2021 Volume 253 Issue 1 Pages 69-76
2021 Volume 253 Issue 1 Pages 69-76
Lead (Pb) and cadmium (Cd) are environmental pollutants and nonessential elements in the body. Both metals induce the development of hypertension which is associated with oxidative stress. Curcumin (CUR) is a polyphenolic compound with strong antioxidant activity. The present study evaluated the effect of CUR on oxidative stress, alteration of vascular responsiveness and hypertension induced by exposure to either Pb, Cd or the combination of Pb and Cd. Male Sprague-Dawley rats were exposed to low level of lead acetate (100 mg/L) and/or cadmium chloride (10 mg/L) in the drinking water for 16 weeks. The control animals received deionized water as drinking water. CUR (100 mg/kg) or propylene glycol as vehicle was intragastrically administered once daily for the last 4 weeks. Exposure to Pb, Cd or the combination induced increases in blood pressure and peripheral vascular resistance, and decreased the blood pressure response to intravenous infusion to acetylcholine. Supplementation with CUR significantly reduced blood pressure, alleviated oxidative stress, and increased plasma nitrate/nitrite and glutathione in the blood. The effects of CUR were associated with the improvement of vascular responsiveness, upregulation of the endothelial nitric oxide synthase and downregulation of the NADPH oxidase expression. Furthermore, CUR reduced the metal levels in blood, aorta, liver and kidney. Altogether, exposure to the combination of Pb and Cd aggravated hypertension and oxidative stress, and CUR effectively ameliorated these adverse events in metal exposed animals. Data indicate that CUR may be useful as a dietary supplement for protection against the noxious effects of the heavy metals.
Lead (Pb) and cadmium (Cd) are the highly toxic metals that are widely distributed, and present in the atmosphere, soil, water and food. The impact of Pb and Cd on human health is principally through occupational exposure, environmental contamination, and accumulation in food (Alissa and Ferns 2011). Pb and Cd have received a particular attention not only as potent hazards to human health, but also given their constant increase in the environment and bioaccumulation throughout the food-chain (Jaishankar et al. 2014). Substantial epidemiologic and experimental evidence supports the role of Pb and Cd in the development of cardiovascular disease (CVD) (Alissa and Ferns 2011; Solenkova et al. 2014). Exposure to Cd or Pb increases the risk of CVD, including hypertension, coronary heart disease, atherosclerosis, stroke, peripheral artery disease, diabetes and chronic kidney disease (Navas-Acien et al. 2007; Tellez-Plaza et al. 2013; Satarug 2019). The exact effects of Pb and Cd on the cardiovascular system still remains unclear, although oxidative stress is one of the major mechanisms underlying the toxicity and CVD.
Hypertension is one of the major risk factors for CVD. Pb or Cd induce hypertension in humans and experimental animals (Vaziri 2008; Messner and Bernhard 2010). The cardiovascular system is probably affected by Pb and Cd through formation of oxidative stress (Prozialeck et al. 2008; Kukongviriyapan et al. 2016). Animals with Pb- or Cd-induced hypertension exhibit oxidative stress that is associated with upregulation of NADPH oxidase, a major source of superoxide anion (O2•−) production (Vaziri et al. 2003; Nwokocha et al. 2013).
Exposure to environmental heavy metal is often initiated by several metals. In animal studies, most studies investigate the toxic effect of a single metal exposure; only a few studies have evaluated the toxic effects of multiple metals (Pandya et al. 2012; Matović et al. 2015). Moreover, the detrimental effects of combined metal exposure, particularly in the combination of Pb and Cd on induction of hypertension and alteration of vascular responsiveness is not well defined since both metals are capable of induction of hypertension (Vaziri 2008; Messner and Bernhard 2010).
A novel therapeutic approach to metal-induced hypertension may be by the suppression of oxidative stress using antioxidant compounds. Curcumin (CUR) is a polyphenolic compound isolated from the rhizome of turmeric (Curcuma longa). It is the active ingredient of the dietary spice turmeric and has been used worldwide as dietary supplements for prevention and treatment of various diseases (Hewlings and Kalman 2017). CUR has a unique conjugated structure with two methoxylated phenols and an enol form of β-diketone (Masuda et al. 2001), and the antioxidative effect comes from its phenolic structure and β-diketone derivative (Aggarwal et al. 2007). Previous studies demonstrated that CUR can protect against Pb-induced hematological, as well as renal, hepatic and cerebellar toxicities by suppression of oxidative stress and inflammation and chelating activity (Abdel-Moneim et al. 2015; Abubakar et al. 2019; Alhusaini et al. 2019). A previous study by our group demonstrated that curcumin (50 or 100 mg/kg) reduced blood pressure and improved vascular function in mice exposed to Cd (100 mg/L) for 8 weeks (Kukongviriyapan et al. 2014). To date, there are no data on the vascular protective effect of CUR against long-term exposure to a multiple metal-induced hypertension. Therefore, the present study was performed to investigate whether exposure of the combined Pb and Cd can initiate the development of high blood pressure and alter the blood pressure response to intravenous infusion of the vasoactive drugs, and whether CUR could protect against oxidative stress, impairment of vascular responsiveness and hypertension in rats exposed to a low level of either Pb or Cd or the combination for 16 weeks. Data from this study provides important information about CUR as a dietary supplement for reduction of cardiovascular risk in the condition of Pb and Cd co-exposure.
Lead (II) acetate (Pb(CH3COO)2) and cadmium chloride (CdCl2), which were of analytical grade, were obtained from Sigma-Aldrich (St. Louis, MO, USA). CUR was generously provided by the Government Pharmaceutical Organization (Bangkok, Thailand).
Animals and experimental designMale Sprague-Dawley rats weighing 180-200 g were obtained from the National Laboratory Animal Center, Mahidol University, Nakornpathom, Thailand. All animals were maintained in a controlled temperature environment (23 ± 2°C) with a 12-h dark/light cycle at the Northeast Laboratory Animal Center (Khon Kaen University, Thailand). All experiments involving animals were performed in accordance with the Animal Welfare Act and were approved by the Institutional Animal Care and Use Committee of Khon Kaen University (AEKKU 4/2555).
After one week of adaptation, rats were assigned randomly to eight groups comprised of 8 animals each, as follows: Group 1 − normal control rats (Con), Group 2 − control rats treated with CUR (Con+CUR), Group 3 − Pb-exposed rats (Pb), Group 4 − Pb-exposed rats treated with CUR (Pb+CUR), Group 5 − Cd-exposed rats (Cd), Group 6 − Cd-exposed rats treated with CUR (Cd+CUR), Group 7 − Pb and Cd-exposed rats (Pb+Cd), and Group 8 − Pb and Cd-exposed rats treated with CUR (Pb+Cd+CUR). Rats in metal exposure groups were provided drinking water containing lead acetate (100 mg/L) and/or cadmium chloride (10 mg/L) dissolved in deionized water for 16 weeks. CUR at a dose of 100 mg/kg b.w. (2 mL/kg b.w.) was intragastrically administered once daily in the morning between 9.00 and 10.00 a.m. for 4 weeks from week 12 to 16 of the exposure period. The unexposed control animals received deionized water as drinking water and were intragastrically administered with propylene glycol (2 mL/kg b.w.) as the vehicle for CUR. The concentrations and durations of Pb and Cd exposures were modified from previous studies (Robles et al. 2007; Yoopan et al. 2008), and based on this study’s preliminary observation that these low levels of Pb and Cd induced hypertension in rats. The concentration of CUR was based our previous study showing that it was sufficient to reduce blood pressure in mice exposed to cadmium chloride (Kukongviriyapan et al. 2014).
Indirect blood pressure measurementTo monitor the changes in arterial blood pressure of rats during metal exposures, blood pressure was measured every two weeks by an indirect method using rat tail plethysmography (Blood pressure analyzer, IITC Life Sciences Inc., CA, USA). Systolic blood pressure (SBP) values of ten consecutive measurements in each rat were recorded.
Assessment of hemodynamics and arterial pressure responsivenessAt the end of experimental period, hemodynamics and arterial pressure responses to vasoactive drugs of all rats were measured using a previously described method (Kukongviriyapan et al. 2014). Briefly, after 6-8 h post-administration of CUR, animals were anesthetized with pentobarbital sodium (60 mg/kg, i.p.). The left femoral arteries were cannulated for continuously monitoring arterial blood pressure and the veins for drug infusion. The SBP, diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) were determined using the data acquisition software (BIOPAC Systems Inc., CA, USA). After obtaining stable baseline measurements, an endothelium-dependent vasodilator, acetylcholine (ACh, 3, 10, 30 nmol/kg) and an endothelium-independent vasodilator, sodium nitroprusside (SNP, 1, 3, 10 nmol/kg) were infused intravenously. The changes in arterial pressure responses to vasoactive drugs were expressed as percentage changes from baseline values obtained immediately before drug administration. After the blood pressure returned to baseline, hindlimb blood flow (HBF) was measured by placing an electromagnetic flow probe around the abdominal aorta (Carolina Medical Electronics Inc., NC, USA). The hindlimb vascular resistance (HVR) was calculated by dividing MAP by HBF. Finally, after animals were sacrificed, blood and tissues from each animal were collected for biochemical and protein expression analysis.
Biochemical assayThe vascular O2•− production was measured in the strip of carotid arteries using the lucigenin-enhanced chemiluminescence method as previously described (Sompamit et al. 2010). The levels of protein carbonyl and malondialdehyde (MDA), markers for protein oxidation and lipid peroxidation, were measured in plasma as previously described (Kukongviriyapan et al. 2014). The reduced glutathione (GSH) and the oxidized form, glutathione disulfide (GSSG) were assessed in whole blood using an enzymatic coupling assay method described previously (Kukongviriyapan et al. 2014). The indicator of the cellular redox state was expressed as GSH/GSSG. The level of nitrate/nitrite, a stable and end product of NO metabolism was measured in plasma as previously described method (Sompamit et al. 2010).
Western blot analysisHomogenates from the aortas were prepared for analysis of protein expressions of endothelial nitric oxide synthase (eNOS) and a transmembrane component of the NADPH oxidase (gp91phox) by a previously described method (Sangartit et al. 2016). The intensities of target proteins were visualized using ImageQuantTM400 imager (GE Healthcare, PA, USA), whereas β-actin was used as a protein loading control.
Assessment of Pb and Cd contentsPb and Cd contents in blood, liver, kidney and aorta were determined as previously described (Sompamit et al. 2010), using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7500 ICP-MS model, CA, USA) according to the manufacturer’s instructions.
Statistical analysisResults are expressed as mean ± SEM. The mean values from different groups were analyzed by one-way ANOVA followed by Newman-Keuls post-hoc test. Statistical significance was defined as a P value < 0.05. The data were analyzed using SigmaStat for Windows® (version 3.11, 2004 Systat Software Inc., USA).
Table 1 shows the SBP of rats in all experimental groups measured by the indirect tail cuff method. Metal exposure significantly increased SBP starting from week 2, continued the rise throughout the exposure period, and reached plateau levels at week 16 in both Pb and Cd groups. SBP in the combination of Pb and Cd group continued rising and were significantly higher than the single metal exposure groups (P < 0.05, Table 1). CUR treatment at the last 4 weeks significantly reduced SBP by 55%, 48% and 53% in Pb, Cd and Pb+Cd groups, compared to their respective metal exposure only groups (P < 0.05, Table 1). The efficacy of the blood pressure lowering effect of CUR was comparable in every treatment group.
Regarding the hemodynamic parameters measured directly via the intravascular catheter, it was shown that Pb and/or Cd significantly increased SBP, DBP and MAP (P < 0.05, Table 2). The blood pressure in animals exposed to the combination of Pb and Cd was higher than single metal exposed animals (Table 2), which was consistent with the measurements by the indirect tail cuff method. Interestingly, increased arterial blood pressure of metal exposed animals was associated with decreased HBF and increased HVR (Table 2). Moreover, it was observed that increased HR was in metal exposed animals (P < 0.05, Table 2). Treatment with CUR improved the hemodynamic status of metal exposed rats by lowering arterial blood pressure, decreasing HR, and increasing HBF, thereby decreasing HVR (P < 0.05, Table 2). The restoration of hemodynamics, especially the peripheral vascular resistance (HVR) after CUR treatment was more pronounced in animals with co-exposure to Pb and Cd.
The effect of heavy metal exposure on the arterial pressure responses to an endothelium-dependent vasodilator, ACh and an endothelium-independent vasodilator, SNP is displayed in Fig. 1. Chronic exposure to Pb and/or Cd attenuated the blood pressure response to intravenous infusion to ACh at 3, 10 and 30 nmol/kg (P < 0.05, Fig. 1A), whereas the response to SNP was unaltered among all groups (Fig. 1B). It is noted that co-exposure to Pb and Cd has a greater effect on attenuation of ACh-induced vasodilation than Pb or Cd alone. Treatment with CUR significantly restored the arterial pressure responses to ACh (Fig. 1A).

Effect of curcumin on systolic blood pressure of rats measured by tail cuff plethysmography during metal exposures for 16 weeks.
Data are expressed as mean ± SEM, n = 8/group. *P < 0.05 compared to control group, †P < 0.05 compared to Pb treated group, ‡P < 0.05 compared to Cd treated group, #P < 0.05 compared to Pb or Cd treated group, §P < 0.05 compared to Pb+Cd treated group, $P < 0.05 compared to Pb or Cd treated group and co-administered with CUR.
Con, control; Cd, cadmium; CUR, curcumin; Pb, lead.

Effect of curcumin on hemodynamic status of rats in all experimental groups after 16 weeks of metal exposures.
Data are expressed as mean ± SEM, n = 8/group. *P < 0.05 compared to control group, †P < 0.05 compared to Pb treated group, ‡P < 0.05 compared to Cd treated group, #P < 0.05 compared to Pb or Cd treated group, §P < 0.05 compared to Pb+Cd treated group, $P < 0.05 compared to Pb or Cd treated group and co-administered with CUR.
Con, control; Cd, cadmium; CUR, curcumin; DBP, diastolic blood pressure; HR, heart rate; HBF, hindlimb blood flow; HVR, hindlimb vascular resistance; MAP, mean arterial pressure; Pb, lead; SBP, systolic blood pressure.

Effect of curcumin on vascular responses to vasoactive drugs in rats after 16 weeks of metal exposure.
Results in all experimental groups are expressed as mean ± SEM, n = 8/group. *P < 0.05 compared to control group, †P < 0.05 compared to Pb treated group, ‡P < 0.05 compared to Cd treated group, §P < 0.05 compared to Pb+Cd treated group, #P < 0.05 Pb+Cd compared to Pb or Cd treated group, $P < 0.05 compared to Pb or Cd treated group and co-administered with 100 mg/kg of CUR.
The restoration of the blood pressure response to intravenous infusion of ACh and SNP by CUR described above may be associated with antioxidant activity of CUR. Exposure to heavy metals caused remarkably elevated vascular O2•− levels, especially in the combination group (Table 3) and that was associated with upregulation of gp91phox expression (Fig. 2B). Treatment with CUR partially suppressed vascular O2•− production in all metal exposed animals (P < 0.05, Table 3) and suppressed the upregulated gp91phox expression (Fig. 2B). On the other hand, nitrate/nitrite, the metabolites of NO in plasma were significantly decreased in heavy metal exposed animals, particularly the combination group (Table 3). The decreased nitrate/nitrite levels were associated with downregulation of eNOS protein expression (Fig. 2A). CUR significantly ameliorated most of the changes by partially restoring nitrate/nitrite levels and expression of eNOS.
Apart from increased ROS production, a marked increase in plasma MDA and protein carbonyl in metal exposed animals, representing increased lipid and protein oxidation was observed (P < 0.05, Table 3). The elevated levels of MDA and protein carbonyl were partially prevented by CUR.
GSH and the redox status were evaluated in heavy metal exposed animals. There was a marked decrease in blood GSH and redox ratio of GSH/GSSG, especially in combination of Pb and Cd (Table 3). It was apparent that the blood GSH and the redox status were markedly improved after CUR treatment, indicating that CUR decreased oxidative stress and improved antioxidant status.

Effect of curcumin on oxidative stress and nitric oxide metabolites of rats in all experimental groups after 16 weeks of metal exposures.
Data are expressed as mean ± SEM, n = 8/group. *P < 0.05 compared to control group, †P < 0.05 compared to Pb treated group, ‡P < 0.05 compared to Cd treated group, #P < 0.05 compared to Pb or Cd treated group, §P < 0.05 compared to Pb+Cd treated group, $P < 0.05 compared to Pb or Cd treated group and co-administered with CUR.
Con, control; Cd, cadmium; CUR, curcumin; GSH, glutathione; GSH/GSSG, glutathione/glutathione disulfide; MDA, malondialdehyde; O2•−, superoxide anion; Pb, lead.

Effect of curcumin on eNOS and gp91phox protein expression in aortic tissues of rats after 16 weeks of metal exposure.
Results in all experimental groups are expressed as mean ± SEM, n = 8/group. *P < 0.05 compared to control group, †P < 0.05 compared to Pb treated group, ‡P < 0.05 compared to Cd treated group, §P < 0.05 compared to Pb+Cd treated group, #P < 0.05 Pb+Cd compared to Pb or Cd treated group, $P < 0.05 compared to Pb or Cd treated group and co-administered with 100 mg/kg of CUR.
Table 4 represents the levels of Pb and Cd accumulations in the blood, liver, kidney and aorta of rats after Pb and/or Cd exposure for 16 weeks. In the unexposed animals, the levels of Pb and Cd were below detection limits. CUR can partially decrease Pb and Cd contents in the blood and tissues (P < 0.05, Table 4). It was noted that blood and tissue levels of metals in the single metal exposure and combination of metal exposure were similar. The same dose of CUR was effective in reducing both metals in the combination of metal group.
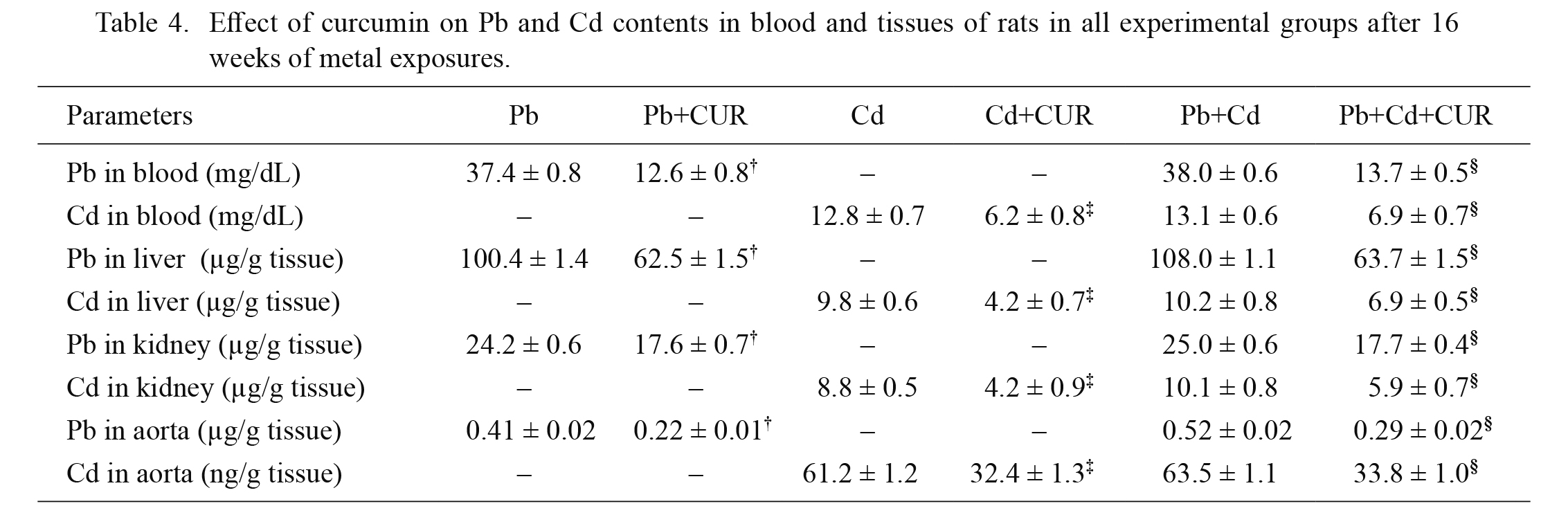
Effect of curcumin on Pb and Cd contents in blood and tissues of rats in all experimental groups after 16 weeks of metal exposures.
Data are expressed as mean ± SEM, n = 8 /group. †P < 0.05 compared to Pb treated group, ‡P < 0.05 compared to Cd treated group, §P < 0.05 compared to Pb+Cd treated group.
Cd, cadmium; CUR, curcumin; Pb, lead
Lead and cadmium are major environmental pollutants which impose several health hazards. Both metals are recognized for their toxicity on the cardiovascular system. This study evaluated the effects of the combination of both metals and CUR in the treatment of the toxicity. It is found that rats developed hypertension after chronic exposure to cadmium chloride (10 mg/L) at a dose 10-fold-lower than previous studies (Sompamit et al. 2010; Kukongviriyapan et al. 2014), and similarly with lead acetate (100 mg/L) alone or in combination with the cadmium chloride for 16 weeks. The impaired vascular response and high blood pressure induced by heavy metals are causally associated with oxidative stress as shown by increased O2•− formation and decreased plasma nitrate/nitrite level, where their surrogated markers in the blood are MDA, protein carbonyl, and the GSH redox state. The changes in vascular tissues concurring oxidative stress were evident by increased expression of gp91phox, and decreased eNOS expression. Treatment with CUR reduced oxidant stress by suppressed the increased formation of O2•−, and increased NO production in association with restoration of hemodynamics in rats.
Pb and Cd exposure induced hypertension accompanied by hemodynamic disturbances shown by the increase in peripheral vascular resistance, thereby raising arterial blood pressure and HR. With regard to the increase in HR after exposure to Pb and Cd, this might be due to the dysregulation of sympathovagal tone in the central nervous system and heart tissues. Previous studies demonstrated that rats chronically exposed to a low level of Pb exhibited arterial hypertension and impaired autonomic control of the cardiovascular system resulting in reduced baroreflex sensitivity and cardiac vagal tone (Simões et al. 2017). CUR treatment slightly decreased HR. This may be due to the effect of the decrease in blood pressure with a subsequently restored baroreflex.
The increase in blood pressure and impairment of vascular response to an endothelium-dependent vasodilator of rats exposed to Pb and Cd may be associated with a reduction in eNOS-derived NO, since NO plays an important role in the function of the vascular tone. Diminished NO bioavailability may be caused by inhibition of eNOS expression, a lack of or inactivation of substrate or a cofactor tetrahydrobiopterin (BH4) for eNOS, and inactivation of NO through the interaction of NO with O2•− leading to the formation of the potent oxidant peroxynitrite (Landmesser et al. 2003). Moreover, impaired blood pressure response to intravenous infusion of ACh has been demonstrated to also be caused by the direct damaging effect of heavy metals on the vascular endothelium (Almenara et al. 2013; Covre et al. 2016).
In the vascular system, NADPH oxidase is the major source of O2•− production. The gp91phox, a membrane-bound NADPH oxidase subunit protein, is expressed in the arterial wall (Görlach et al. 2000). In the current study, it was found that gp91phox protein was upregulated in animals exposed to Pb and/or Cd. An imbalance between endothelium-derived NO and ROS may promote endothelial dysfunction and lead to hypertension (Higashi et al. 2014). It should be noted that the oxidative stress status is slightly higher in the combination of metal exposure than single metal exposure as indicated by the larger increase in vascular O2•−, plasma MDA and protein carbonyl in animals exposed to Pb plus Cd. In this study, the decrease in blood pressure response to intravenous infusion of ACh of rats exposed to Pb and/or Cd was restored after treatment with CUR by increased NO bioavailability as shown by the upregulation of eNOS and downregulation of gp91phox proteins, thereby increasing plasma nitrate/nitrite levels. These data support the previous studies that found that CUR supplementation improved the blood pressure response to intravenous infusion of ACh by increasing NO bioactivity in metal exposed animals in accompanied with decreased oxidative stress (Kukongviriyapan et al. 2014; Zhai et al. 2015).
Furthermore, GSH is a tripeptide, which is the important component of the cellular antioxidant system. GSH and its redox status regulates various critical redox-sensitive enzymes, transcription factors and structural proteins (Townsend et al. 2003). Heavy metals can bind strongly to sulfhydryl groups and deplete GSH stores (Matović et al. 2015), thereby altering the GSH redox state. In this study, the GSH and GSH/GSSG redox ratio in the blood were greatly suppressed in Pb and Cd exposed animals. Interestingly, CUR greatly alleviated oxidative stress by reduction of ROS formation and the decrease in lipid and protein oxidation concurrent with an increased cellular GSH and its redox ratio. Therefore, this study indicates that CUR possessed strong antioxidant and free radical scavenging activities thereby maintaining the cellular redox status and various body redox-sensitive processes.
Pb and Cd were detected in the blood and tissues in exposed animals. It is noted that levels of Pb and Cd in the blood and tissues in single metal and combined metal exposure were not different, suggesting that there is no interference between two metals in the pharmacokinetic process. It is reported that the enol form of CUR is a good chelator of positively charged metals (García-Niño and Pedraza-Chaverrí 2014). Accordingly, current data confirm the chelating property of CUR. This metal chelating activity may contribute to the antihypertensive and antioxidant effects of CUR.
In conclusion, the present study is apparently the first work reporting the beneficial effect of CUR against hypertension, hemodynamic disturbance, the reduction in blood pressure response to intravenous infusion of ACh, and oxidative stress in rats with long-term exposure to a low level of the highly toxic metals, Pb and Cd, either alone or in combination. The combination of metals exposure shows an increase in the adverse effect on the cardiovascular system. The plausible mechanisms by which CUR confers these effects might be the ROS scavenging effect, the increase in NO bioavailability, the suppression of NADPH oxidase, and the maintenance of the GSH redox state. CUR also acts as a metal chelating compound, thereby reducing the metal load in the body. Overall, CUR may be useful as a dietary supplement to reduce the risk of Pb and Cd-induced hypertension and cardiovascular disease.
This work was supported by the Invitation Research Fund (IN61217), Faculty of Medicine and the Khon Kaen University Research Grants (590061 & 600036). Akarachai Tubsakul was supported by a Joint Funding (591H217) under the Program for Supporting Lecturer to Admit High Potential Student to Study and Research on His Expert Program from the Graduate School, Khon Kaen University, Thailand. We would like to thank Professor James Arthur Will for editing the manuscript via the Publication Clinic KKU, Thailand.
The authors declare no conflict of interest.