Abstract
In response to the COVID-19 pandemic caused by SARS-CoV-2 in 2020, we conducted drive-through nasopharyngeal swab testing for COVID-19 in Sendai city, Japan, since April 2020. All tested individuals were judged in advance by public health centers for the necessity of undergoing the test with possible contact history and/or symptoms suggestive of COVID-19. In this study, to identify the predictors of SARS-CoV-2 test positivity for more efficient and evidenced selection of suspected individuals, we enrolled 3,540 consecutive individuals, tested in the first 7 months of the testing program, with data regarding to the history of close contact with COVID-19 patients, including those involved in cluster outbreaks. This cohort included 284 foreign students (257 males and 27 females) from a vocational school involved in the largest cluster outbreak in the area. Close contact history was present in 952 (26.9%) of the participants. The reverse transcription-polymerase chain reaction (RT-PCR) test results showed that 164 participants (4.6%) were positive and 3,376 participants (95.4%) were negative for the SARS-CoV-2 nucleocapsid gene (N2). In the univariate and multivariate analyses, history of close contact with COVID-19 patients, higher age, cough symptoms, and non-native ethnicity were predictors for SARS-CoV-2 test positivity. However, the significance of age and foreign nationality disappeared or declined upon excluding the foreign students from the aforementioned largest cluster outbreak. In conclusion, a history of close contact with COVID-19 patients and the presence of cough symptoms are significant predictors of SARS-CoV-2 test positivity.
Introduction
The novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome- coronavirus 2 (SARS-CoV-2), is currently a major public health crisis worldwide with a significantly higher fatality rate than typical seasonal influenza (Fauci et al. 2020; Gates 2020). Japan has been among the countries affected by the virus since the early phase of the pandemic (Mizumoto et al. 2020; Moriarty et al. 2020). In the face of the COVID-19 outbreak in Japan in early 2020, there emerged a need to safely and rapidly test a large number of people across the country to implement effective quarantine measures and detect clusters to prevent further virus transmission in communities (Frieden and Lee 2020; Furuse et al. 2020). Simultaneously, we needed to avoid unnecessary concentration of suspected cases in the testing centers that could result in cluster outbreaks (Lu et al. 2020; Wee et al. 2020). To address these requirements, as requested by the local government (Miyagi Prefecture and Sendai City), we established a drive-through outpatient clinic for the testing of COVID-19 (Tohoku University Medical Office) at a location away from the Tohoku University Hospital in Sendai city. We started performing drive-through nasopharyngeal swab testing for COVID-19, collaborating with the university hospital and the local government, and checked more than 4,000 participants in the first seven months of the program since April 2020. In this report, after presenting how we established and managed a drive-through outpatient clinic for COVID-19 testing, we describe the details of the tested participants and the COVID-19 testing results.
Methods
Process of the drive-through nasopharyngeal swab test
Fig. 1 presents a flowchart of the drive-through nasopharyngeal swab test for COVID-19 at our outpatient clinic. A person who wanted to undergo a nasopharyngeal swab test first had to make a phone call to the health centers managed by the local government in advance. Then, the public health center guided the callers about the appointment time (usually the next day) and the location of the drive-through outpatient clinic. In addition to these participants with their spontaneous willingness to take the test, the public health center also recruited participants who had been confirmed to have contacted COVID-19 patients based on contact tracing by announcing them with phone calls. Upon occurrence of cluster events, the drive-through testing center performed screening test for the individuals with suspected contact histories. Pregnant women without certain contact history who wanted to be checked for COVID-19 were also recruited.
The testing schedule was planned with 10-20 individuals per hour to prevent long queues of waiting cars that could hamper the traffic flow and increase the risk of intrafamilial infection by being on board the same car (Shah et al. 2020). Then, the callers visited the clinic by car (single person or family), bus (clusters in dormitories or nursing homes), or bicycles (if the caller does not own a car). The major reason behind the callers taking the nasopharyngeal swab test was the possible history of contact with COVID-19 patients, whereas the other participants were tested because of the presence of suspicious symptoms such as fever or cough. None of the 326 pregnant women who underwent nasopharyngeal RT-PCR swab tests at our outpatient clinic had a history of preceding contact with COVID-19 patients.
The actual sampling scenes at the testing site are shown in Fig. 2. At the testing site, a nasopharyngeal swab test was performed by the testers (doctors) over the half-opened car window, with the tested individuals inside the car with the engine turned off. All medical staff wore protective clothing, face shields, and N95 masks. The testers wore two-layered disposable latex hand gloves and changed the outer gloves after each sampling to avoid contamination or iatrogenic spread of the infection. The swab test sample at the clinic was transferred swiftly to the local government-run public health centers for real-time reverse transcription-polymerase chain reaction (RT-PCR) testing, the mainstay of presently used tests for detecting SARS-CoV-2 viral RNA (Zitek 2020). The next day, the individuals were notified of the results of their RT-PCR test by a phone call, while providing the necessary guidance and advice to those who tested positive for infection with the virus.
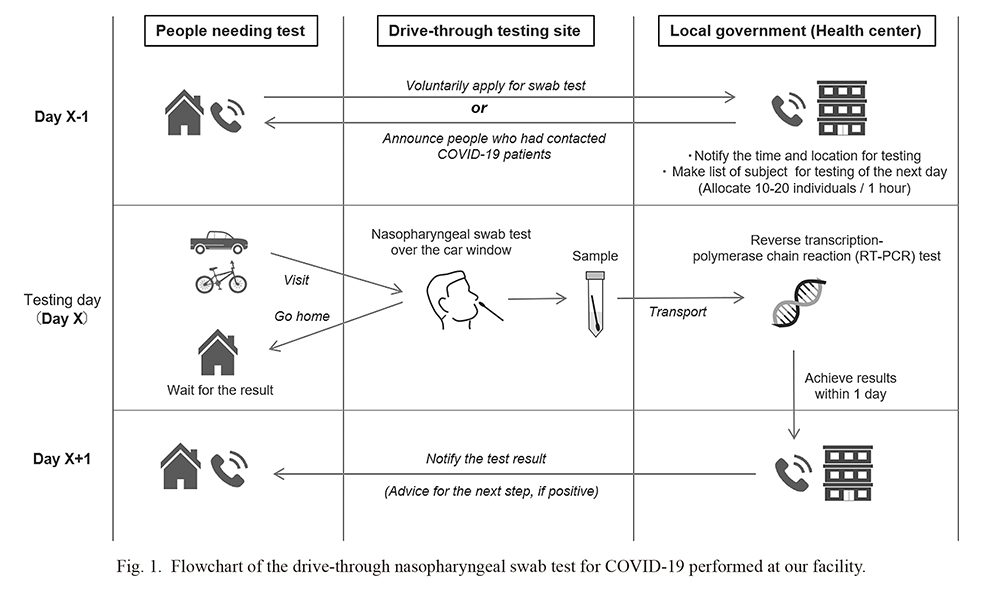
In this study, we enrolled all consecutive subjects who underwent drive-through nasopharyngeal swab testing at our outpatient clinic since July 16, 2020 (i.e., the day when we started to collect comprehensive testing related data including the close contact history), for whom complete data related to sex, age, contact history with COVID-19 patients, body temperature, and presence of cough and dyspnea were available at the outpatient clinic. The history of “close contact” was defined by the following four criteria: (1) contact with a COVID-19 patient 2 days before the onset of symptoms, (2) not wearing masks, (3) less than 1 meter, and (4) more than 15 min of contact.
Real-time RT-PCR
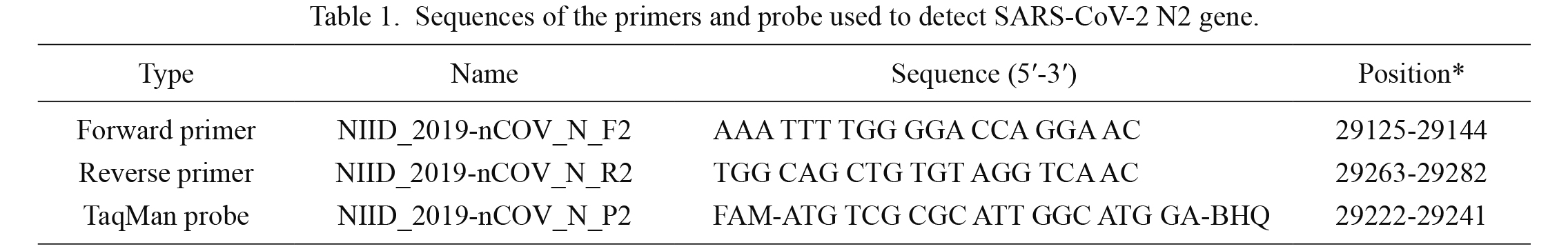
To detect SARS-CoV-2 viral RNA, real-time RT-PCR for detecting the nucleocapsid protein set no. 2 (N2) gene was performed using a protocol reported by the National Institute of Infectious Diseases in Japan (NIID) (Shirato et al. 2020). The reaction mixture comprised 4× TaqMan Fast Virus 1-Step Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), primer/probe set for N2 detection designed by NIID, and nuclease-free water. Then, the nucleic acid sample was added to the reaction mixture. The thermal cycling conditions used were as follows: 50°C for 5 min (reverse transcription), 95°C for 20 s (initial denaturation), followed by 45 cycles of 95°C for 3 s (denaturation) and 60°C for 30 s (annealing and extension). Sequences of the primers and probe containing a 5′-6-carboxyfluorescein (FAM) and a 3′- black hole quencher (BHQ) reporter dyes used to detect the N2 region of the nucleocapsid gene in this study are listed in Table 1.
Statistical analyses
The quantitative variables between the two groups were compared using either Student’s t-test or Mann-Whitney U-test according to the distribution patterns of the variables. Categorical variables were compared between the two groups using the chi-square test or Fisher’s exact test according to the sample size in each cell. In the multivariate analysis to identify the significant predictors of SARS-CoV-2 RT-PCR positivity, binary logistic regression analysis was performed. In the multivariate analysis, the RT-PCR test result was used as the dependent variable, and other variables of particular clinical interest or those having a significant impact (p < 0.10) on the subsequent positive result for COVID-19 in the univariate analysis were used as the independent variables. P values less than 0.05 were considered statistically significant in this study. Statistical analyses were performed using IBM SPSS Statistics 22.0 (IBM Corp., USA).
Ethics
This study was approved by the Institutional Review Board of Tohoku University Graduate School of Medicine (IRB approval number: 2020-1-535). All the procedures in this study were performed in accordance with the ethical principles outlined in the current version of the Declaration of Helsinki.
Results
Demographic and clinical data stratified by SARS-CoV-2 positivity
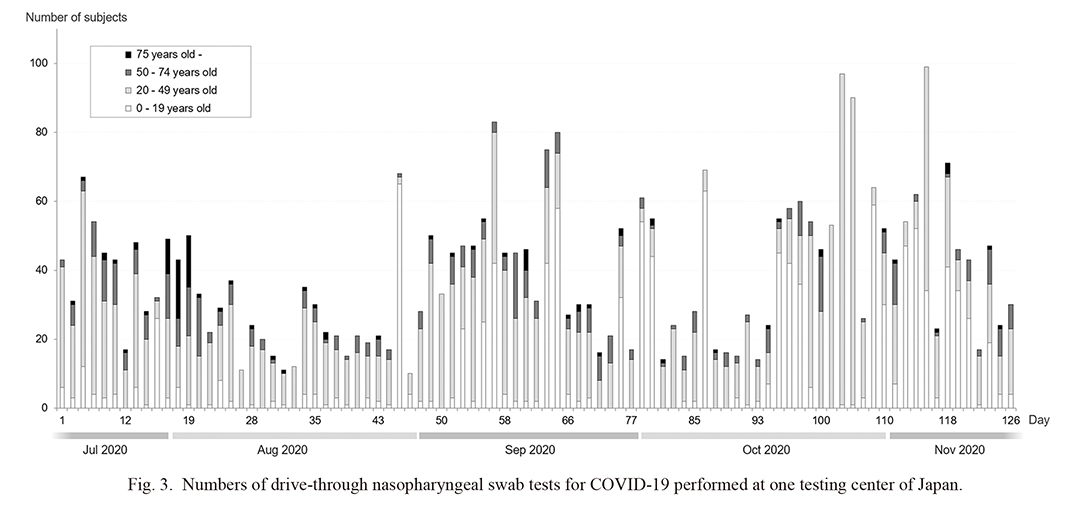
A total of 3,540 consecutive participants who underwent nasopharyngeal RT-PCR swab tests at our drive-through outpatient clinic were enrolled in this study. Complete eligible data were available for all the participants. The number of participants tested each day by age group is shown in Fig. 3.
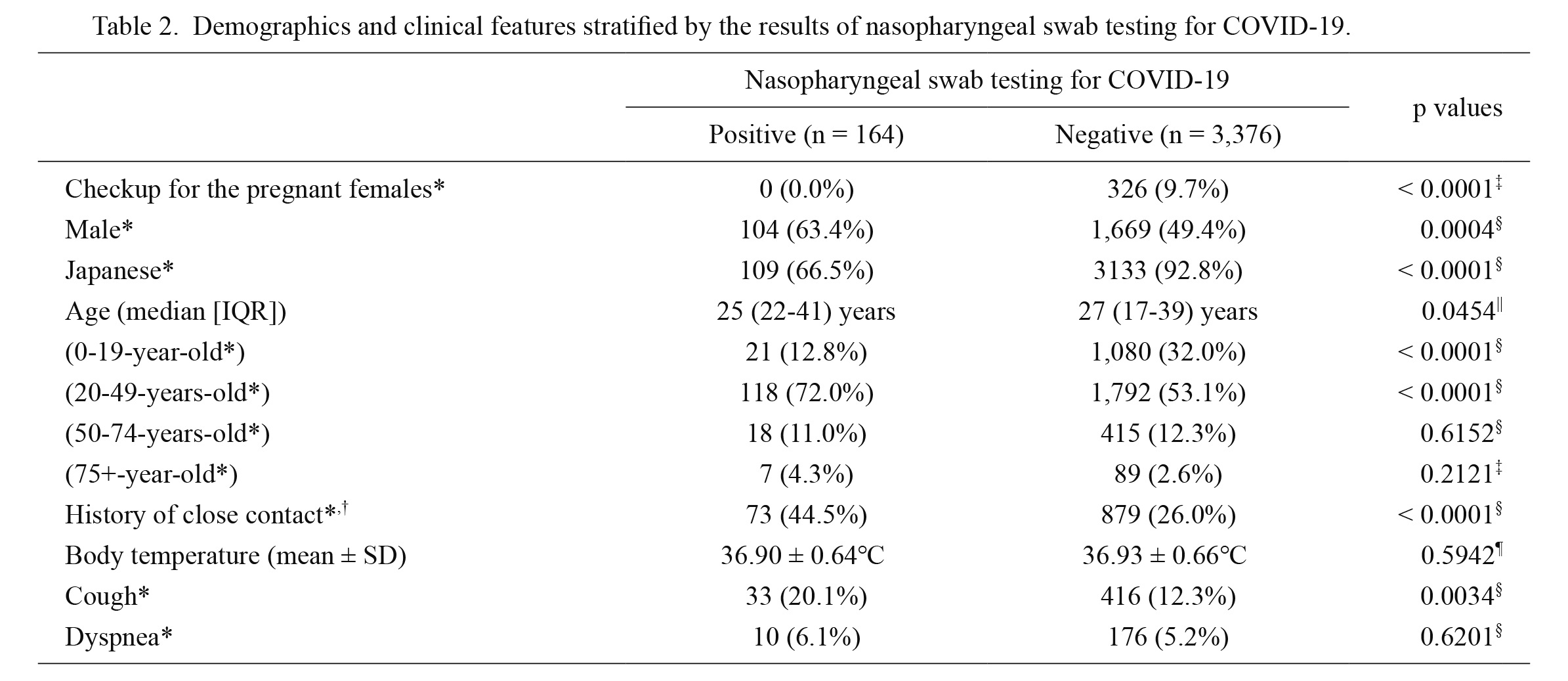
The demographic and clinical data of the participants stratified by the SARS-CoV-2 test results are listed in Table 2. More than 80% of the participants were below 50 years. Male (p = 0.0004), non-native ethnicity (p < 0.0001), age between 20-49 years (p < 0.0001), history of close contact (p < 0.0001), and the presence of cough at the time of testing (p = 0.0034) were significant predictors of SARS-CoV-2 test positivity in the univariate analyses. Among these predictors, male predominance can be attributed to the large cluster outbreak at an industrial vocational training school for foreign students in Sendai city during day 99-106 of the testing program. This cohort of cluster outbreak involved 284 foreign students (257 males and 27 females) who were tested in our testing center, and 53 of them tested positive for SARS-CoV-2. Upon excluding these 284 cases of this cluster outbreak, the male predominance disappeared (male: 53/111 [47.7%] in the test-positive group vs. 1,463/3,145 [46.5%] in the test-negative group; p = 0.7986). Importantly, the high prevalence of non-native residents among those with positive results for COVID-19 was also strengthened by this large cluster outbreak in the vocational school for foreign students. After removing the 284 students from the analysis, the observed tendency for non-native predominance disappeared (prevalence of non-native participants: 1.8% in the test-positive group vs. 0.4% in the negative group; p = 0.0798). In addition, after removing the 284 foreign students, the prevalence of participants of 20-49 years was no longer a significant predictor for SARS-CoV-2 test positivity (rate of participants between 20 and 49 years: 58.6% in the test-positive group vs. 49.7% in test-negative group; p = 0.0665). None of the 326 pregnant women (none had a preceding history of contact with COVID-19 patients) who were tested in our drive-through clinic were positive for SARS-CoV-2.
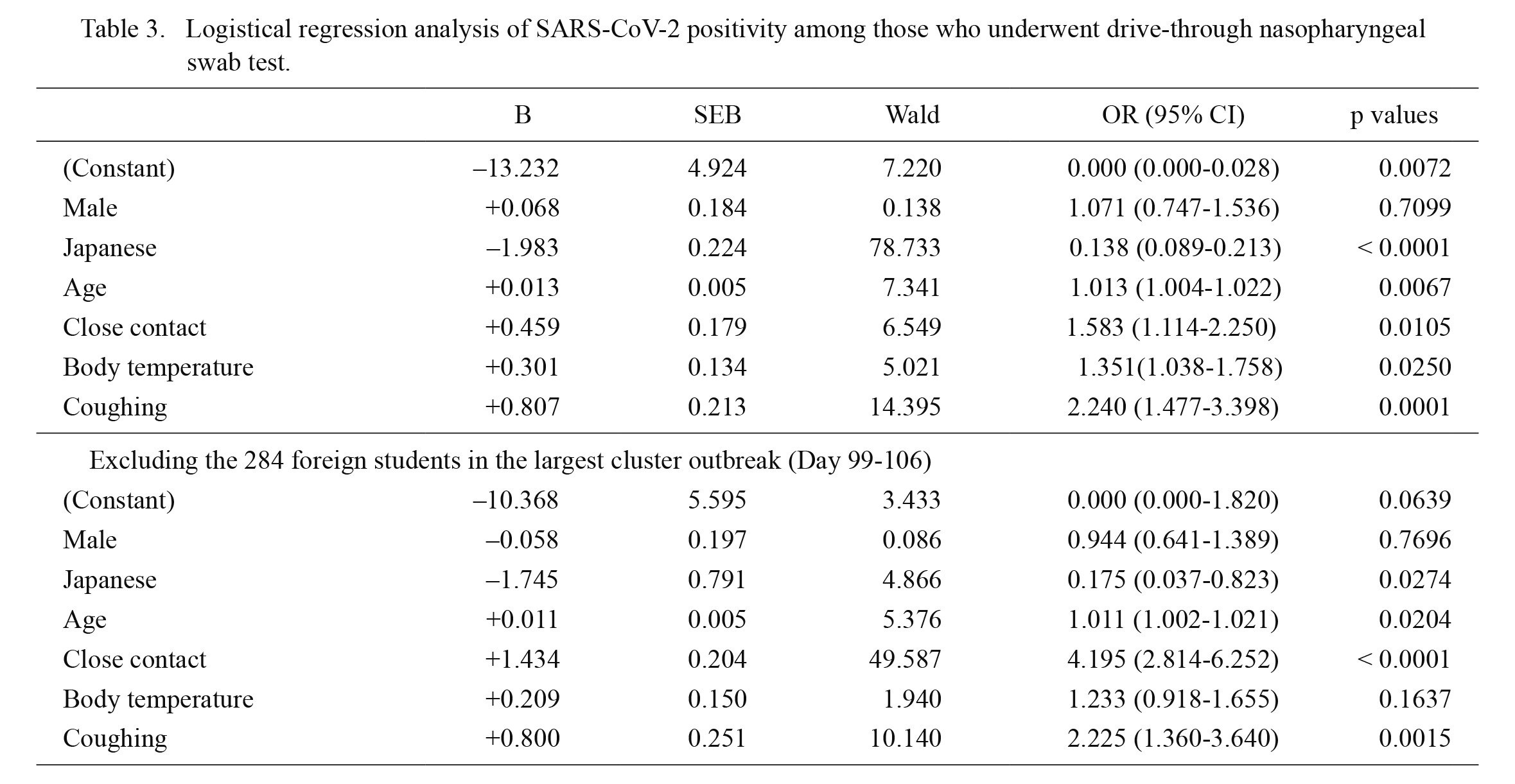
Based on the results of the univariate analyses, we performed a multivariate analysis with binary logistic regression to predict SARS-CoV-2 test positivity by employing variables of particular clinical interest or with p values < 0.10 as independent variables. Age and body temperature were both used as numeric variables and not as ordinal variables. The results of the multivariate analysis are shown in the first half of Table 3. Male predominance disappeared in the multivariate analysis, whereas other variables (namely foreign nationality, age above 19 years, close contact history, body temperature, and cough symptoms) showed statistical significance in predicting SARS-CoV-2 test positivity.
Since the results could have been significantly biased by the largest cluster outbreak in the vocational school for foreign students, we also performed a binary logistic regression analysis after excluding the 284 foreign students involved in the cluster outbreak. The results after excluding the data from the largest cluster outbreak are shown in the second half of Table 3. The significance of foreign nationality and age above 19 years was significantly weakened, whereas that of body temperature disappeared. Meanwhile, the significance of close contact history with COVID-19 patients was further strengthened by excluding the cluster outbreak.
Details of 164 COVID-19 positive patients
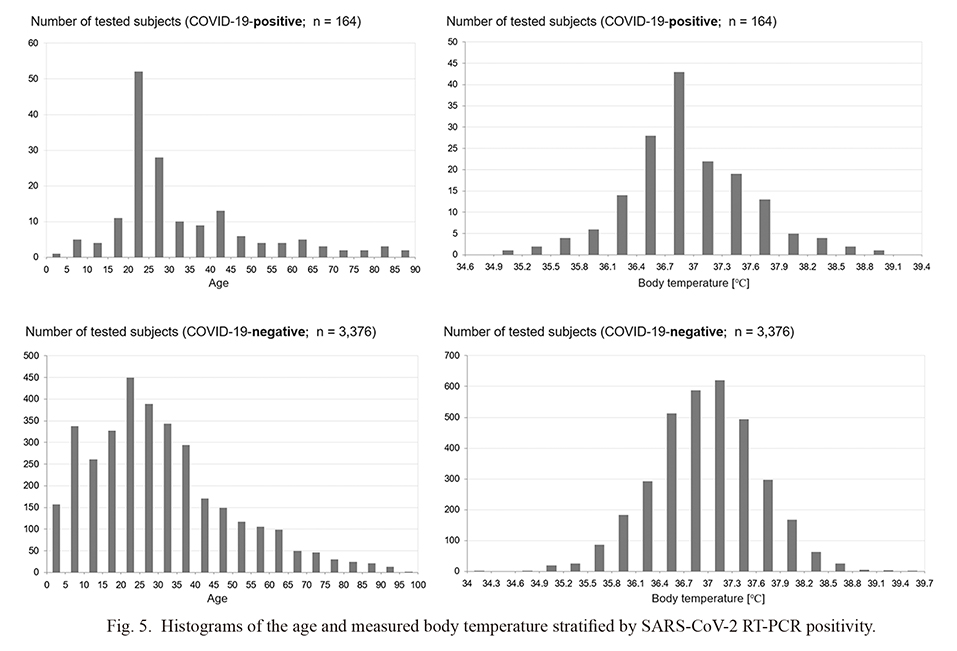
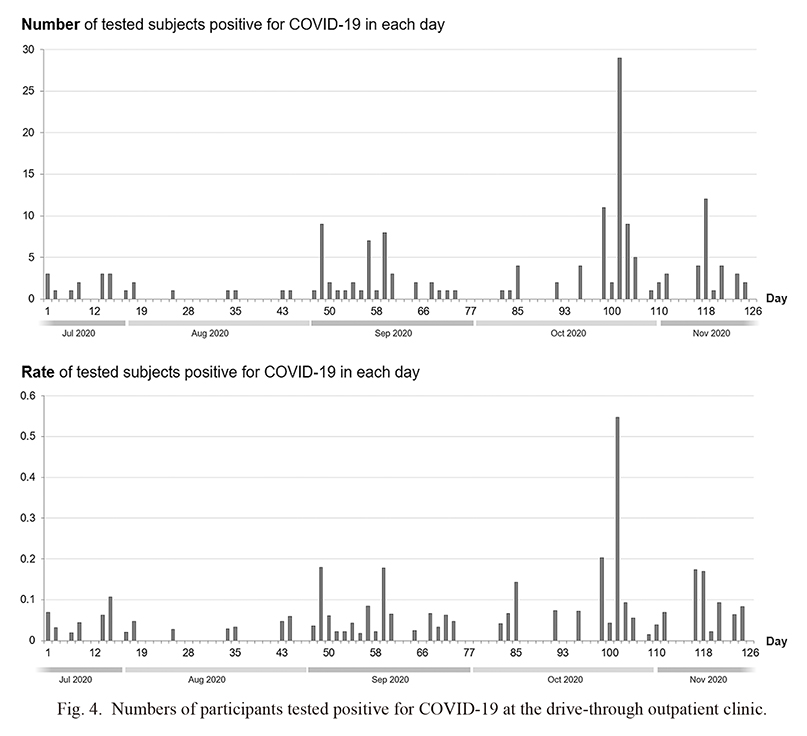
First, the changes in the number and rate of COVID-19-positive cases on each day are shown in Fig. 4. We could demonstrate at least six apparent independent cluster occurrences, with the largest occurring around day 103 (i.e., the aforementioned cluster outbreak in a vocational school for foreign students). Next, the histograms of the age and body temperature measured at the drive-through outpatient clinic of the COVID-19-positive cases, together with those of the COVID-19-negative cases, are shown in Fig. 5. The age distribution of COVID-19-positive patients who visited our outpatient clinic was partly similar to the peak (i.e., 20-25 years) age of the COVID-19-negative participants who visited the clinic. The distribution of body temperature was not significantly different between those with COVID-19-positive and COVID-19-negative cases. It should be noted that since the body temperature was measured outdoor using a non-contact thermometer, the measured values could be different from those obtained indoor using a contact thermometer.
Discussion
Here, we present an overview of the drive-through nasopharyngeal RT-PCR swab test for COVID-19 performed at our drive-through outpatient clinic. We also evaluated the demographic and clinical data collected at the clinic to determine factors that could predict positive test results for COVID-19. To the best of our knowledge, this is the first report on the current status of a drive-through testing site for COVID-19 with the achieved test results. The results provide several important insights that could be useful for operating drive-through testing centers in other countries. First, the number of testing participants largely fluctuated each day as shown in Fig. 3, suggesting that the required minimum number of medical staff can be different on each day. Another notable finding was that none of the 326 pregnant women (none had contact history with COVID-19 patients) who underwent medical checkups were positive for COVID-19. Performing swab tests for all pregnant women without a contact history or suspicious symptoms may have a limited role in the present pandemic situation in Japan. This study also demonstrated the validity and usefulness of checking for a history of close contact with COVID-19 patients. Regardless of the presence of suspicious symptoms, individuals with close contact with COVID-19 patients should preferably undergo testing for the virus as the government presently recommends.
A limitation of this study is that it was based on data from a single testing site in Japan. Consequently, the generalizability of our results is limited. Similar reports from other countries are needed to confirm the validity of this study. Another limitation is that information about dysgeusia was not collected at the testing site. The clinical importance of dysgeusia as a predictor of COVID-19-positivity needs to be evaluated.
In conclusion, the obtained data of drive-through nasopharyngeal swab RT-PCR test show that close contact history and cough symptoms are significant predictors of SARS-CoV-2 test positivity. Individuals with these predisposing factors should preferentially undergo testing for SARS-CoV-2 viral RNA.
Acknowledgments
The authors deeply thank all medical staffs and local government staffs (Sendai City, Miyagi Prefecture) who joined and cooperated to this drive-through RT-PCR testing project. Doctors from the Departments of General Medicine, Kampo Medicine, Dentistry, Otolaryngology-Head and Neck Surgery, Pediatrics, Emergency and Critical Care Medicine, Hematology and Rheumatology, Psychiatry, and Geriatric Medicine and Neuroimaging of Tohoku University Graduate School of Medicine, Disaster Medical Science Division of the International Research Institute of Disaster Science (IRIDeS) of Tohoku University, and Graduate Medical Education Center of Tohoku University Hospital contributed to collect the samples. Co-medical staffs from the Nursing Department, Infection Control Office, Pharmaceutical Department, Clinical Technology Department, Administration Department, Medical Engineering Center, and Community Medical Cooperation Center of the Tohoku University Hospital contributed to manage and support the operation of this project. Also, we thank Dr. Shinichi Fukushige (Tohoku University) for reviewing the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Fauci,
A.S.,
Lane,
H.C. &
Redfield,
R.R.
(2020) Covid-19: navigating the uncharted. N. Engl. J. Med., 382, 1268-1269.
-
Frieden,
T.R. &
Lee,
C.T.
(2020) Identifying and interrupting superspreading events-implications for control of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis., 26, 1059-1066.
-
Furuse,
Y.,
Sando,
E.,
Tsuchiya,
N.,
Miyahara,
R.,
Yasuda,
I.,
Ko,
Y.K.,
Saito,
M.,
Morimoto,
K.,
Imamura,
T.,
Shobugawa,
Y.,
Nagata,
S.,
Jindai,
K.,
Imamura,
T.,
Sunagawa,
T.,
Suzuki,
M., et al.
(2020) Clusters of coronavirus disease in communities, Japan, January-April 2020. Emerg. Infect. Dis., 26, 2176-2179.
-
Gates,
B.
(2020) Responding to Covid-19: a once-in-a-century pandemic?. N. Engl. J. Med., 382, 1677-1679.
-
Lu,
D.,
Wang,
H.,
Yu,
R.,
Yang,
H. &
Zhao,
Y.
(2020) Integrated infection control strategy to minimize nosocomial infection of coronavirus disease 2019 among ENT healthcare workers. J. Hosp. Infect., 104, 454-455.
-
Mizumoto,
K.,
Kagaya,
K.,
Zarebski,
A. &
Chowell,
G.
(2020) Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill., 25, 2000180.
-
Moriarty,
L.F.,
Plucinski,
M.M.,
Marston,
B.J.,
Kurbatova,
E.V.,
Knust,
B.,
Murray,
E.L.,
Pesik,
N.,
Rose,
D.,
Fitter,
D.,
Kobayashi,
M.,
Toda,
M.,
Cantey,
P.T.,
Scheuer,
T.,
Halsey,
E.S.,
Cohen,
N.J., et al.
(2020) Public health responses to COVID-19 outbreaks on cruise ships: worldwide, February-March 2020. MMWR. Morb. Mortal. Wkly. Rep., 69, 347-352.
-
Shah,
A.,
Challener,
D.,
Tande,
A.J.,
Mahmood,
M.,
O’Horo,
J.C.,
Berbari,
E. &
Crane,
S.J.
(2020) Drive-through testing: a unique, efficient method of collecting large volume of specimens during the SARS-CoV-2 (COVID-19) pandemic. Mayo Clin. Proc., 95, 1420-1425.
-
Shirato,
K.,
Nao,
N.,
Katano,
H.,
Takayama,
I.,
Saito,
S.,
Kato,
F.,
Katoh,
H.,
Sakata,
M.,
Nakatsu,
Y.,
Mori,
Y.,
Kageyama,
T.,
Matsuyama,
S. &
Takeda,
M.
(2020) Development of genetic diagnostic methods for detection for novel coronavirus 2019(nCoV-2019) in Japan. Jpn. J. Infect. Dis., 73, 304-307.
-
Wee,
L.E.,
Conceicao,
E.P.,
Sim,
X.Y.J.,
Aung,
M.K.,
Tan,
K.Y.,
Wong,
H.M.,
Wijaya,
L.,
Tan,
B.H.,
Ling,
M.L. &
Venkatachalam,
I.
(2020) Minimizing intra-hospital transmission of COVID-19: the role of social distancing. J. Hosp. Infect., 105, 113-115.
-
Zitek,
T.
(2020) The appropriate use of testing for COVID-19. West. J. Emerg. Med., 21, 470-472.