2020 年 252 巻 2 号 p. 103-107
2020 年 252 巻 2 号 p. 103-107
Coronavirus disease 2019 (COVID-19) is a global public health concern that can be classified as mild, moderate, severe, or critical, based on disease severity. Since the identification of critical patients is crucial for developing effective management strategies, we evaluated clinical characteristics, laboratory data, treatment provided, and oxygenation to identify potential predictors of mortality among critical COVID-19 pneumonia patients. We retrospectively utilized data from seven critical patients who were admitted to our hospital during April 2020 and required mechanical ventilation. The primary endpoint was to clarify potential predictor of mortality. All patients were older than 70 years, five were men, six had hypertension, and three ultimately died. Compared with survivors, non-survivors tended to be never smokers (0 pack-years vs. 30 pack-years, p = 0.08), to have higher body mass index (31.3 kg/m2 vs. 25.3 kg/m2, p = 0.06), to require earlier tracheal intubation after symptom onset (2.7 days vs. 5.5 days, p = 0.07), and had fewer lymphocytes on admission (339 /μL vs. 518 /μL, p = 0.05). During the first week after tracheal intubation, non-survivors displayed lower values for minimum ratio of the partial pressure of oxygen to fractional inspiratory oxygen concentration (P/F ratio) (44 mmHg vs. 122 mmHg, p < 0.01) and poor response to intensive therapy compared with survivors. In summary, we show that obesity and lymphopenia could predict the severity of COVID-19 pneumonia and that the trend of lower P/F ratio during the first week of mechanical ventilation could provide useful prognostic information.
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a novel beta coronavirus (Zhou et al. 2020) with a high affinity for the angiotensin-converting enzyme-2 (ACE-2) receptor present in the alveolar epithelium (Liu et al. 2020). Moreover, as the ACE-2 receptor is expressed in various cell types and tissues, such as vascular endothelial cells, nerves, intestines, and kidneys (Danser et al. 2020), patients infected with SARS-CoV-2 suffer both lung damage and multiple organ failure, depending on receptor expression profile and susceptibility of each patient (Dixon et al. 2020). Endothelial dysfunction is the most important determinant of the severity of COVID-19 pneumonia as patients with severe COVID-19 pneumonia often develop venous or arterial thrombosis (Bikdeli et al. 2020). Furthermore, severe hypercoagulability has been described, which complicates severe COVID-19 pneumonia among patients admitted to intensive care units (ICUs) (Spiezia et al. 2020).
Several studies have described the epidemiology, clinical characteristics, and thoracic imaging findings of COVID-19 pneumonia (Chen et al. 2020; Wang et al. 2020), and while approximately 80% of COVID-19 cases are self-limiting ( Kim et al. 2020), severe or critical COVID-19 pneumonia can follow a relentless clinical course.
Therefore, our study aimed to evaluate trends in gas exchange parameters and to identify crucial clinical factors in critically ill COVID-19 pneumonia patients.
This was a single-center retrospective cohort study that was conducted between April 1 and 30, 2020, and was approved by the Institutional Review Board with a waiver for informed consent (OCH 2020. No.20). Data from critical COVID-19 pneumonia patients at the Okinawa Chubu Hospital who required mechanical ventilation were used. COVID-19 diagnosis was considered confirmed if the reverse transcription-polymerase chain reaction of nasopharyngeal swab samples was positive for SARS-CoV-2. In response to the SARS-CoV-2 outbreak, we launched a COVID-19 intubation team of anesthesiologists, emergency medicine physicians, trained ICU nursing staff, infectious disease physicians, and pulmonologists. As COVID-19 pneumonia can progress rapidly, the anesthesiologist conducted a one-time rapid sequence oral intubation with video laryngoscopy if a patient’s oxygen requirement exceeded 5 L/min. We identified seven critical COVID-19 pneumonia patients who met our intubation criteria of 1) severe acute distress, 2) respiratory rate > 30 min, 3) oxygen requirement greater than 5 L/min when delivered by mask, and 4) altered consciousness. Data on demographics, underlying diseases, and serial laboratory values, including white blood count (WBC) C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, D-dimer, oxygenation data, and chest computed tomography (CT) findings were extracted from our electronic medical records. Oxygenation data included the serial values for (P/F) ratio, which refers to the ratio of the partial pressure of oxygen to fractional inspiratory oxygen concentration. Blood samples were obtained upon admission and chest CT findings were evaluated at baseline. We defined symptom onset as the date when patients noticed relevant symptoms such as fever, cough, dyspnea, and fatigue. Data on prognosis and treatment were updated on April 30, 2020. The severity of COVID-19 pneumonia was defined according to the second edition of the Guidance of Treatment for Novel Coronavirus Infection: COVID-19, issued by The Ministry of Health, Labor Standards, Japan. Our cohort of critical COVID-19 pneumonia patients satisfied the criteria for mechanical ventilation and intensive care unit monitoring.
Measurements and outcomesThe primary outcome was hospital discharge or death by April 30, 2020. For predictors of critical COVID-19 pneumonia, serum inflammatory biomarker such as WBC, lymphocyte, CRP and ferritin showed no statistically significant difference. We therefore focused on the P/F ratio during the first week after mechanical ventilation, wherein the maximum and minimum values for P/F ratio were defined as the highest and lowest values recorded during the first week of artificial mechanical ventilation, respectively.
Statistical analysisContinuous variables are expressed as means with standard deviations, and categorical variables are reported as numbers. Patients were divided into two groups: survivors and non-survivors, and comparisons between the two groups were analyzed using the Student t-test. All data analyses were conducted using STATA (version 11.2, Stata Corp, College Station, TX, USA).
We evaluated the data from the seven critical COVID-19 pneumonia patients whose average age was 74 years. There were five men in the cohort, and six patients had hypertension (Table 1). Six patients had fever, and four reported exertional dyspnea, and ultimately, three of them died because of progressive respiratory failure due to COVID-19 pneumonia. Among the seven patients, three survived patients were ever smokers.
Compared with survivors, non-survivors tended to (i) have high body mass index (31.3 kg/m2 vs. 25.3 kg/m2, p = 0.06) and (ii) require earlier tracheal intubation after symptom onset (2.7 days vs. 5.5 days; p = 0.07; Table 1). Furthermore, in initial laboratory findings, non-survivors tended to have lower lymphocyte counts (339 /μL vs. 518 /μL, p = 0.05) than survivors. Other laboratory data such as WBC, CRP, LDH, ferritin, and D-dimer were not significantly different between the two groups (Table 2). After tracheal intubation, compared with survivors, non-survivors had lower maximum P/F ratio and minimum P/F ratio values (max P/F, 221 vs. 342 mmHg, p = 0.02; min P/F, 44 vs. 122 mmHg, p < 0.01; Table 2).
Imaging findings included multi-focal ground-glass opacities, which were often observed on chest CT at admission. Representative CT images from a surviving patient (Fig. 1) and a patient who did not survive (Fig. 2) showed a typical bilateral dense consolidation resembling acute respiratory distress syndrome (ARDS) in the latter. All patients in this cohort were administered favipiravir via a nasogastric tube to block virus replication from the day of admission, whereas three patients were administered tocilizumab for cytokine storm syndrome and two of them were survivors. The ventilation management strategy for all patients followed the ARDS management protocol, i.e., low tidal volume with high positive end-expiratory pressure, and three patients received prone position ventilation and one of them survived. All patients were provided heparin prophylaxis to prevent deep vein thrombosis. Mean hospital stay of survivors and non-survivors were 25 days and 13 days.

Demographics of critical COVID-19 pneumonia.
Data are expressed as mean and range.
BMI, body mass index.
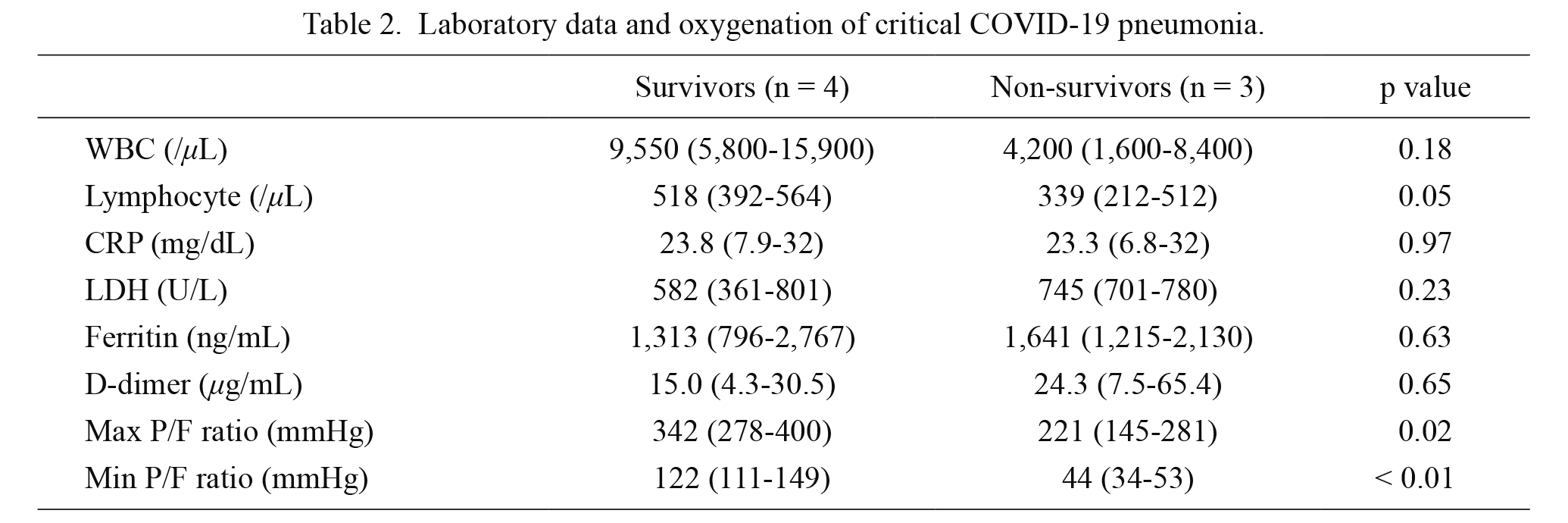
Laboratory data and oxygenation of critical COVID-19 pneumonia.
Data are expressed as mean and range.
WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase; P/F ratio, ratio of partial pressure of oxygen and fractional inspiratory oxygen concentration.
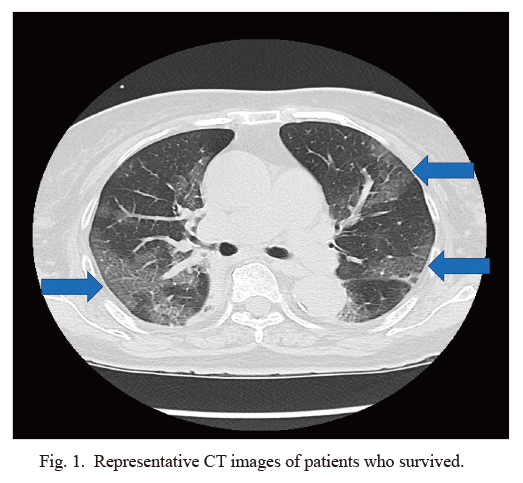
Representative CT images of patients who survived.
Thoracic CT image of a 77-year-old woman demonstrated multi-focal ground-glass opacity (GGO) with interlobular septal thickening. Arrow indicates GGO.
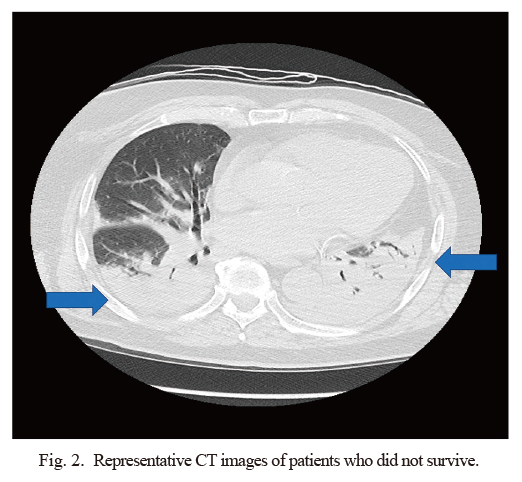
Representative CT images of patients who did not survive.
Thoracic CT image of a 78-year-old man showed bilateral dorsal consolidation with air-bronchogram on day 1 of mechanical ventilation. Arrow indicates consolidation.
We describe the clinical characteristics and oxygenation profile of patients with critical COVID-19 pneumonia who required mechanical ventilation and show that serial monitoring of P/F ratio in these patients was useful for predicting their short-term prognosis. Obesity and hypertension, which are recognized risk factors for critical COVID-19 pneumonia (Tamara and Tahapary 2020; Hong et al. 2020), were also more prevalent among non-survivors in our cohort. Importantly, obese patients have also been reported to be at increased risk for H1N1 influenza-associated pneumonia (Honce and Schultz-Cherry 2019).
Adipocytes produce several cytokines, including interleukin-6, which are associated with the development of extensive inflammation (Casimiro et al. 2020). Lymphopenia might signify lower T-lymphocyte counts in the elderly or could reflect the recruitment of lymphocytes to the lungs as a defense mechanism against COVID-19 (Diao et al. 2020). Serum parameters such as CRP, LDH, ferritin, and D-dimer showed marked elevations, which is in accordance with the observations from previous reports (Colafrancesco et al. 2020; Breakey and Escher 2020). Significant elevations in both CRP and ferritin are associated with cytokine storm syndrome during critical COVID-19 pneumonia (Nile et al. 2020); therefore, these inflammatory markers can also predict the severity and prognosis of COVID-19 pneumonia. Furthermore, serum D-dimer has been reported to be a useful marker of the progression of COVID-19 pneumonia. In cases of severe to critical COVID-19, phenomena such as cerebral infarction, COVID toe, and several other thrombotic complications have also been reported (Klok et al. 2020).
We focused on changes in P/F ratio as a readout for oxygenation after endotracheal intubation in our cohort, especially during the first week after mechanical intubation, because we noticed that this trend may be predictive of the clinical outcome in critical COVID-19 pneumonia. The P/F ratio is a well-recognized parameter for determining the severity of ARDS. In our study, serial monitoring of the P/F ratio helped distinguish survivors from non-survivors, and two patients who died were treated with prone position ventilation and intensive therapy using anti-viral, anti-inflammatory, and anti-cytokine drugs as they appeared to have profound inflammation and possible thrombosis.
From a physiological viewpoint, hypoxemia with a widening alveolar-arterial oxygen difference (A-aDO2) is associated with ventilation–perfusion mismatch, intrapulmonary shunting, or decreased carbon dioxide diffusion capacity of the lung (DLco). However, as A-aDO2 can also become very large when pAO2 exceeds 100 mmHg or if FiO2 is high, it cannot be used in the evaluation of gas exchange abnormalities during high FiO2 or mechanical ventilation. We therefore did not evaluate A-aDO2 in the present study.
Chest imaging findings showed that non-survivors typically had more consolidation during the early phase. We assumed that this dense dorsal consolidation on CT during the early phase of illness might be associated with intrapulmonary shunting rather than ventilation-perfusion mismatch or reduced DLco. In contrast to non-survivors, patients who survived showed a trend toward improvement in both laboratory biomarkers and oxygenation during the first week after tracheal intubation.
There are several limitations to our study. First, it is a small, single-center study. Second, the dates of maximum and minimum oxygenation were not standardized across among all patients, which led to tracheal intubation. However, we frequently evaluated the P/F ratio during the first week after mechanical intubation as ascertaining the maximum and minimum P/F ratios during this critical period is useful. Third, as there remains no standard treatment strategy for COVID-19 pneumonia, treatment choices may have affected the clinical course of critical COVID-19 pneumonia and it is not known whether COVID-19 management strategy or host defense is a crucial determinant of disease outcomes.
To summarize, we show that obesity and lymphopenia could predict the severity of COVID-19 pneumonia and that serial monitoring of oxygenation during the first week of mechanical ventilation could provide useful prognostic information. Specifically, in our cohort, during the first week of mechanical ventilation, a maximum P/F ratio > 300 mmHg might be associated with good prognosis, whereas a minimum P/F ratio < 100 mmHg might be associated with poor prognosis. A multi-center study is necessary to validate our results.
We thank Dr. Kenneth N.M. Sumida, University of Hawaii John A. Burns School of Medicine, Dr. Rita McGill, University of Chicago and Enago for English editing of our manuscript.
The authors declare no conflict of interest.