2020 Volume 251 Issue 2 Pages 125-133
2020 Volume 251 Issue 2 Pages 125-133
Polymyalgia rheumatica (PMR) is an inflammatory disorder in the elderly and is characterized by pain in the shoulders and lower back. Previous studies from western countries have shown that relapse is frequent; however, there are only a few reports on the relapse rate in Japan. Here we examined the relapse rate, and sought to identify factors that predict recurrence in patients with PMR. Of 110 patients who fulfilled the Bird’s criteria for PMR between May 2011 and June 2019, 21 patients were excluded, and the remaining 89 patients were followed up until July 2019. Relapse was defined when clinical symptoms were exacerbated and serum C-reactive protein level increased. The relapse-free survival curves were plotted using the Kaplan-Meier method, and log-rank test was used for statistical analysis. The mean age of the 89 patients (50 males and 39 females) was 71.8 years. The mean dose of initial prednisolone (PSL) was 11.8 mg/day. The 1-, 3-, and 5-year relapse-free survival rates were 81.6%, 58.0%, and 52.3% (N = 59, 21, and 7), respectively. In patients who experienced recurrence, the 1- and 3-year second relapse-free survival rates were 58.3% and 27.3% (N = 18 and 3), respectively. Immunosuppressants, such as methotrexate and tacrolimus, were added to PSL in 19 of 30 patients who experienced relapse at the discretion of the attending physicians; however, none of the immunosuppressants worked for preventing second relapses and had steroid-sparing effects. These results indicate that effective immunosuppressants are required to suppress relapse in the treatment of PMR.
Polymyalgia rheumatica (PMR) is an inflammatory disorder exclusively affecting elderly patients over 50 years of age (Gonzalez-Gay et al. 2017). It is characterized by symmetric neck, peri-scapular, and lumbar pain and stiffness that lasts for more than a month. Patients often display systemic symptoms such as fever, general malaise, anorexia, weight loss, and depression (Buttgereit et al. 2016). In addition, laboratory findings show an increase in acute phase reactants such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Examinations using ultrasound and magnetic resonance imaging reveal synovitis and bursitis of the shoulder and hip in almost all patients (Gonzalez-Gay et al. 2017). Most patients respond to a low dose of oral glucocorticoids, with a range of 12.5-25 mg/day of prednisolone (PSL) equivalent (Dejaco et al. 2015).
PMR is closely related to giant cell arteritis (GCA), which is an autoimmune inflammatory vasculopathy categorized as large vessel vasculitis (Weyand and Goronzy 2014; Watanabe et al. 2017). Previous reports from Europe and the United States (US) have shown that 40%-50% of GCA cases are associated with PMR, whereas 15%-20% of patients with PMR go on to develop GCA (Brooks and McGee 1997; Gonzalez-Gay et al. 2009). Studies using genomic sequencing have revealed that PMR and GCA share a human leukocyte antigen (HLA) polymorphism, leading to the assumption that they have a shared etiological mechanism (Weyand et al. 1994). On the other hand, in Asia, Takayasu’s arteritis is common among large vessel vasculitis, and the prevalence of GCA is extremely low (Weyand and Goronzy 2014; Yoshida et al. 2016). However, PMR is a common disease that is frequently encountered in daily practice in Japan. Therefore, the complication rate of PMR and GCA in Japan may differ from that in Europe and the US.
Bird’s criteria have long been used for the diagnosis of PMR (Bird et al. 1979); however, the European League Against Rheumatism (EULAR) issued new criteria in 2012 (Dasgupta et al. 2012). In both criteria, excluding other diagnoses, particularly elderly-onset rheumatoid arthritis (RA), is critical. Generally, rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (ACPA) are negative in patients with PMR, but positive results cannot rule out the diagnosis of PMR (Dasgupta et al. 2012). If treated with PSL alone, joint destruction progresses in patients with RA (Smolen et al. 2016); therefore, despite their similarities, differentiation between the two is essential (Caporali et al. 2001).
The high rate of recurrence is a significant problem in the treatment of PMR (Gonzalez-Gay et al. 2017). Remission can be achieved with small amounts of PSL; however, recurrences often occur during tapering. Moreover, few studies have been reported on the relapse rate of patients with PMR in Japan. In this study, we examined the relapse rate of patients with PMR in our hospital, and sought to identify factors that predict recurrence. Furthermore, we investigated whether currently available immunosuppressants suppressed second relapse after first relapse.
Patients who visited our department from May 2011 to June 2019 and fulfilled Bird’s criteria for classifying PMR (Bird et al. 1979) were recruited in this study. Bird’s criteria include seven components: (1) bilateral shoulder pain or stiffness, (2) onset of illness < 2 weeks’ duration, (3) initial ESR greater than 40 mm/h, (4) duration of morning stiffness exceeding 1 hour, (5) age ≥ 65 years, (6) depression and/or weight loss, and (7) bilateral tenderness in the upper arms. If any three or more of these criteria are fulfilled, the patient is diagnosed with probable PMR (Bird et al. 1979). The follow-up period was defined as the time from diagnosis to either the date of death or the latest visit to our hospital. The follow-up of the patients was conducted until July 2019. The study protocol was approved by the Ethics Committee of the Osaki Citizen Hospital (No. 20190822-24) and was performed in accordance with the Declaration of Helsinki.
Clinical evaluationWe retrospectively reviewed the medical records and obtained data regarding age at onset, sex, concomitant diagnosis of GCA, history of malignancies, and medications including PSL and immunosuppressants. Laboratory results, such as white blood cell (WBC) counts, hemoglobin level, platelet counts, and CRP levels were also obtained.
Diagnosis of GCADiagnosis of GCA was based on the American College of Rheumatology (ACR) 1990 criteria for GCA (Hunder et al. 1990). The criteria include five components: (1) age at disease onset ≥ 50 years, (2) new headache, (3) temporal artery abnormality, (4) elevated ESR, (5) abnormal artery biopsy. If at least three of these six criteria are present, the patient is diagnosed with GCA (Hunder et al. 1990).
TreatmentAfter the diagnosis of PMR, PSL was initiated at 10 to 20 mg/day. The initial dose of PSL was maintained for 2-4 weeks, and tapered to 10 mg/day within 8 weeks in most cases. Then, daily oral PSL was tapered by 1 mg every 4-8 weeks. Below 5 mg/day, oral PSL was tapered more slowly. Immunosuppressants were administered before relapse in some cases, but were added after recurrence in many cases. The choice of immunosuppressant, including methotrexate (MTX), tacrolimus, azathioprine, salazosulfapyridine (SASP), and mizoribine was at the discretion of the attending physicians.
Definition of relapseRelapse was defined when clinical symptoms derived from PMR, such as shoulder and lumbar pain, worsened and serum CRP levels were elevated. Relapse was mostly, but not always, associated with the administration of an increased dose of PSL. Simply initiating immunosuppressive agents was not regarded as relapse.
Statistical analysisStatistical analysis was performed using GraphPad Prism 8 (San Diego, California, USA) and EZR software (Kanda 2013). Fisher’s exact test was used for binary data, and Mann-Whitney U-test was used for continuous data (Tomiyama et al. 2016). The survival curves and the relapse-free survival curves were plotted for a maximum of 5 years in each patient using the Kaplan-Meier method, and the log-rank test was used to compare the survival rates between patient groups, as previously described (Ishizuka et al. 2016; Yoshida et al. 2016). The relapse-free survival curve was drawn from the timing of diagnosis, whereas the second-relapse free survival curve was drawn after the first relapse. In relapse-free survival curves, patients who relapsed were censored and excluded from subsequent follow-up. P values less than 0.05 were considered significant. The Benjamini-Hochberg procedure was applied for multiple testing with the false-discovery rate of 0.05, as previously described (Zhang et al. 2017).
Of the 110 patients who met the Bird’s criteria for PMR between May 2011 and June 2019, 18 patients were finally diagnosed with RA (Aletaha et al. 2010), and 3 patients were excluded because of other diagnoses (psoriatic arthritis, mixed connective tissue disease, and vasculitis). The remaining 89 patients, including 50 males and 39 females, were enrolled in this study (Fig. 1). The mean age of the patients at the time of diagnosis was 71.8 years, and the complication of GCA was observed in 2 patients (2.2%) according to the ACR 1990 criteria (Hunder et al. 1990). History of malignancies were found in 18 patients (20.2%), which included prostate cancer (7 patients), gastric cancer (4 patients), acute myeloid leukemia (2 patients), breast, lung, renal, bladder, and skin cancer (1 patient each). PMR was diagnosed after the diagnosis of malignancies in 11 patients, whereas malignancies were detected during the follow-up of PMR in 7 patients. None of the patients were thought to have developed PMR-like symptoms as a result of paraneoplastic syndromes because clinical symptoms were not resolved by cancer therapy. The mean WBC counts, hemoglobin levels, platelet counts, and CRP levels at disease onset were 9,850/µL, 12.0 g/dL, 30.5×104/µL, and 8.09 mg/dL, respectively. The mean dose of initial PSL was 11.8 mg/day.
Patients enrolled in the study.
In total, 110 patients who visited our hospital from May 2011 to June 2019 fulfilled Bird’s criteria for polymyalgia rheumatica (PMR). A total of 21 patients were excluded because their final diagnosis was rheumatoid arthritis (N = 18) and other diseases (N = 3). The remaining 89 patients who were diagnosed with PMR were enrolled in this study.
The survival and relapse-free survival curves are shown in Fig. 2. During the follow-up, three patients died from rupture of abdominal aortic aneurysm, exacerbation of acute myeloid leukemia, and cerebral infarction, and five patients were lost to follow-up as a result of changing hospital (Fig. 2A). Thus, the follow-up rate was 94.3% (84/89) and the overall 1-, 3-, and 5-year survival rates, including death and loss of follow-up, were 98.8%, 90.4%, and 82.5% (N = 88, 83, and 81), respectively.
On the other hand, the 1-, 3-, and 5-year relapse-free survival rates were 81.6%, 58.0%, and 52.3% (N = 59, 21, and 7), respectively (Fig. 2B). While steroid-free remission was achieved in 28 of the 89 patients (31.4%) after an average of 26.5 months, approximately half of the patients with PMR experienced recurrence of the disease during 5-year follow-up. In particular, there were many relapses in the first 3 years.

Survival rate and relapse-free survival rate estimated by the Kaplan-Meier method.
(A) The survival curve was plotted up to 5 years using the Kaplan-Meier method. During follow-up, three patients died, and five patients were lost to follow-up as a result of a change in hospital. The overall 1-, 3-, and 5-year survival rates, including death and loss of follow-up, were 98.8%, 90.4%, and 82.5% (N = 88, 83, and 81), respectively. The solid line represents survival alone, whereas the dashed line represents survival and loss of follow-up. (B) The relapse-free survival curve was plotted up to 5 years using the Kaplan-Meier method. The 1-, 3-, and 5-year relapse-free survival rates were 81.6%, 58.0%, and 52.3% (N = 59, 21, and 7), respectively. Patients who relapsed were censored and excluded from the subsequent follow-up.
We compared the clinical characteristics between patients who experienced relapse during the follow-up and those who did not. As shown in Table 1, none of the clinical parameters obtained in this study, such as concomitant GCA, history of malignancies, laboratory findings, and initial dose of PSL, were distinguishable between the groups. The dose of PSL at 1 year was 6.1 mg/day in patients with relapse and 3.4 mg/day in patients without relapse, respectively (P = 0.0002). In addition, the cumulative amount of PSL for first 1 year was 2.9 g and 2.1 g, respectively (P = 0.01). Therefore, we could not conclude that a rapid reduction of oral PSL was associated with the recurrence in this study. Regarding age at onset, the mean age in patients who relapsed was 71.4 years, whereas that in patients who did not relapse was 72.0 years (Table 1). However, the median age in the former group was 70 years, whereas that in the latter group was 73.0 years.
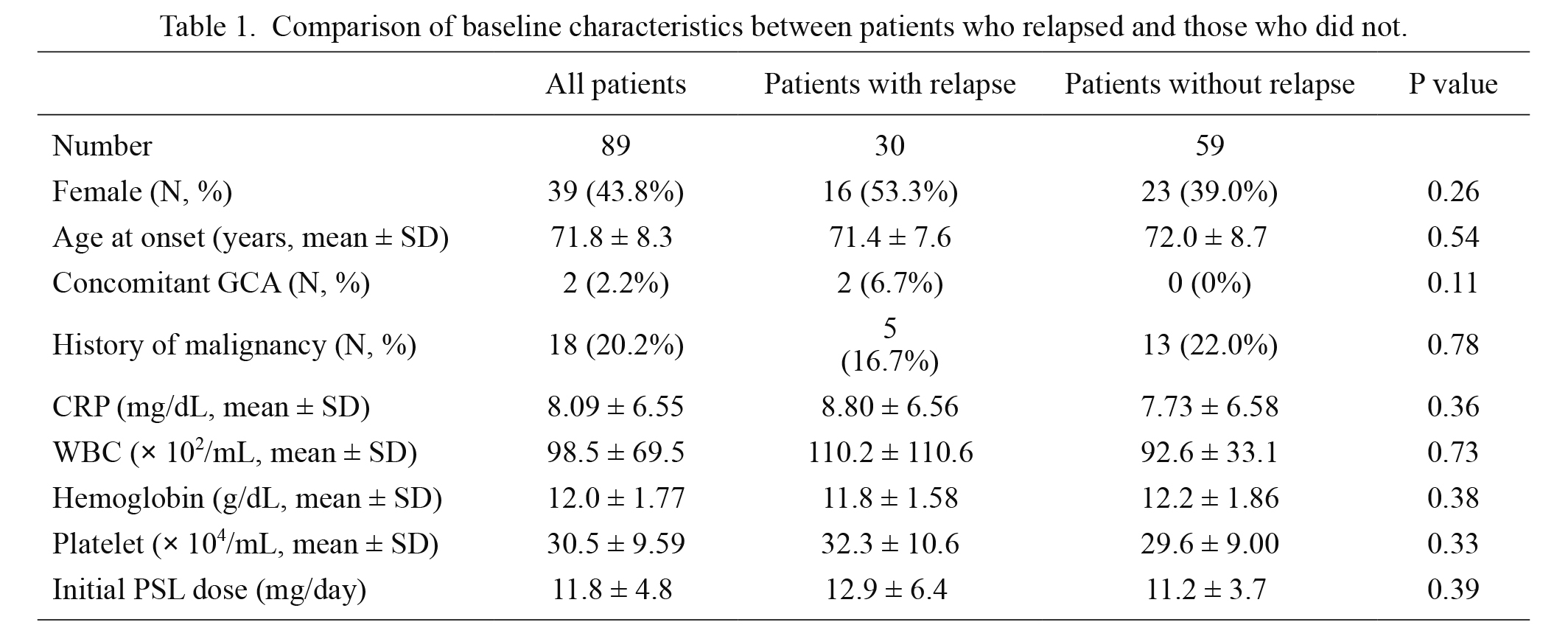
Comparison of baseline characteristics between patients who relapsed and those who did not.
CRP, C-reactive protein; GCA, giant cell arteritis; PSL, prednisolone; WBC, white blood cell.
Although there was no significant difference in clinical characteristics between the relapsed and non-relapsed groups (Table 1), we plotted the relapse-free survival curves based on different clinical parameters (Fig. 3). Sex, history of malignancies, and serum CRP level at disease onset did not significantly impact the relapse-free rate (Fig. 3A-C); however, patients who had relatively young-onset of PMR (≤ 72 years) had a higher relapse rate than those who had elderly-onset of PMR (> 72 years, P = 0.047) (Fig. 3D). We attempted to identify the optimal cut-off value using receiver operating curve analysis, but it did not yield values indicating significant sensitivity and specificity. Therefore, we applied the mean age for this analysis, in which the P value showed borderline significance. However, after multiple testing with the Benjamini-Hochberg procedure, the p value did not remain significant.
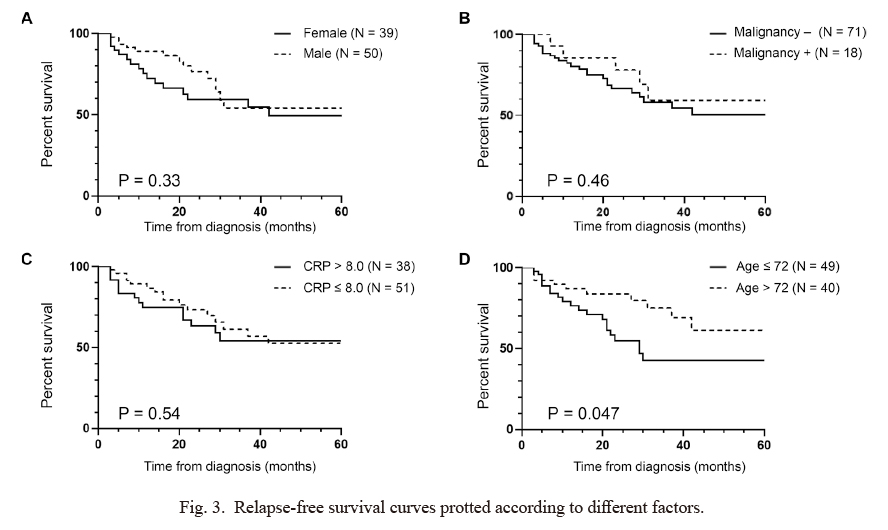
Relapse-free survival curves protted according to different factors.
The relapse-free survival curve depicted in Fig. 2B was re-plotted based on various factors; (A) sex, (B) history of malignancy, (C) C-reactive protein (CRP) level, and (D) age at onset using the Kaplan-Meier method. P values were determined using log-rank test. After multiple testing with the Benjamini-Hochberg procedure, all P values did not remain significant.
Since we observed a weak correlation between age at disease onset and relapse (Fig. 3D), we compared the clinical characteristics of patients with relatively young-onset (≤ 72 years) and those with elderly-onset (> 72 years). The baseline clinical characteristics between the two groups were indistinguishable other than age of onset (Table 2).

Comparison of baseline characteristics between patients with relatively young-onset (age ≤ 72) and those with elderly-onset (age > 72).
CRP, C-reactive protein; GCA, giant cell arteritis; PSL, prednisolone; WBC, white blood cell.
Finally, we examined how frequently patients who had first relapse had a second relapse (Fig. 4). We plotted the second relapse-free survival curve after the first relapse (Fig. 4A). The incidence of second relapse was extremely high; the 1- and 3-year second relapse-free survival rates in such patients were 58.3% and 27.3% (N = 18 and 3), respectively, and the second relapse-free survival curve dropped within 4 years (Fig. 4A). After the first relapse, the decision of treatment was made by the attending physicians. Of the 30 patients who experienced first relapse, only the dosage of PSL was increased in 11 patients; in others, immunosuppressants, such as MTX, tacrolimus, SASP, azathioprine, and mizoribine were added to PSL (Fig. 4B). We plotted the curves based on the treatment strategies including PSL alone (N = 11), PSL plus MTX (N = 9), and PSL plus other immunosuppressants (N = 10). We found that none of them were successful in preventing second relapse (Fig. 4C). While the mean dose of PSL at first relapse was 5.0 mg/day, the mean dose of PSL at second relapse was 6.4 mg/day, which indicated that none of the immunosuppressants had steroid-sparing effects (Fig. 4D).
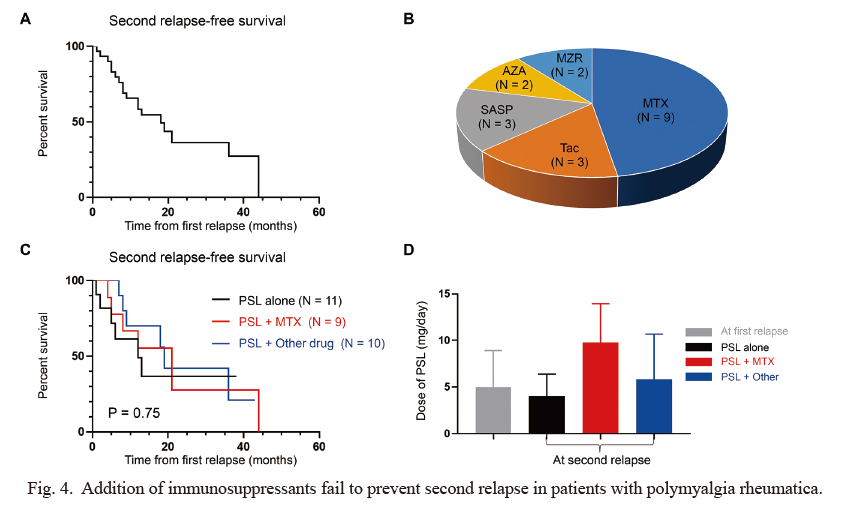
Addition of immunosuppressants fail to prevent second relapse in patients with polymyalgia rheumatica.
The second-relapse free survival curve after first relapse was plotted using the Kaplan-Meier method in 30 patients who experienced relapse. The 1- and 3-year second relapse-free survival rates in such patients were 58.3% and 27.3% (N = 18 and 3), respectively. Patients who relapsed were censored and excluded from the subsequent follow-up. (B) Diagram showing immunosuppressants added to prednisolone (PSL) after first relapse. (C) The second relapse-free survival curve depicted in (A) was re-plotted according to different treatment strategies. The black line represents PSL alone (N = 11), the red line represents PSL plus MTX (N = 9), and the blue line represents PSL plus other immunosuppressive agents (N = 10). (D) The dose of PSL at relapse. Gray bar represents the dose at first relapse. Black bar (PSL alone, N = 11), red bar (PSL plus MTX, N = 9), and blue bar (PSL plus other immunosuppressants, N = 10) represent the doses at second relapse based on the treatment strategies. Bar graphs show the mean ± SD. The P values were determined using log-rank test.
AZA, azathioprine; MTX, methotrexate; MZR, mizoribine; SASP, sarazosulfapyridine; Tac, tacrolims.
In this study, we retrospectively examined the recurrence rate of patients with PMR in Japan and found that the rate was as high as 50% over the course of a 5-year follow-up (Fig. 2B). Moreover, almost all patients who relapsed once experienced a second relapse within 4 years (Fig. 4A). The addition of currently available immunosuppressants to low-dose PSL were unable to prevent second relapse (Fig. 4C) and had no steroid-sparing effects (Fig. 4D). The most important message in this study is that effective immunosuppressants that can prevent PMR recurrence are required.
Previous studies from Europe and the US have reported that the frequency of relapse in the first year is 20%-55% (Kremers et al. 2005; Hernandez-Rodriguez et al. 2009; Gonzalez-Gay et al. 2017). Even if patients were treated with PSL plus MTX from the beginning of treatment, almost half of the patients had at least one relapse within 76 weeks (Caporali et al. 2004). The incidence of relapse in Japanese patients with PMR also ranged from 31% to 49% during the first 2 years if treated with PSL alone (Fukui et al. 2016; Hayashi et al. 2019). Although our study results showed that the relapse rate at 1 year was relatively low compared to these previous studies, the rate at 5 years was still high. A rapid reduction of oral glucocorticoids after remission induction is considered as a risk factor for recurrence (Behn et al. 1983). In our hospital, the dosage of PSL may have been gradually tapered because of the recognition that this disease has a high recurrence rate. However, as described in the result resection, we could not conclude that the reduction speed of oral PSL contributed to the recurrence in this study.
We found a weak correlation between age at disease onset and relapse (Fig. 3D). Patients who developed PMR at an age of 72 years or younger had a slightly higher relapse rate than those who developed PMR at an age over 72 years old; however, the baseline clinical characteristics between the two groups were indistinguishable (Table 2) and the multiple testing with the Benjamini-Hochberg procedure revealed that the P value was false-positive. In addition, we applied multivariate logistic regression analysis to identify prognostic factors for relapse; however, age at onset was not associated with relapse. On the other hand, Charpentier et al. (2018) reported that patients younger than 60 years old were more dependent on glucocorticoids and MTX was more necessary than in those older than 60 years (Charpentier et al. 2018). The reason why “young-onset” patients required more glucocorticoids than “elderly-onset” patients remains unclear; however, the prevalence of smokers was higher in the “young-onset” group than in the “elderly-onset” group, which might contribute to the difference (Charpentier et al. 2018). Further studies are needed to examine the correlation between age at disease onset and relapse.
Glucocorticoids remain the mainstay of treatment for PMR. As described above, a range of 12.5-25 mg/day of PSL is mostly effective as an initial therapy (Dejaco et al. 2015). In case of relapse, an increase of oral PSL to the pre-relapse dose, and tapering it to the dose at which the relapse occurred within 4-8 weeks is currently recommended (Dejaco et al. 2015). Regarding immunosuppressive agents, MTX is conditionally recommended in patients at a high risk for relapse, whereas anti-tumor necrosis factor (TNF) alpha inhibitor is discouraged (Dejaco et al. 2015). A randomized, double-blind, placebo-controlled trial showed that PSL plus MTX treatment enabled shorter treatment duration and glucocorticoid sparing than treatment with PSL alone (Caporali et al. 2004). In our study, MTX was the most widely used immunosuppressant (Fig. 4B). However, MTX has substantial side effects such as interstitial pneumonia and renal dysfunction. Therefore, administration of MTX is contraindicated in some patients. In such patients, non-MTX immunosuppressants, such as tacrolimus, SASP, azathioprine, and mizoribine were used off-label in combination with PSL, but their ability to suppress relapse was limited. In this study, all 9 patients who were added MTX after first relapse experienced second relapse (Fig. 4C) and MTX showed no steroid-sparing effects (Fig. 4D). However, the average dose of MTX added to PSL was 6 mg/week. Therefore, although we should be cautious about the dose of MTX because elderly patients often have potentially diminished renal function, the maximum dose of MTX should have been given when patients experienced recurrence.
Although the results of randomized controlled trials using anti-TNF alpha inhibitors were disappointing, there is growing evidence of the efficacy of tocilizumab, a humanized antibody to the interleukin-6 receptor, for the treatment of PMR (Izumi et al. 2015; Devauchelle-Pensec et al. 2016; Akiyama et al. 2020). Given the fact that PMR is closely related to GCA, and that the efficacy of tocilizumab for GCA has been proven (Stone et al. 2017), the effect of tocilizumab for PMR is to be expected. Currently, a multitude of new drugs are being tested as therapeutics for GCA (Watanabe et al. 2016; Dejaco et al. 2017; Weyand et al. 2019). For example, Janus kinase inhibitor seems promising in the mouse model of GCA (Zhang et al. 2018). It may be possible that these drugs are used as treatments for PMR, and we hope that effective therapeutic options for PMR will become available in the near future.
The prevalence of GCA in patients with PMR was lower than expected in our cohort. This may be because we made use of the ACR 1990 criteria for diagnosing GCA which emphasized headache symptoms. Recently, it has become clear that approximately half of patients with GCA have aortic lesions (Kermani et al. 2015; Watanabe et al. 2018). In our study, of the 87 patients who were not diagnosed with GCA, 4 patients had evidence of aortic involvement, but did not fulfill the ACR 1990 criteria. Therefore, in total, 6 out of 89 patients (6.7%) can be diagnosed as probable GCA. Still, it may be lower than the complication rate of PMR and GCA in Europe and the US. The 4 patients with aortic involvement in our study did not recur during the course; thus, the presence of aortic lesions was not associated with relapse.
There are several limitations of this study. First, this was a retrospective study in a single center, which potentially had a selection bias. Second, because we recruited patients from 2011, we applied the classic Bird’s criteria instead of the new 2012 classification criteria. Third, not all patients were examined for RF and ACPA; 7 of 79 tested patients had positive results for RF, whereas 1 of 75 examined patients was positive for ACPA. Of note, the one ACPA-positive patient (41.6 U/mL) was a 70-year-old female treated with low-dose PSL, which resulted in a good response without evidence of joint destruction. However, this patient should be closely monitored because ACPA is generally considered as a hallmark of RA and is also associated with joint destruction in RA (Koga et al. 2017).
In conclusion, despite these limitations, this study clearly showed that the recurrence rate of PMR is high in Japan, and that, while steroid-free remission can be achieved in some patients, others experience recurrence multiple times. Effective immunosuppressive agents are required in such patients.
We would like to thank Ms. Kaori Sasaki for her assistance with ethical approval, Ms. Saori Hanashima for the data collection, and Enago (https://www.enago.jp) for the language editing.
S.O. and R.W. conceived the study design. S.O. collected the data and wrote the manuscript. R.W. analyzed the data and wrote the manuscript. K.H., M.K., H.H. and H.F. participated in the study design and discussion. H.H. and H.F. finalized the manuscript. All authors reviewed and approved the manuscript.
The authors declare no conflict of interest.