2020 Volume 252 Issue 3 Pages 193-197
2020 Volume 252 Issue 3 Pages 193-197
Puberty is the transitional period from childhood to adult that leads to growth spurt, sexual maturation and attainment of reproductive capacity. Precocious puberty is defined when secondary sexual characteristics develop before the age of eight for girls and nine for boys. Central precocious puberty (CPP) is diagnosed when the process is driven by premature activation of hypothalamic gonadotropin-releasing hormone (GnRH) secretion. Many factors promote CPP, and the thyroid function is thought to be one of them. In our previous study, thyroid stimulating hormone (TSH) was higher in the CPP group than that of the participants without CPP. This elevation of TSH in CPP is said to be associated with pubertal luteinizing hormone (LH) elevation. The aim of this study was to evaluate the causal relationship between TSH and LH in CPP patients. A total of 221 girls diagnosed with CPP and treated with GnRH agonists were included. All participants except one showed LH suppression (peak LH < 3 IU/L), and serum levels of follicle stimulating hormone (FSH) were also lower after the treatment. These results indicate that puberty has slowed down and that the patients were successfully treated for CPP. As for thyroid hormones, TSH was significantly lower and free thyroxine (fT4) levels were higher after 12 months of GnRH agonist treatment compared with baseline. With GnRH agonist treatment, the serum levels of LH and TSH were decreased, suggesting that the increase in serum TSH levels is associated with premature LH elevation in girls with CPP.
Puberty is a period of change. From a biological perspective, puberty is a process of sexual maturation and the physical change is initiated by hormonal signals from the brain. Pulsatile gonadotropin-releasing hormone (GnRH) production activates the hypothalamic-pituitary-gonadal (HPG) axis. GnRH from hypothalamus releases gonadotropins such as luteinizing hormone (LH) and follicle stimulating hormone (FSH) from anterior pituitary which in turn stimulates ovary and testis to mature. As GnRH and gonadotropin secretion become marked, secondary sex characteristics appear (Burt Solorzano and McCartney 2010). Precocious puberty is defined when these pubertal changes happen before the age of eight in girls and nine in boys, and it is one of the most common conditions encountered in pediatric endocrinology practice. Many factors promote early puberty, such as race, environment, body mass index (BMI) and endocrinological abnormality (Carel and Léger 2008). Precocious puberty due to endocrinological abnormality can be further divided into central and peripheral precocious puberty (PPP) depending on the site of hormone production. Central precocious puberty (CPP) is defined when the hormonal production begins early due to premature activation of the hypothalamic-pituitary-gonadal (HPG) axis. PPP is defined when the hormones are produced through a process other than HPG activation, such as adrenal gland or gonadal tumors.
CPP is more common than PPP, and the incidence and prevalence of CPP is increasing rapidly. According to a study by Kim et al. (2019), the prevalence of CPP increased 8.6 times in girls and 10.4 times in boys from 2008 to 2014 in Korea. CPP is far more common in girls, and 90% of girls have an idiopathic form of the condition. When CPP is suspected, the onset and extent of body odor, acne, breast budding, pubic and axillary hair, and growth velocity are examined with thorough history taking and physical examination. In addition, biochemical markers, including LH and FSH levels are tested before a diagnosis is made. Precocious puberty leads to early secondary sexual development, which can cause problems such as sexual abuse that can bring psychosocial burden to the family and society (Tremblay and Frigon 2005; Klein et al. 2017). Therefore, once diagnosed with precocious puberty, patients begin treatment to delay puberty, and most are treated with a GnRH agonist (Chen and Eugster 2015). GnRH analogues are used to suppress the HPG axis (Berberoglu 2009). Continuous stimulation of GnRH receptor by a GnRH agonist causes the downregulation and desensitization of GnRH receptor in pituitary gland that leads to suppression of gonadotropin secretion. The safety and effectiveness of GnRH analogues have been proven over the years.
In addition to management approaches, factors that promote early puberty have been studied. Onset of puberty is influenced by genetic factors and many environmental cues. Obesity is one of them and there have been numerous studies that show relationship between childhood obesity and early puberty (Kaplowitz 2008).
Obesity is a metabolic disease that affects the endocrine system. It is linked to many endocrine abnormalities, including thyroid dysfunction (Pacifico et al. 2012). Studies have shown a positive relationship between BMI and thyroid stimulating hormone (TSH) levels (Biondi 2010; Cho et al. 2018). As obesity is related to TSH levels and precocious puberty, it can be assumed that TSH and precocious puberty are related. However, relationship between TSH and CPP are not well known.
In our previous study, the CPP group had higher TSH and lower free thyroxine (fT4) levels than the participants without CPP, and hyperthyrotropinemia was related to age and LH level (Jung et al. 2019). In older patients and those with higher peak LH levels, TSH levels were higher. It has been suggested that an elevated peak LH is related to elevated TSH levels. However, a causal relationship between the two has not been established. We have hypothesized that peak LH level and TSH level are decreased after GnRH agonist treatment. The aim of this study was to evaluate the causal relationship between thyroid function and CPP.
This was a retrospective study. Girls diagnosed with CPP who visited the Pediatric Endocrinology Clinic at Korea University Ansan Hospital between January 2017 and May 2019 were included. A total 221 girls aged 6.0 to 8.9 years with CPP who had been treated with a GnRH agonist for 12 months were included. Participants with clinical hyperthyroidism, hypothyroidism, or CPP with any organic disease were excluded from this study.
Clinical data collectionPatient data was collected from medical records through a retrospective review. Age, height, weight, body mass index (BMI), bone age, serum TSH, fT4, LH, and FSH levels before and after 12 months of GnRH agonist treatment were collected. Among a total of 211 patients, 199 subjects were given 3.75 mg leuprolide every month, 18 subjects were given 3.75 mg triptorelin every month, and 4 subjects were given 11.25 mg triptorelin every three months. Height was measured to the nearest 0.1 cm using a rigid stadiometer, and weight was measured to the nearest 0.1 kg using a calibrated balance scale. Height standard deviation score (SDS), weight SDS and BMI SDS were calculated by using the Cole’s Lambda Mu Sigma method according to 2017 Korean National Growth Chart (Kim et al. 2018). Bone age was measured by a single pediatric endocrinologist using the Greulich and Pyle method. In order to diagnose CPP, GnRH stimulation test was performed by administering GnRH 0.1 mg. Then, blood samples were taken at 30, 45, 60, and 90 minutes to measure the LH and FSH levels. Serum LH and FSH levels were measured using electrochemiluminescence technology (Cobas 6000, Roche Diagnostics, Indianapolis, IN, USA). Serum TSH and fT4 levels were measured using radioimmunoassay methods (Gamma Pro, Seyeong NDS Co., Seoul, Korea).
DefinitionCPP was diagnosed when secondary sex characteristics developed before the age of eight, when LH levels during the GnRH stimulation test peaked above 5 IU/L, and when bone age was advanced more than one year compared to chronological age before the age of nine. Hyperthyrotropinemia was defined as elevated TSH with normal fT4 (TSH ≥ 5.0 mIU/L and 0.8 ng/dL < fT4 < 1.9 ng/dL).
Statistical analysisData are expressed as mean ± standard deviation. Paired t-tests were used to compare clinical characteristics and lab results before and after the GnRH agonist treatment. The McNemar test was used to compare the prevalence of hyperthyrotropinemia before and after GnRH agonist treatment. Pearson’s correlation test was used to analyze the relationships between multiple characteristics and TSH concentration. A multiple linear regression analysis was used to investigate the independent factors influencing TSH levels. p values under < 0.05 were considered statistically significant. IBM SPSS Statistics version 20.0 (IBM Co., Armonk, NY, USA) was used to conduct statistical analyses.
Ethical StatementThis was a retrospective study approved by the Institutional Review Board at Korea University Ansan Hospital in Ansan, South Korea (approval number: 2020AS0133).
Clinical characteristics and lab results were compared after 12 months of GnRH agonist treatment (Table 1). After treatment, there was a significant decrease in TSH (pre-treatment: 2.16 ± 1.01, post-treatment: 1.65 ± 0.84, p < 0.001), LH (pre-treatment: 13.10 ± 11.09, post-treatment: 1.57 ± 0.92, p < 0.001), and FSH levels (pre-treatment: 15.00 ± 4.87, post-treatment: 4.04 ± 2.83, p < 0.001). Height and weight were significantly increased after treatment, and there were increases in weight standard deviation score (SDS), BMI, BMI SDS, bone age, and fT4 levels, while height SDS was decreased. Of all participants, 208 out of 211 girls (93.3%) showed LH suppression (peak LH < 3 IU/L) after GnRH agonist treatment. While 7 out of 211 subjects (3.1%) had hyperthyrotropinemia before GnRH agonist treatment, only one participant had hyperthyrotropinemia after the treatment (p < 0.001; Table 1).
Change in TSH level (△TSH, TSH at 12 months-TSH at baseline) after the 12 months of treatment was compared with clinical characteristics and lab data (Table 2). △TSH had a positive correlation with basal TSH. However, △TSH had no significant correlation with age, height SDS, weight SDS, BMI SDS, bone age, alkaline phosphatase (ALP), peak LH, peak FSH, △fT4, △LH, △FSH, △bone age, and △ALP (Table 2).
Multiple linear regression analysis showed factors associated with TSH change (Table 3). Age, TSH, fT4, ALP, LH, △LH, and △ALP were analyzed. Baseline TSH (β = −0.589, p < 0.001); and ALP (β = 0.002, p = 0.041) levels were associated with △TSH levels.
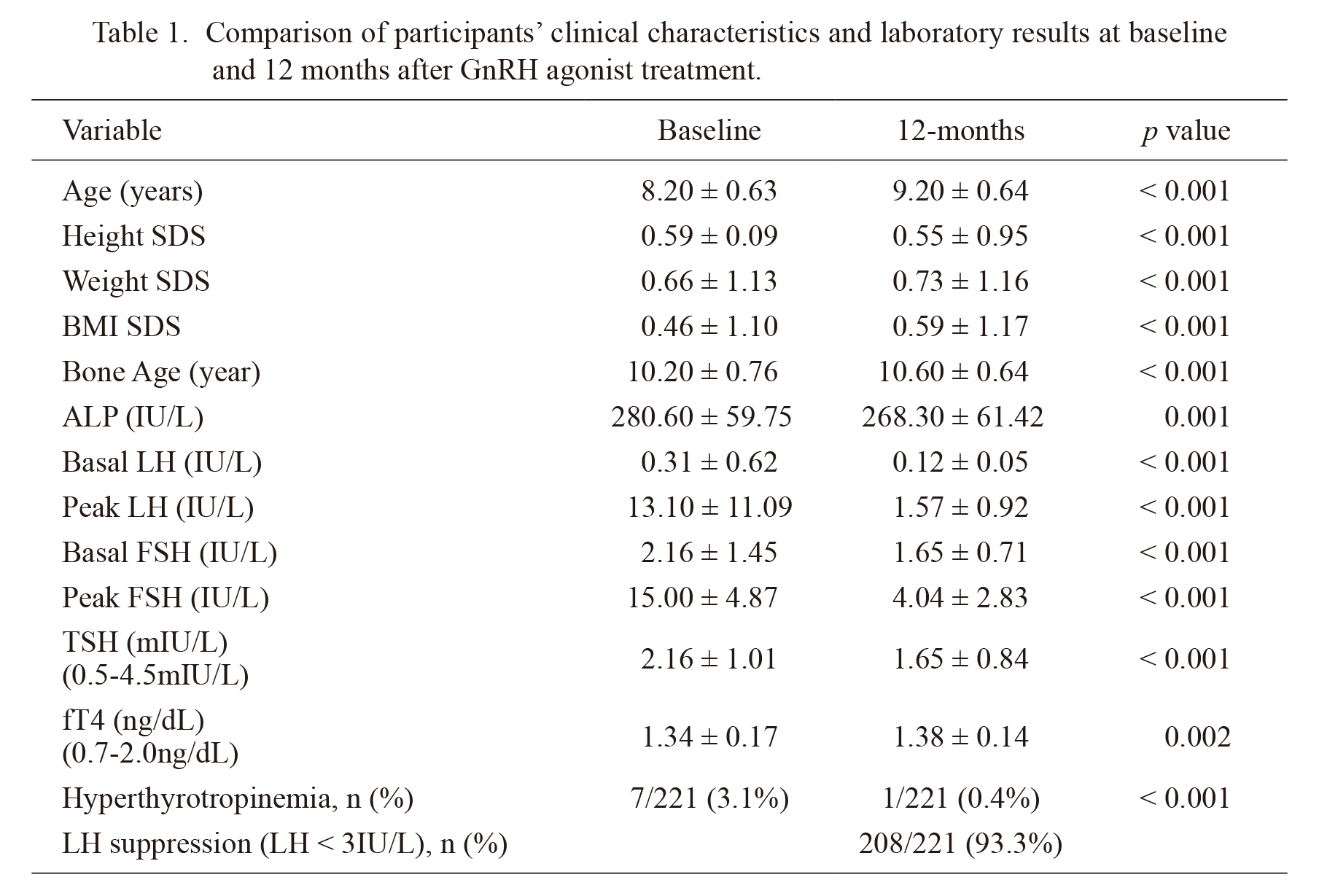
Comparison of participants’ clinical characteristics and laboratory results at baseline and 12 months after GnRH agonist treatment.
Values are presented as mean ± SD or number (%).
SDS, standard deviation score; BMI, body mass index; LH, luteinizing hormone; FSH, follicle stimulating hormone; TSH, thyroid stimulating hormone; Free T4, free thyroxine.
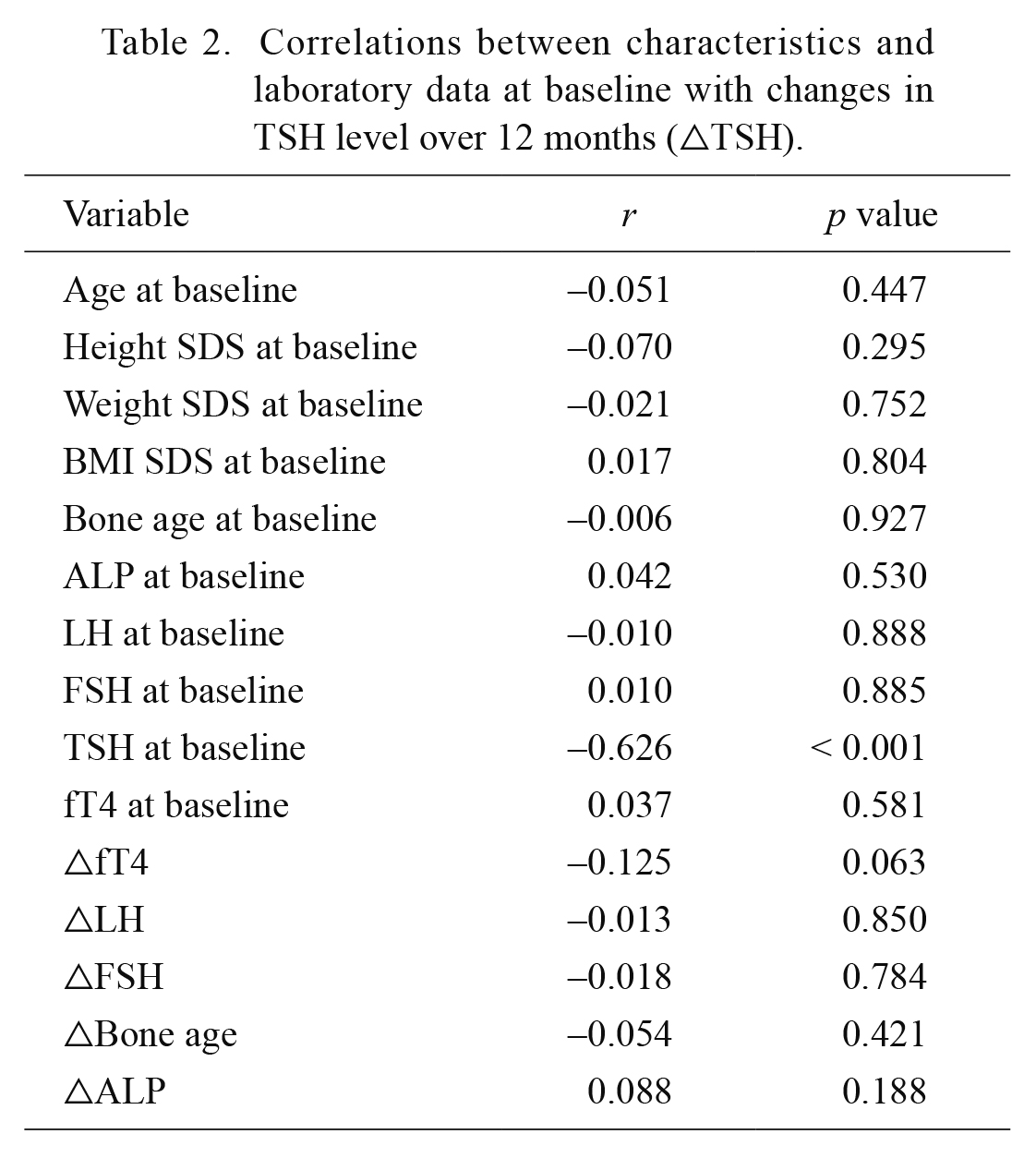
Correlations between characteristics and laboratory data at baseline with changes in TSH level over 12 months (△TSH).
TSH, thyroid stimulating hormone; SDS, standard deviation score; BMI, body mass index; LH, luteinizing hormone; FSH, follicle stimulating hormone; Free T4, free thyroxine; △, change over 12 months of treatment; r, coefficient of correlation.
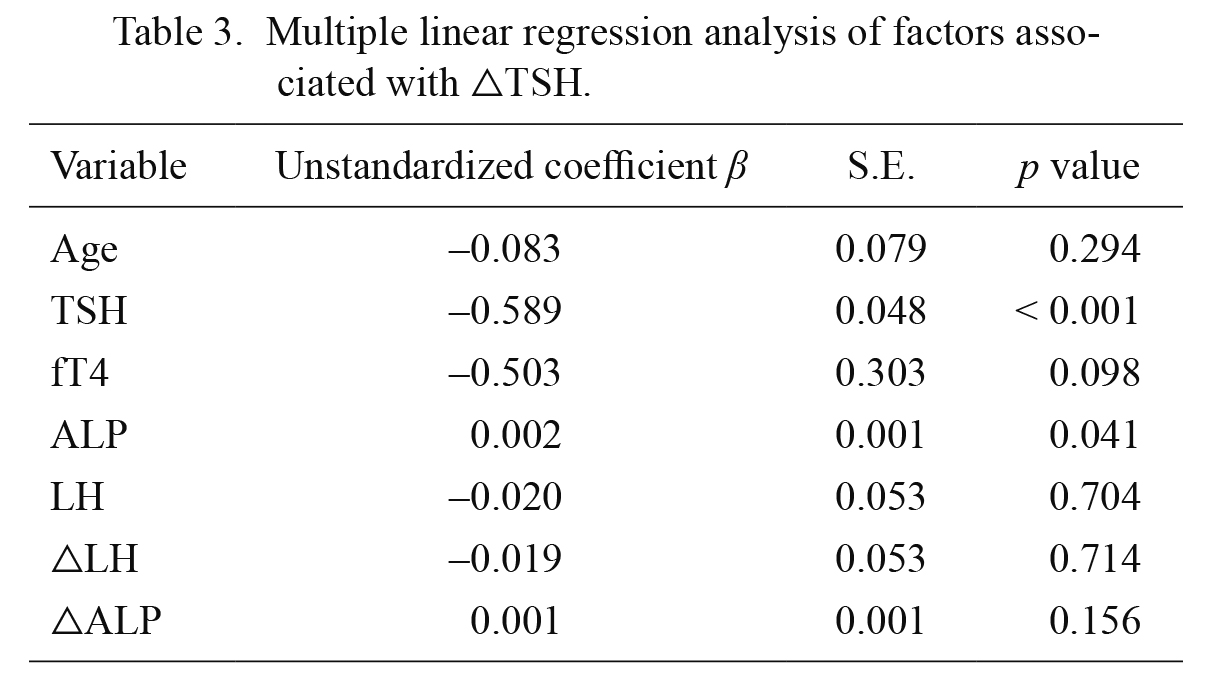
Multiple linear regression analysis of factors associated with △TSH.
TSH, thyroid stimulating hormone; Free T4, free thyroxine; LH, luteinizing hormone; △, change over 12 months of treatment; S.E., standard error.
Little research has investigated associations between obesity, CPP, and thyroid function. To our knowledge, this is one of the first studies to investigate the relationship between thyroid function and LH elevation in CPP patients. In this study, TSH level in CPP girls decreased as LH level decreased after GnRH agonist treatment. In addition, fT4 increased, and the incidence of hyperthyrotropinemia decreased significantly. Therefore, hyperthyrotropinemia in CPP girls is associated with LH surge before puberty.
Puberty starts, as the HPG axis becomes activated. The HPG axis is active from mid-fetal development to early infancy and enters a juvenile pause during childhood, when the axis becomes relatively quiescent (Nathan and Palmert 2005). The HPG axis is governed by stimulatory and inhibitory signals, such as GnRH, LH, and FSH. During childhood, GnRH secretion progressively increases, and puberty starts when the frequency and amplitude of GnRH secretion leads to the pulsatile secretion of LH and FSH, and the HPG axis is reactivated (Buck Louis et al. 2008). Precocious puberty occurs when HPG activation starts prematurely and when pubertal changes occur before the age of eight in girls.
Much research has been done to understand the mechanism behind precocious puberty. Environmental and genetic factors can promote the early activation of the HPG axis (Parent et al. 2003). Obesity is a well-known factor that promotes early puberty (Ahmed et al. 2009). Obesity is not only related to CPP but also elevated TSH levels. Cho et al. (2018) found that obesity and an increase in triglyceride were related to a per-unit increase in TSH. There are many hypotheses behind this phenomenon. Some possible factors are TSH receptor gene mutation, thyroid hormone resistance, and an adaptation process to increase energy expenditure (Reinehr et al. 2008). Leptin also appears to be a key factor in regulating TSH. A study showed that leptin modulates serum TSH level, which, in turn, regulates leptin pulses (Iacobellis et al. 2005).
It can be postulated that since CPP patients are obese, they tend to show TSH elevation. The mechanism for this is still unclear, but there is a clear association between CPP and thyroid function (Bastemir et al. 2007). In this context, our previous study showed that TSH levels in the CPP group were higher than in the non-CPP group. In addition, age and peak LH level were independently predictive of serum TSH concentration. We concluded that the onset of puberty was related with TSH elevation (Jung et al. 2019).
To further investigate our previous findings (Jung et al. 2019) and to determine the causal relationship between TSH and LH levels, we collected the data from 221 girls with precocious puberty and compared their TSH and LH levels before and after GnRH agonist treatment. Our data revealed that patients’ peak LH levels and TSH levels were decreased after GnRH agonist treatment. Paired t-test showed statistically significant changes in other clinical characteristics and lab values as well after the treatment. Basal TSH level had a positive relationship with △TSH, and baseline TSH and ALP levels were independent factors. ALP, a marker of bone formation, increases during puberty as growth is accelerated. When puberty is suppressed with GnRH agonist treatment, ALP levels are decreased. ALP levels are higher in patients with advanced puberty, and this baseline ALP was positively correlated with △TSH. These findings indicate that puberty and thyroid function are related.
Additionally, the serum levels of TSH were decreased together with LH levels, even though weight and BMI were increased after 12 months of GnRH agonist treatment. Our results suggested that TSH is more closely related to LH level than obesity. The TSH decrease in this study is probably due to LH decrease from GnRH agonist treatment. These findings are supported by a study led by Massart and coworkers which showed that TSH levels were decreased with GnRH agonist treatment without causing thyroid dysfunction (Massart et al. 2007). Interaction between LH and TSH can be explained by their molecular similarity, since they are glycoprotein hormones composed with a common α subunit and specific β subunits (Vassart et al. 2004). Because of this similarity, LH can potently bind to TSH receptor (Carayon et al. 1980; Yoshimura et al. 1993). TSH can also bind to LH or FSH receptor and cause ovarian hyperstimulation which leads to precocious puberty. This mechanism is seen in Van Wyk-Grumbach syndrome which is characterized by primary hypothyroidism that leads to hyperstimulation of the pituitary gland. This in turn causes ovarian hyperstimulation and precocious puberty.
This study has limitations. No control group was included, and clinical data was only collected from girls with CPP, so data from a normal healthy control group were not gathered and compared. In addition, LH level was measured differently at the time of diagnosis and at the 12-month follow-up. At the time of diagnosis, samples were taken every 30 minutes during the 90-minute period after GnRH injection, and the peak level was collected for the data. However, at the 12-month follow-up, the LH sample was taken 30 minutes after the injection of a GnRH analogue. Checking peak LH level by taking blood every 30 minutes during the 90-minute period is the most accurate way. However, this is time consuming and needs experienced personnel for precise sampling. It can be useful when making a diagnosis but due to the drawbacks mentioned above, LH is measured 30 minutes after GnRH injection at follow-up visits. A single 30-minute LH sample is simpler, less cumbersome, and as useful in evaluating response to treatment (Cavallo et al. 1995).
In conclusion, this study has shown that serum TSH is decreased with LH suppression after GnRH agonist treatment. We therefore suggest that hyperthyrotropinemia is associated with premature LH elevation in girls with CPP.
The authors declare no conflict of interest.