Abstract
Graft-versus-host disease (GVHD) is a potentially life-threatening complication of allogeneic stem cell transplantation (Allo-SCT). Chronic GVHD, which typically presents more than 100 days after Allo-SCT, can resemble manifestations of autoimmune disease; however, there are only a few reports on the development of Crohn’s disease (CD) after Allo-SCT. Here, we report a case of steroid-refractory CD after umbilical cord blood transplantation (CBT), which was dramatically improved with administration of anti-tumor necrosis factor-alpha (anti-TNF-alpha) antibodies. A 21-year-old woman with refractory Hodgkin lymphoma underwent CBT and achieved complete remission. About 1 year after CBT, she complained of intermittent abdominal pain and bloody diarrhea, and colonoscopy revealed multiple longitudinal colonic ulcers with a cobblestone appearance; thus, based on the colonoscopy findings, she was diagnosed with CD. We considered a CD-like manifestation of gastrointestinal GVHD and initially administered steroids, but the therapeutic effect was poor. Then, we administered anti-TNF-alpha antibodies, infliximab, and then adalimumab, which resulted in rapid improvement of abdominal symptoms, with no recurrence despite discontinuation of this therapy. Anti-TNF-alpha antibodies are effective for CD after Allo-SCT, which can be considered as a subsequent complication of GVHD.
Introduction
Allogeneic stem cell transplantation (Allo-SCT) is a potentially curative option for hematological malignancies in relapsed or refractory settings. One of the major complications of Allo-SCT is graft-versus-host disease (GVHD), in which donor T-cell mediated inflammation induces a variety of symptoms including autoimmune disease-like manifestations (Tyndall and Dazzi 2008). Anti-tumor necrosis factor-alpha (anti-TNF-alpha) antibodies have shown effectiveness for steroid-refractory acute GVHD, especially in intestinal GVHD (Couriel et al. 2004). They are also established as a standard treatment for refractory inflammatory bowel disease (IBD), which is composed of Crohn’s disease (CD) and ulcerative colitis (UC). Although the pathogenesis has not been fully elucidated, IBD is induced by a dysregulated immune response in the intestinal tract. Several studies have shown the association of IBD with human leukocyte antigen (HLA) haplotypes (Fernando et al. 2008), implying that an alloimmune reaction will eventually lead to IBD in Allo-SCT recipients.
Here, we report a case of steroid-refractory CD after umbilical cord blood transplantation (CBT), which was dramatically improved with administration of anti-TNF-alpha antibodies.
Case Presentation
A 21-year-old Japanese woman was admitted to our hospital due to a mediastinal mass. She had no past or family history of autoimmune disease or IBD. From pathological analysis of the mass, she was diagnosed with nodular sclerosis classical Hodgkin lymphoma, the most common subtype of Hodgkign lymphoma (Ann Arbor stage IIE) and received six cycles of ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) therapy. She soon relapsed and received salvage therapy followed by autologous stem cell transplantation plus involved-field radiotherapy to the residual mass. She relapsed again immediately and then received four cycles of brentuximab vedotin, an antibody-drug conjugate directed against the CD30 antigen expressed on Hodgkin lymphoma, and subsequently underwent 5/8 allele-matched CBT, which resulted in complete remission. The recipient had alleles of HLA-A*24:02/*26:02; HLA-B*39:01/*40:02; HLA-Cw*07:02/*15:02; HLA-DR*08:03/*15:01, while the donor had HLA-A*24:02/*26:02; HLA-B*39:01/*51:01; HLA-Cw*07:02/*14:02; HLA-DR*08:03/*11:01. The mismatched donor HLA loci are shown in bold. Although acute GVHD was not present, she suffered severe chronic GVHD (cGVHD) from 4 months after CBT; oral cGVHD with lichenoid changes and xerostomia, and skin cGVHD with lichenoid lesions, scored 3 and 1 respectively by National Institutes of Health (NIH) criteria. She had difficulty in eating and weight loss, then increased tacrolimus and prednisolone 25 mg/day was administered, resulting in improvement of her symptoms. After that, the severity of cGVHD was well controlled to mild grade (NIH criteria) with prednisolone 10 mg/day and tacrolimus (trough concentration, 5-7 ng/mL). About 1 year after CBT, she complained of intermittent abdominal pain, bloody diarrhea, and pyrexia, without new clinical signs of GVHD and exacerbation of pre-existing oral and skin cGVHD. Laboratory tests showed an increased level of c-reactive protein (CRP). Stool culture detected only Enterococcus species and no Clostridium toxins. Positron emission tomography showed fluorodeoxyglucose uptake by the intestinal wall including that of the ileum. Colonoscopy revealed multiple erosions and ulcers over the colon, suggesting IBD. Her symptoms were resolved after the administration of oral prednisolone, 20 mg/day; beclomethasone dipropionate; and mesalazine. While tapering prednisolone to 5 mg/day over 6 months, the patient’s abdominal pain exacerbated. Colonoscopy revealed multiple colonic ulcers with characteristic longitudinal arrangement and cobblestone appearance, with an intervening area of normal mucosa (Fig. 1A-1, A-2) and multiple small aphthous ulcerations (Fig. 1A-3), and the pathological analysis showed that the intestinal mucosa was highly infiltrated by lymphocytes and plasma cells, with gland necrosis, without granulomas or crypt cell apoptosis (Fig. 1B-1). Histochemical analysis was negative for latent membrane protein-1, cytomegalovirus, and Ziehl-Neelsen stain, and thus a diagnosis of CD was made. Prednisolone was increased to 40 mg/day, and subsequently, infliximab, a mouse-human chimeric anti-TNF-alpha antibody, was started and tacrolimus was tapered, after which the patient’s abdominal symptoms were rapidly resolved concomitant with a decreasing CRP level. However, she presented with a persistent cough after the fourth administration of infliximab, and chest computed tomography revealed ground-glass opacities in both lungs. With no infectious microorganisms detected in the bronchoalveolar lavage, we diagnosed organizing pneumonia (OP), which was confirmed by transbronchial lung biopsy specimens. A drug-induced lymphocyte stimulation test was positive for infliximab (stimulation index, 373%; a positive result is defined as a stimulation index > 180%), indicating infliximab-induced OP. We then switched infliximab to adalimumab, a fully human anti-TNF-alpha antibody. The patient received adalimumab every 2 weeks for 4 months while discontinuing tacrolimus, and no abdominal pain and exacerbation of oral and skin GVHD has since been observed even after adalimumab was discontinued. Consistent with the abdominal symptom improvement, colonoscopy showed complete remission with scarring and with regeneration of normal glands (Fig. 1A-4, B-2). The patient’s clinical course since the onset of abdominal pain is illustrated in Fig. 2.

Informed consent was obtained from the patient for publication of this case report.
Discussion
Here, we described a case of steroid-refractory CD after CBT, which was dramatically resolved after administration of anti-TNF-alpha antibodies. CD exhibits characteristic endoscopic features comprising transmural inflammation and skip lesions, with longitudinal ulcers and cobblestone appearance, which are the clearest diagnostic findings. In the present case, any obvious pathological findings indicating intestinal GVHD were not detected; however, late-acute or chronic intestinal GVHD can have nonspecific histological features (Shulman et al. 2006). Thus, CD coexisting with intestinal GVHD would be possible.
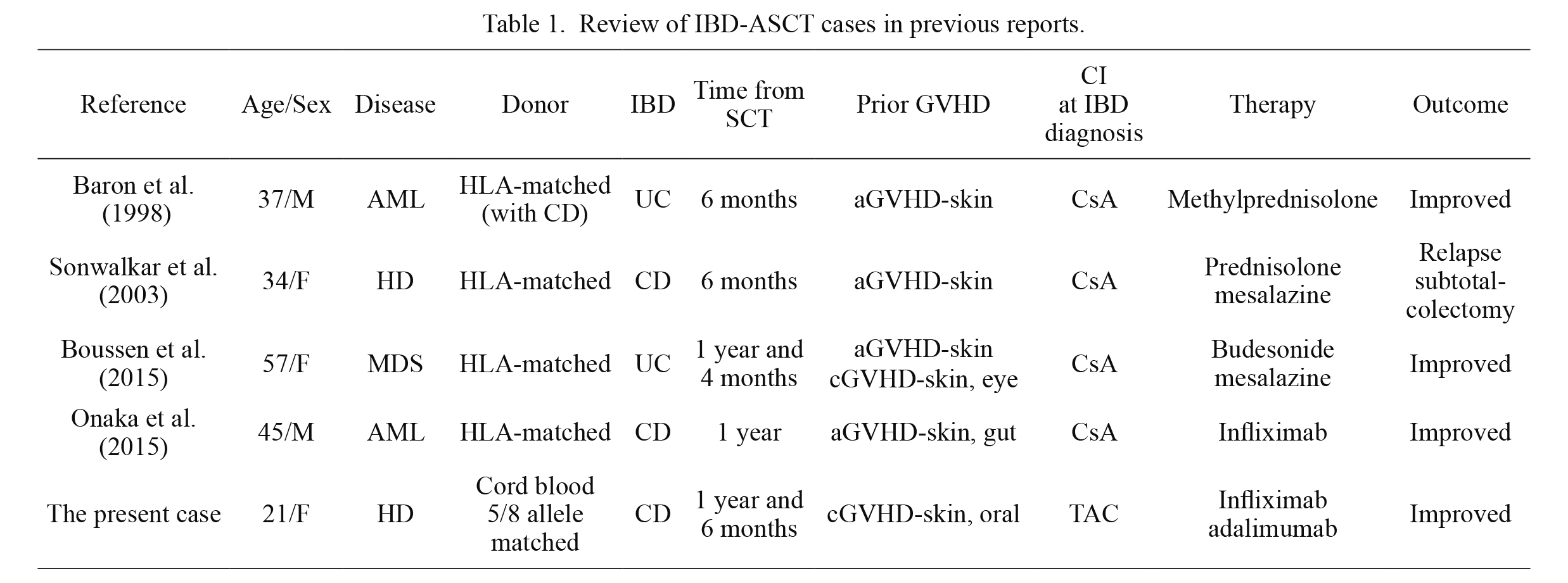
Uncovering the pathophysiology of CD after Allo-SCT is challenging. TNF-alpha is responsible for the pathogenesis of IBD and the initiation and progression of GVHD (Schmaltz et al. 2003; Abraham and Cho 2009), which implies that the pathophysiology of IBD after Allo-SCT (IBD-ASCT) can overlap with that of GVHD. Nevertheless, IBD-ASCT has been reported in only four other cases so far (Baron et al. 1998; Sonwalkar et al. 2003; Boussen et al. 2015; Onaka et al. 2015) (Table 1). Interestingly, these cases, including the present case, share the common features: all had prior acute or chronic GVHD and had been maintained on calcineurin inhibitors (CIs). Furthermore, the period from Allo-SCT to IBD diagnosis had a chronic course of > 6 months in all cases.
Notably, recurrent or more aggressive courses of pre-existing IBD after solid organ transplantation were reported despite the continuation of a CI, which occurred most commonly with tacrolimus (Verdonk et al. 2006). This seems to be contradictory because tacrolimus shows remission-induction efficacy for refractory IBD; however, its efficacy is dose-dependent and is usually tapered off after obtaining remission. Regulatory T-cells (Tregs) decrease in patients with IBD, and their compensatory immunosuppression is disturbed in the intestinal mucosa (Maul et al. 2005). CIs reduce interleukin-2 (IL-2) production by effector T-cells, which subsequently reduces the pool of Tregs, and impairs its reconstitution and immune tolerance in the Allo-SCT setting (Zeiser et al. 2006). Recently, low-dose IL-2 therapy has been shown to restore Tregs and lead to clinical improvement for refractory GVHD and autoimmune diseases (Klatzmann and Abbas 2015; Matsuoka 2018). We speculate that maintaining a dose of CIs to control GVHD cannot prevent and indeed may contribute to inducing IBD in Allo-SCT patients, as seen in the present case.
Genetic predisposition is likely to be a major factor in the pathogenesis of IBD-ASCT, since IBD develops in only a limited number of patients receiving Allo-SCT. Association of HLA polymorphisms with CD susceptibility has been shown, although with variations among different races (Fernando et al. 2008). Interestingly, clinical implications resulting from the adoptive transfer of donor-derived autoimmune diseases including IBD have been reported in Allo-SCT recipients (Baron et al. 1998; Berisso et al. 1999; Neumeister et al. 2000; Sonwalkar et al. 2003). In the present case, the donor had recipient-mismatched HLA haplotypes, HLA-B*51:01 and HLA-Cw*14:02, which are identified as high-risk haplotypes for CD susceptibility in the Japanese population (Oryoji et al. 2015). Considering imbalanced effector T cells on the intestinal mucosa, especially the predominance of Th1 and Th17 cells with excessive secretion of TNF-alpha, interferon-γ and interleukin-17, involved in the pathogenesis of CD (Bouma and Strober 2003), the interaction of high-risk HLA haplotypes for CD with certain patterns of HLA alleles mismatches, which could lead to excessive antigen presentation to effector T cells and subsequent activation of GVHD, may play an important role in the development of CD after Allo-SCT, although it is unclear whether the adoptive transfer of CD susceptibility contributed in the present case. Further accumulation of cases is needed to identify high-risk mismatched HLA alleles associated with an increased risk of IBD-ASCT in an era increasingly turning to alternative-donor transplantation.
In summary, anti-TNF-alpha therapies were effective for steroid-refractory CD after Allo-SCT. All the reported cases of IBD-ASCT occurred in recipients who had prior acute or chronic GVHD and were maintained on CIs. Thus, IBD-ASCT can be considered as a subsequent complication of GVHD. Maintaining CIs and receiving a transplant with specific mismatched HLA alleles could be contributing to the risk. Further investigations are required to clarify the pathogenesis and risk factors of IBD-ASCT, and its relevance to GVHD.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Abraham,
C. &
Cho,
J.H.
(2009) Inflammatory bowel disease. N. Engl. J. Med., 361, 2066-2078.
-
Baron,
F.A.,
Hermanne,
J.P.,
Dowlati,
A.,
Weber,
T.,
Thiry,
A.,
Fassotte,
M.F.,
Fillet,
G. &
Beguin,
Y.
(1998) Bronchiolitis obliterans organizing pneumonia and ulcerative colitis after allogeneic bone marrow transplantation. Bone Marrow Transplant., 21, 951-954.
-
Berisso,
G.A.,
van Lint,
M.T.,
Bacigalupo,
A. &
Marmont,
A.M.
(1999) Adoptive autoimmune hyperthyroidism following allogeneic stem cell transplantation from an HLA-identical sibling with Graves’ disease. Bone Marrow Transplant., 23, 1091-1092.
-
Bouma,
G. &
Strober,
W.
(2003) The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol., 3, 521-533.
-
Boussen,
I.,
Sokol,
H.,
Aractingi,
S.,
Georges,
O.,
Hoyeau-Idrissi,
N.,
Hugot,
J.P.,
Mohty,
M. &
Rubio,
M.T.
(2015) Inflammatory bowel disease after allogeneic stem cell transplantation. Bone Marrow Transplant., 50, 1365-1366.
-
Couriel,
D.,
Saliba,
R.,
Hicks,
K.,
Ippoliti,
C.,
de Lima,
M.,
Hosing,
C.,
Khouri,
I.,
Andersson,
B.,
Gajewski,
J.,
Donato,
M.,
Anderlini,
P.,
Kontoyiannis,
D.P.,
Cohen,
A.,
Martin,
T.,
Giralt,
S., et al.
(2004) Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood, 104, 649-654.
-
Fernando,
M.M.,
Stevens,
C.R.,
Walsh,
E.C.,
De Jager,
P.L.,
Goyette,
P.,
Plenge,
R.M.,
Vyse,
T.J. &
Rioux,
J.D.
(2008) Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet., 4, e1000024.
-
Klatzmann,
D. &
Abbas,
A.K.
(2015) The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol., 15, 283-294.
-
Matsuoka,
K.I.
(2018) Low-dose interleukin-2 as a modulator of Treg homeostasis after HSCT: current understanding and future perspectives. Int. J. Hematol., 107, 130-137.
-
Maul,
J.,
Loddenkemper,
C.,
Mundt,
P.,
Berg,
E.,
Giese,
T.,
Stallmach,
A.,
Zeitz,
M. &
Duchmann,
R.
(2005) Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology, 128, 1868-1878.
-
Neumeister,
P.,
Strunk,
D.,
Apfelbeck,
U.,
Sill,
H. &
Linkesch,
W.
(2000) Adoptive transfer of vitiligo after allogeneic bone marrow transplantation for non-Hodgkin’s lymphoma. Lancet, 355, 1334-1335.
-
Onaka,
T.,
Kitagawa,
T.,
Mori,
M.,
Yonezawa,
A. &
Imada,
K.
(2015) Infliximab therapy for Crohn’s-like gastrointestinal lesions after allogeneic bone marrow transplantation. Rinsho Ketsueki, 56, 2452-2455.
-
Oryoji,
D.,
Hisamatsu,
T.,
Tsuchiya,
K.,
Umeno,
J.,
Ueda,
S.,
Yamamoto,
K.,
Matsumoto,
T.,
Watanabe,
M.,
Hibi,
T. &
Sasazuki,
T.
(2015) Associations of HLA class I alleles in Japanese patients with Crohn’s disease. Genes Immun., 16, 54-56.
-
Schmaltz,
C.,
Alpdogan,
O.,
Muriglan,
S.J.,
Kappel,
B.J.,
Rotolo,
J.A.,
Ricchetti,
E.T.,
Greenberg,
A.S.,
Willis,
L.M.,
Murphy,
G.F.,
Crawford,
J.M. &
van den Brink,
M.R.
(2003) Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood, 101, 2440-2445.
-
Shulman,
H.M.,
Kleiner,
D.,
Lee,
S.J.,
Morton,
T.,
Pavletic,
S.Z.,
Farmer,
E.,
Moresi,
J.M.,
Greenson,
J.,
Janin,
A.,
Martin,
P.J.,
McDonald,
G.,
Flowers,
M.E.,
Turner,
M.,
Atkinson,
J.,
Lefkowitch,
J., et al.
(2006) Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: II. Pathology Working Group Report. Biol. Blood Marrow Transplant., 12, 31-47.
-
Sonwalkar,
S.A.,
James,
R.M.,
Ahmad,
T.,
Zhang,
L.,
Verbeke,
C.S.,
Barnard,
D.L.,
Jewell,
D.P. &
Hull,
M.A.
(2003) Fulminant Crohn’s colitis after allogeneic stem cell transplantation. Gut, 52, 1518-1521.
-
Tyndall,
A. &
Dazzi,
F.
(2008) Chronic GVHD as an autoimmune disease. Best Pract. Res. Clin. Haematol., 21, 281-289.
-
Verdonk,
R.C.,
Dijkstra,
G.,
Haagsma,
E.B.,
Shostrom,
V.K.,
Van den Berg,
A.P.,
Kleibeuker,
J.H.,
Langnas,
A.N. &
Sudan,
D.L.
(2006) Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am. J. Transplant., 6, 1422-1429.
-
Zeiser,
R.,
Nguyen,
V.H.,
Beilhack,
A.,
Buess,
M.,
Schulz,
S.,
Baker,
J.,
Contag,
C.H. &
Negrin,
R.S.
(2006) Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood, 108, 390-399.