2020 Volume 251 Issue 3 Pages 175-181
2020 Volume 251 Issue 3 Pages 175-181
The novel coronavirus disease 2019 (COVID-19) is now officially declared as a pandemic by the World Health Organization (WHO), and most parts of the world are taking drastic measures to restrict human movements to contain the infection. Millions around the world are wondering, if there is anything that could be done, other than maintaining high personal hygiene, and be vigilant of the symptoms, to reduce the spread of the disease and chances of getting infected, or at least to lessen the burden of the disease, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The National and International health agencies, including the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), and the WHO have provided clear guidelines for both preventive and treatment suggestions. In this article, I will briefly discuss, why keeping adequate zinc balance might enhance the host response and be protective of viral infections.
The Coronavirus disease 2019 (COVID-19) is triggered by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On March 11th, 2020, the current coronavirus-mediated infection was officially declared a global pandemic by the World Health Organization (WHO). The enormous physical, emotional, social, and economic impact of the COVID-19 pandemic let the health professionals invest time, intellect, and resources to develop meaningful therapeutic approaches to reduce the disease burden. In addition, the need for identifying the factors to reduce the risk of SARS-CoV-2 infection that could be adopted by a large population at a low cost with minimal risk is a medical priority at this time of crisis. Zinc is one of the micronutrients that could be consumed to reduce the intensity of SARS-CoV-2 infection and perhaps lessen the respiratory tract infection through the antiviral actions (Eby 1997; Hemila 2015; Read et al. 2019). Zinc supplementation against rhinovirus infection, or “common cold” viruses including influenza virus has shown promising antiviral effects with reduced disease burden. It is important to mention that the amount of ionic zinc present at the oral and nasal mucosa (site of infection) positively correlated with the study outcome (Eby 1997, 2010); a 42% reduction of ‘cold duration’ was estimated with a higher dose of ionic zinc (Hemila 2011, 2015). Of relevance, SARS-CoV-2 also takes a similar route to get entry to the body, including the lungs. Whether the presence of a higher concentration of zinc at and around the site of infection would reduce the intensity of the SARS-CoV-2 infection, is an area that surely needs further clinical validation. However, in a pandemic situation, a courageous attempt to use zinc to reduce disease burden is worth trying. More importantly, consuming around 25-40 mg zinc per day is affordable, and less likely to induce human toxicity, as more than 200 to 400 mg per day of zinc consumption has shown to induce adverse effects, including nausea, vomiting, epigastric pain, lethargy, and fatigue (Brown et al. 1964; Fosmire 1990). In a follow-up study, a relatively higher risk of prostate cancer was detected in men who consumed more than 100 mg/day of supplemental zinc for 10 or more years, as compared to the nonusers (Leitzmann et al. 2003). Since the tolerable upper intake level, UL, for zinc is 40 mg, as determined by the Institute of Medicine (IOM), consuming up to 40 mg of zinc per day might provide an additional shield against the COVID-19 pandemic, possibly by increasing the host resistance to viral infection to minimize the burden of the disease. Some studies, however, reported the requirement of up to 150 mg/day of zinc supplementation to reduce viral infections (Acevedo-Murillo et al. 2019).
Importantly, SARS-coronavirus (SARS-CoV) replication has shown to be inhibited by zinc (te Velthuis et al. 2010). Using the combination of zinc ions and the zinc-ionophore, pyrithione, the investigation has shown that the replication of SARS-CoV could be significantly reduced by increasing the intracellular concentration of zinc. This inhibition of SARS-CoV was achieved by zinc-mediated inactivation of the core replication enzyme, the RNA-dependent RNA polymerase (RdRp) (te Velthuis et al. 2010). Viral proteases are essential for the life cycle of many viruses, including for the virulence of SARS-CoV. Replication of the genomic RNA of SARS-CoV is driven by replicase polyproteins, which are generated by the proteolytic processing of two viral proteases, papain-like protease (PLpro) and 3C-like protease (3CLpro). Studies have convincingly shown that SARS-CoV PLpro2 is the molecular target for zinc-mediated inhibition (Han et al. 2005). Similarly, a zinc-conjugated compound could selectively inhibit SARS-CoV 3CLpro (Hsu et al. 2004). Whether zinc could exert similar inhibitory effects on proteases of SARS-CoV-2 needs additional studies, as proteases might present an attractive drug target for reducing SARS-CoV-2 load. Of relevance, the potential antiviral drug development against SARS-CoV-2 is likely to focus on 1) blocking the synthesis of viral RNA, 2) inhibiting virus replication by disrupting the essential viral enzymes, 3) preventing the viral binding to the host cell receptors, or 4) stopping the endogenous viral self-assembly system by targeting the structural proteins (Wu et al. 2020). There are four major structural proteins detected in coronavirus: spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N) (Fig. 1) (Bosch et al. 2003).
Zinc ionophore pyrrolidine dithiocarbamate has also shown to reduce the in vitro replication potential of influenza (PR/8/34) virus (Korant et al. 1974). Zinc oxide nanoparticles demonstrated promising antiviral effects against H1N1 influenza virus infection (Ghaffari et al. 2019). In a similar line of study, zinc salt inhibited the replication of the respiratory syncytial virus (Suara and Crowe 2004); the investigators have shown an 800-fold reduction in respiratory syncytial virus with the exposure of 10 M concentration of zinc salt (Suara and Crowe 2004). Antiviral effects of zinc are also shown in the hepatitis C virus (HCV), where zinc has shown to reduce the HCV replication (Yuasa et al. 2006). More importantly, zinc supplementation in HCV-infected patients reduced hepatitis, and enhanced the response to antiviral treatment (Murakami et al. 2007; Matsuoka et al. 2009; Matsumura et al. 2012). Similarly, zinc salts (sulfate and acetate) could also block hepatitis E virus replication by inhibiting the viral RdRp activity, but no such inhibition was detected with magnesium salts (sulfate and acetate), suggesting that not all micronutrients have antiviral effects (Kaushik et al. 2017). Zinc supplementation significantly improved both cutaneous and genital warts that are induced by human papillomavirus (HPV) (Raza and Khan 2010; Simonart and de Maertelaer 2012). Pyrithione, known as a zinc ionophore, has shown to inhibit the growth of herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). Pyrithione treatment could induce cellular zinc influx, which facilitated the dysregulation of the ubiquitin-proteasome system to reduce viral titer (Qiu et al. 2013). Similarly in human studies, using a zinc oxide-based cream, the skin lesions caused by HSV-1 have shown to be improved, along with the lesser intensity of pain and reduced itching, as compared to the untreated HSV-1 patients (Godfrey et al. 2001). Zinc-mediated inhibition has also shown with Vaccinia virus (Zaslavsky 1979). Although studies have shown the inhibitory effects of zinc on the viral transcription of Human Immunodeficiency Virus-1 (HIV-1) (Haraguchi et al. 1999), additional studies are needed to determine the clinical benefits of using zinc for HIV-1-infected patients. Furthermore, in developing countries, zinc supplementation in children significantly reduced the prevalence of pneumonia (Bhutta et al. 1999; Prasad et al. 2000). How zinc exerts its antiviral effects are not yet clear, it could inhibit viral binding to the nasal and oral mucosa and subsequent viral replications.
At present, there is no specific drug available to treat patients with COVID-19. With limited success, several drugs are being used as either therapeutics or prophylactics, including IL-6 inhibitors (tocilizumab and sarilumab), interferon, lopinavir-ritonavir (HIV protease inhibitors), chloroquine (anti-malarial), ivermectin (anti-parasite), azithromycin, doxycycline, dexamethasone, favipiravir, remdesivir, and convalescent plasma therapy to minimize SARS-CoV-2-related infection. The utilities of some of these drugs are questionable and would need further clinical validations. For the COVID-19 pandemic, broadly three major therapeutic strategies are employed to provide the treatment: 1) To try the existing broad-spectrum anti-viral agents, including interferons, ribavirin; the known pharmacokinetics and pharmacodynamics of these drugs are added advantage, while non-specific effects against SARS-CoV-2 is an obvious disadvantage. 2) To try high-throughput screening of existing molecular databases to identify molecules that might exert therapeutic effects against SARS-CoV-2; the identification of anti-HIV infection drug, lopinavir-ritonavir is a good example of this strategy. 3) To use viral genotype and phenotype to develop de novo drug, specific against SARS-CoV-2; despite time-consuming, offers the best therapeutic strategy (Wu et al. 2020; Zumla et al. 2016).
Chloroquine is a commonly used antimalarial drug that has been used in the treatment of SARS-CoV-2 infection (Centor et al. 2020; de Sena et al. 2019; Devaux et al. 2020; Ferner and Aronson 2020; Huang et al. 2020). In vitro studies found that chloroquine could inhibit the replication of SARS-CoV-2 in Vero E6 cells (effective concentration EC90 of 6.90 µM) (Wang et al. 2020b). Chloroquine is also a zinc ionophore that could enhance cellular zinc uptake in a concentration-dependent manner (Xue et al. 2014); the chloroquine-induced effects on COVID-19 patients might be partly mediated by the intracellular antiviral effects of zinc. Nevertheless, further analysis of controlled trials will be needed to have a better understanding of how chloroquine might act against SARS-CoV-2 infection. Chloroquine is a cardiotoxic drug and a better alternative is necessary to treat SARS-CoV-2 infection (Blignaut et al. 2019). Of clinical importance, due to adverse effects, WHO has recommended discontinuing the ongoing clinical trials using chloroquine or hydroxychloroquine to treat SARS-CoV-2 infected patients. In fact, on June 15, 2020, the Food and Drug Administration revoked its emergency use authorization for hydroxychloroquine and chloroquine for the treatment of COVID-19.
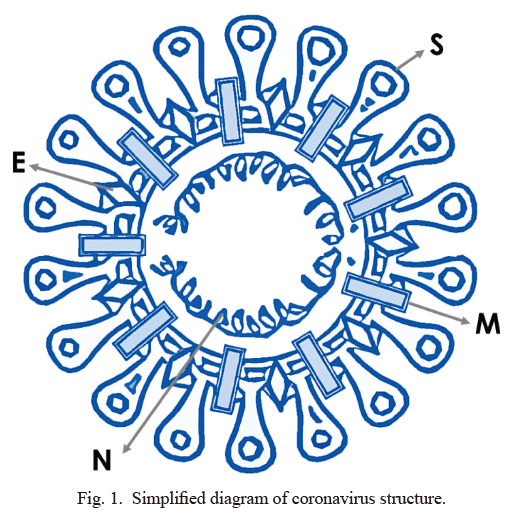
Simplified diagram of coronavirus structure.
S, spike protein; M, membrane protein; E, envelope protein; N, nucleocapsid protein (Stadler et al. 2003).
Micronutrients, in general, play an important role in maintaining adequate immune activity. Impairment of micronutrient balance adversely affects the immune system to increase the susceptibility to various bacterial and viral microorganisms. Studies have shown how a wide range of vitamins and micronutrients influence the functionality of various immune cells (Field et al. 2002; Prasad 2007). Zinc is an important dietary trace mineral that can influence the functions of the immune cells (Field et al. 2002; Overbeck et al. 2008). Zinc also acts in the activation and inactivation of over 300 enzymes and coenzymes that are involved in vital cellular functions, including energy metabolism, DNA synthesis, RNA transcription, etc. (Field et al. 2002; Overbeck et al. 2008). Of relevance, zinc is the main structural component of around 750 zinc-finger transcription factors (Barazandeh et al. 2018; Lambert et al. 2018a, b), and incorporated into about 10% of all human proteins (Andreini et al. 2006). Meat and seafood, such as lamb, beef, chicken, oyster, and lobster are good sources of zinc; for better absorption of zinc, these should be eaten together with vegetables (Kaur et al. 2014). Black rice, black sesame, soy foods, mushroom, celery, legumes, lentils, nuts, sunflower seeds, and almonds are also good sources of zinc (Uwitonze et al. 2020).
Therapeutic strategies against COVID-19 are likely to be focused on either 1) to enhance the human immune system to minimize the intensity of SARS-CoV-2 infection, or 2) to develop specific virucidal agents against SARS-CoV-2. Our innate immunity plays a vital role in regulating the replication and infection potentials of the SARS-CoV and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Of note, SARS-CoV, SARS-CoV-2, and MERS-CoV have similar sequences and encode structurally analogous RdRp (Morse et al. 2020). Earlier studies have shown that zinc deficiency can hinder host‐defense systems (Fraker et al. 2000) to increase the susceptibility to various viral and bacterial infections (Prasad 2007). The human genetic disorders related to zinc malabsorption are commonly associated with severe fungal, viral, or bacterial infections, along with immune system dysregulation (Prasad et al. 1988). Acrodermatitis enteropathica is a rare genetic disorder with decreased zinc absorption causing low plasma zinc concentrations; patients suffering from acrodermatitis enteropathica experienced fewer infections when treated with a high dose of zinc supplementation (Hambidge et al. 1977; Neldner and Hambidge 1975). Studies conducted in vitro, have shown that low concentrations of zinc can induce apoptotic cell death of mouse CD4+CD8+ thymocytes, (Telford and Fraker 1995), while, a higher concentration of zinc can block such apoptosis (Fraker and Telford 1997). Zinc deficiency not only reduced lymphocyte counts but also impaired T and B lymphocyte functions; when murine T- and B-lymphocytes were challenged with mitogens, markedly reduced proliferative activities were noted in zinc-deficient mice, as compared to controls (Fraker et al. 1978, 1986). In a similar line of observation, zinc supplementation resulted in a higher proportion of CD4+CD3+ cells in peripheral blood, with enhanced T‐cell‐mediated immunity in supplement-treated children (Sazawal et al. 1997). Of relevance, analyzing 339 patients with COVID-19, the CD4+ and CD8+ cells were found to be significantly reduced in all the studied patients. Compared to the survived patients (n = 274), the lymphocyte, monocyte, and platelet counts were significantly lower in the non-survived patients (n = 65); recovered patients have higher lymphocyte count, and claimed to a predictive marker for better outcome (Wang et al. 2020a). Similar results were also obtained by separate groups, compared with the normal values, relatively lower white blood cell counts, lymphopenia, and/or thrombocytopenia were detected in patients with COVID-19; elevated C-reactive protein level was detected in these patients (Chen et al. 2020; Lescure et al. 2020).
In vivo experimental studies have shown that zinc deficiency could compromise B‐cell development (Shankar and Prasad 1998) with low IgG production (DePasquale-Jardieu and Fraker 1984), leading to an increased rate of infection, and higher mortality (Fraker et al. 1982). Maternal zinc deficiency in animal studies also resulted in reduced antibody production in the offspring (Fraker et al. 1984). Of clinical importance, such impaired antibody‐mediated responses could be restored by zinc supplementation (Fraker et al. 1984). Moreover, studies have shown that in inadequate zinc microenvironment, macrophages have lowered the phagocytic ability against the parasites, and zinc treatment could restore the phagocytic ability of the macrophages (Sazawal et al. 1997). Higher intracellular zinc concentration has shown to increase monocyte resistance to apoptosis via suppressing the activation of caspase 3 (Perry et al. 1997). It is, however, needed to mention that a high concentration of zinc or zinc compound could also induce apoptosis in certain cancer cells (Li and Liu 2020; Li et al. 2019; Wang et al. 2018; Zangeneh et al. 2019).
Yearlong consumption of 45 mg elemental zinc per day has markedly reduced the incidence of infection in elderly individuals, ranging from 55 years to 87 years (Prasad et al. 2007). In vitro studies have shown the potent antiviral effects of free zinc, which are validated in human trials with high free zinc-containing creams, lozenges, and supplements. Whether gargling with zinc-containing fluid would reduce, viral load in oral and pharyngeal areas will need clinical evaluation. Of relevance, in Asian countries, including Japan, gargling is recommended and commonly practiced for influenza virus infection (Ide et al. 2014, 2017). In a randomized trial conducted on upper respiratory tract illness, a 36% reduction of infection was documented in patients who gargled with water, as compared with a non-gargling control group (Satomura et al. 2005).
The clinical zinc inadequacy is more common in elderly individuals (Fraker et al. 2000), and the global prevalence of zinc deficiency is estimated to be around 20% (Wessells and Brown 2012; Wessells et al. 2012). Of concern, elderly individuals with comorbid conditions, including hypertension and diabetes are usually zinc diffident (Anderson et al. 2001). Elderly individuals are commonly infected by the SARS-CoV-2 (Armitage and Nellums 2020; Kunz and Minder 2020), and whether impaired zinc balance is causing these individuals to be more vulnerable to infection is an area that needs further clinical studies. However, existing evidence shows that zinc could exert antiviral effects: 1) by suppressing viral replication and 2) by boosting immune responses (Fig. 2). As mentioned, zinc possesses antiviral properties and may provide inexpensive and effective adjunct therapy for some viral species, that can induce a wide range of infections, including respiratory tract infections (Read et al. 2019).
In conclusion, maintaining adequate zinc balance is important to protect from microorganisms, including viral infections. As stated, the tolerable upper intake level for zinc is 40 mg/day, and consuming such amount of zinc is likely to be beneficial against the SARS-CoV-2 infection, possibly by enhancing the host resistance against the viral infection. Of note, zinc sulfate contains around 23% elemental zinc, consequently, to have about 50 mg of zinc, one would need to take 220 mg of zinc sulfate (National Institute of Health 2001). Again, the potential beneficial role of zinc in COVID-19 needs further clinical validation, in this pandemic situation, using zinc to reduce disease burden would be a well-intentioned trial (Rahman and Idid 2020). In an uncontrolled case series of laboratory-confirmed COVID-19 patients with clinical features (CDC case definition), a high dose of zinc salt lozenges claimed to show symptomatic and objective improvements (Finzi 2020). Carefully designed clinical trials would need to determine the potential benefits of zinc in minimizing the risk and disease burden of COVID-19. ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world, and the WHO’s International Clinical Trials Registry Platform (WHO ICTRP) have enlisted around 15 different ongoing clinical studies where zinc is being tested on COVID-19 patients. Some of these studies are using high dose intravenous zinc as an intervention to minimize the disease burdens that come with SARS-CoV-2 infection. The forthcoming results of these studies in the USA, Europe, and other parts of the world would shed better light on the utility of zinc, either as a prophylactic or as an adjuvant therapy for the SARS-CoV-2 infection.
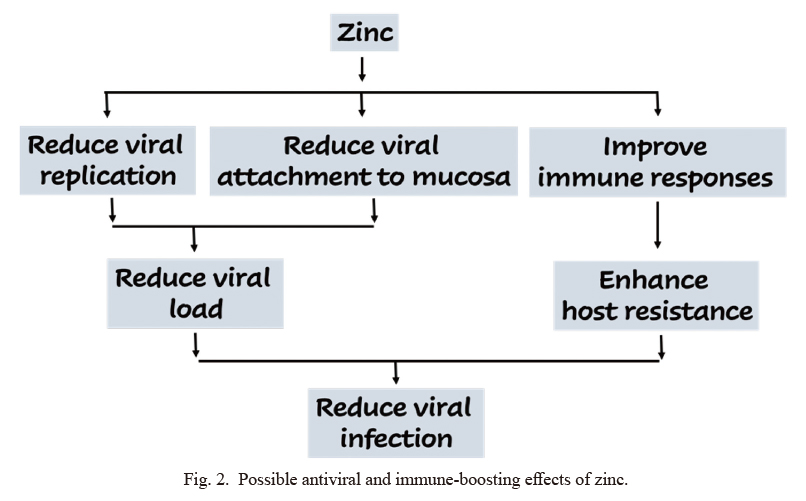
Possible antiviral and immune-boosting effects of zinc.
Zinc could exert antiviral effects by suppressing viral replication and by enhancing immune responses.
Thanks to Mr. M. Muhit Razzaque, Ms. Rufsa H. Afroze, Ms. Zinnia Mosharraf, and Ms. Peace Uwambaye for carefully reading the manuscript and providing useful suggestions.
The author declares no conflict of interest.