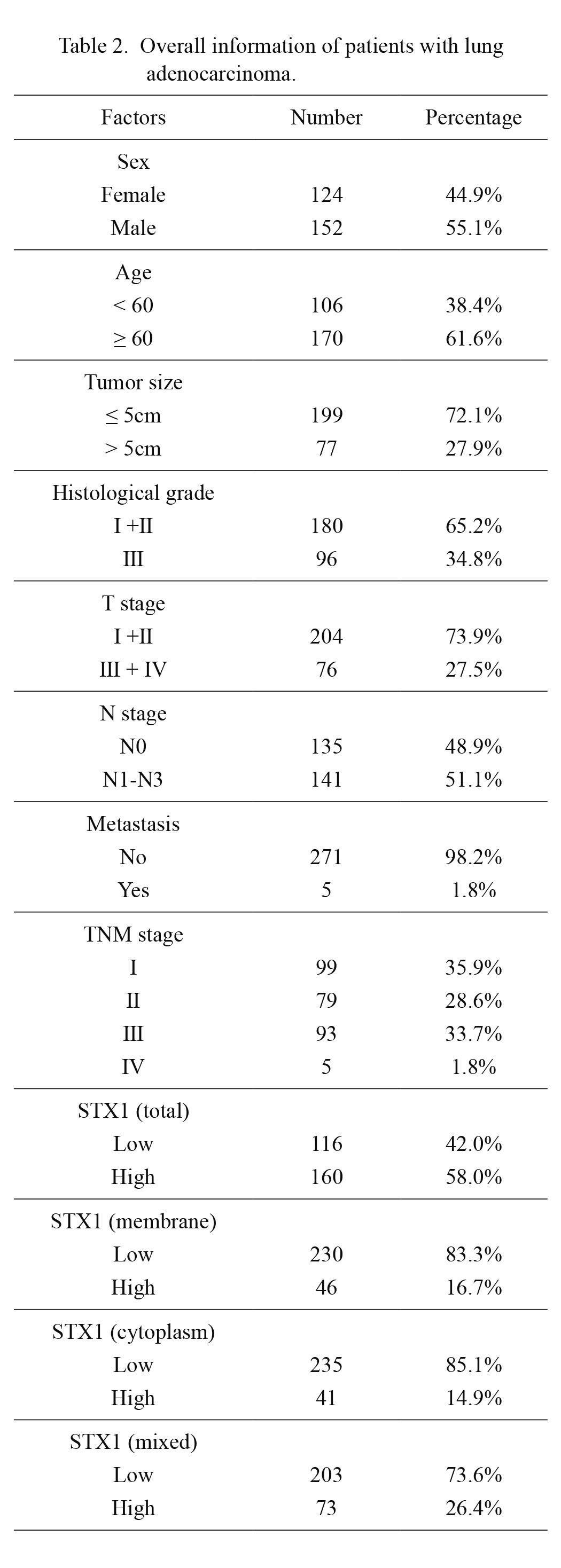
Overall information of patients with lung adenocarcinoma.
The correlations between STXBP1 expression and other clinical variables of lung adenocarcinoma were analyzed with the Chi-square test (Table 3). The clinical variables included the sex and age of patients, tumor size, histological grade, tumor T, N, M and TNM stage. Consequently, male patients tended to have high STXBP1 expression, with an insignificant statistical tendency (P = 0.053). Since STXBP1 showed different expression types in lung adenocarcinoma, we further stratified the patients based on the intracellular location of STXBP1. However, neither membrane, cytoplasm nor mixed expression of STXBP1 had prominent correlation with the clinical variables including sex, age, tumor size, histological grade, T stage, N stage, M stage and TNM stage.

Correlations between different intracellular location of STXBP1 and the clinical factors.
*Analyzed with the Chi-square test.
Although STXBP1-regulated cellular secretion is a basic function of all kinds of cells, the function of STXBP1 was rarely studied in tumor prognosis. Here we further estimated the correlation between STXBP1 expression and the OS rate. In our study, the 5-year OS rates of low and high expression of STXBP1 were 35.5% and 25.5%, respectively (Fig. 2A). The whole STXBP1 expression was significantly related to OS of lung adenocarcinoma (P = 0.006). Besides STXBP1, patients’ sex, tumor size, T stage, N stage and TNM stage were all prognostic factors in our study (Table 4). Moreover, we analyzed the prognostic value of different phenotypes of STXBP1 expression. Interestingly, STXBP1 expression on membrane was correlated with unfavorable prognosis (P = 0.008) (Fig. 2B), while its expression in cytoplasm had no obvious effect on OS (P = 0.989) (Fig. 2C). Patients with STXBP1 expression in both membrane and cytoplasm seemed to had poorer prognosis, but the statistical significance was not predominant enough (P = 0.274) (Fig. 2D).
Furthermore, we enrolled the prognostic factors into multi-variate analyses for the identification of independent prognostic biomarkers. Patients’ sex, tumor size, T stage and N stage were selected into the Cox-regression model, and the overall expression and membranal expression of STXBP1 were analyzed separately (Table 5). Male (P = 0.017), advanced T stag (P = 0.008) and positive lymphatic metastasis (P < 0.001) were all confirmed as independent prognostic factors of lung adenocarcinoma. Tumor size was not an independent risk, which may be attributed to its overlap with the T stage. In addition, the overall STXBP1 expression was also an unfavorable independent biomarker of lung adenocarcinoma (P = 0.048), with a risk ratio as high as 1.34. In addition, the prognostic value of membrane expression of STXBP1 was also analyzed. Membrane expression also could be predictive of the poor prognosis of patients, and the hazard ratio of membrane expression was higher than the overall expression (1.52 vs. 1.34).

The STXBP1 expression in membrane indicates unfavorable prognosis of lung adenocarcinoma.
In 276 lung adenocarcinomas, the survival curves of patients with whole STXBP1 expression (A) and different STXBP1 expression in membrane (B), cytoplasm (C), and both membrane and cytoplasm (D) were drawn with the Kaplan-Meier method. The statistical significance of different curves was analyzed with the log-rank test.

Total and membrane expression of STXBP1 was correlated with low 5-year OS.
*Analyzed with log-rank test.

Total and membrane expression of STXBP1 were independent prognostic factors of lung adenocarcinoma.
*Analyzed with the Cox-regression model.
Adenocarcinoma is the major histological subtype of the lung cancer. Lung adenocarcinoma and squamous carcinoma were sorted into the same type-the non-small-cell lung cancer (NSCLC), because of the similar treatment. However, adenocarcinoma and squamous carcinoma are distinct histotypes and had different biological features. In our study, we separated adenocarcinoma from NSCLC and investigated its prognostic biomarker with 276 cases. As a result, we demonstrated that high STXBP1 was a prognostic biomarker of lung adenocarcinoma, indicating that STXBP1 is a potential drug target and may help develop new therapies. Almost two-thirds of patients with non-small cell lung cancer have at least one oncogenic mutation, and half of them have a therapeutically targetable site (Rotow and Bivona 2017). Since the first target drug epidermal growth factor receptor (EGFR) inhibitor gefitinib was used to treat lung cancer (Ciardiello and Tortora 2001), a number of more personalized therapies are developed thanks to that new biotechnologies are applied to identify potential biomarkers for diagnosis or prognosis of lung cancer. The treatment of lung cancer has made great progression due to the identification of targetable mutations and molecular classification (Le and Gerber 2017). Patients with lung cancers processing the druggable oncogenic alterations are highly responsive to targeted therapies. However, drug resistance to target therapy is still a severe problem to patients in an advanced stage, making the exploration of new biomarkers as a continuous mission.
STXBP1 is essential in exocytosis, especially in synaptic vesicle release (Verhage et al. 2000), which is a fundamental biological events and involved in important processes like cell communication, development, migration, etc (Wu et al. 2014). Although the study of STXBP1 is rare regarding to tumor progression, the function of the main target of STXBP1, syntaxin, has been reported to be involved in the drug resistance, progression or prognosis in several types of cancers including prostate cancer, bladder cancer and papillary renal cell carcinoma (Peak et al. 2019, 2020; Raja et al. 2019). STXBP1 is previously reported to regulate the assemble of SNARE, which is the main machinery for secretion. However, the function of SNARE was reported in other physiological processes like the biogenesis of lysosome, autolysosome and exosome, and the dysregulation of these processes are widely reported in tumorigenesis and progression (Gu et al. 2019). The intracellular location of STXBP1 was an indication of its function. Generally, the assembly of STXBP1 around cell membrane was a sign of cell secretion, which was also a hallmark of tumors. That is why the expression of STXBP1 in normal lung tissues was weak and concentrated in cytoplasm instead of membrane. Here we demonstrated that STXBP1 expression, especially when its location was near cellular membrane, was a prognostic factor of lung adenocarcinoma, but we did not mention the molecular mechanisms and elucidate the role of STXBP1 in the progression of lung adenocarcinoma. STXBP1 participates in many ectopic processes of tumor such as autophagy and exosome secretion (Wei et al. 2017; Dolai et al. 2018).
The expression of STXBP1 was previously believed to be expressed predominantly in the brain. The mutations in STXBP1, including missense, frameshift, splice site, and nonsense mutations, and intragenic and whole gene deletions have been demonstrated to be associated with different diseases including epileptic encephalopathy, and intellectual disability, and autism, Dravet syndrome and West syndrome (Lanoue et al. 2019), most of which are neural disorders. Here we first described the expression of STXBP1 in lung adenocarcinoma and demonstrated that STXBP1 was associated with poor prognosis of lung adenocarcinoma. Our findings expanded the disease spectrum of STXBP1 and broadened the understanding of STXBP1 in oncology, providing new and intriguing results of STXBP1 as a potential biomarker and drug target in cancer therapy.
In summary, we demonstrate that STXBP1 is highly expressed in lung adenocarcinoma cells, accounting for 58.0% of all cases. STXBP1 expression in lung adenocarcinoma is significantly higher than that in normal lung tissues. The intracellular localization of STXBP1 is on membrane, cytoplasm, or both membrane and cytoplasm. The overall STXBP1 expression and membrane expression are independent prognostic factors of lung adenocarcinoma. STXBP1 detection can help screen patients who may have poor prognosis and strengthen the adjuvant therapy more precisely.
The study was funded by Program of Health Commission of Weifang (wfwsjk_2019_237).
The authors declare no conflict of interest.