Abstract
Mesenteric panniculitis is a chronic inflammatory disease characterized by non-specific inflammation of the adipose tissue in the mesentery. Hemophagocytic lymphohistiocytosis is a life-threating disease associated with aberrant macrophage overactivation, in which infections can be a leading cause in immunocompromised hosts. Here, we report a rare case of mesenteric panniculitis and hemophagocytic lymphohistiocytosis complicated by disseminated Mycobacterium intracellulare. A 71-year-old male with mesenteric panniculitis was admitted to our hospital for fever and pancytopenia. He was treated with oral prednisolone (15 mg/day) and cyclosporin A (150 mg/day) at presentation. Physical and laboratory examinations revealed disseminated infection with nontuberculous mycobacteria; Mycobacterium intracellulare was detected in cultures of cerebrospinal fluid, blood, sputum, and gastric fluid. Patient signs and symptoms fulfilled the five criteria for a diagnosis of hemophagocytic lymphohistiocytosis, including fever, cytopenia, hemophagocytosis, hyperferritinemia, and high soluble interleukin-2 receptor levels. Therefore, the diagnosis of nontuberculous mycobacteria-associated hemophagocytic lymphohistiocytosis was established. An anti-mycobacterial chemotherapy including chloramphenicol (800 mg/day), rifampin (450 mg/day) and ethambutol (750 mg/day) together with streptomycin (750 mg twice per week) was initiated at 30 days after admission; maintenance doses of prednisolone were increased to 60 mg/day. Fever and pancytopenia improved in response to anti-mycobacterial chemotherapy. The present case suggests that mesenteric panniculitis could be complicated with hemophagocytic lymphohistiocytosis caused by immunosuppressive therapy-associated infections as well as underlying disease activity. In conclusion, the possibility of disseminated nontuberculous mycobacteria infection with hemophagocytic lymphohistiocytosis should be considered if unexplained fever or hematological dyscrasia were presented in patients of mesenteric panniculitis.
Introduction
Mesenteric panniculitis (MP) is a chronic inflammatory disease characterized by non-specific inflammation of the adipose tissue of the mesentery (Issa and Baydoun 2009). The main symptoms of MP are fever, non-specific abdominal complaints, and malaise, however, some patients are asymptomatic at diagnosis (Araújo-Filho et al. 2016). Although specific treatment modalities have not been established, immunosuppressive therapies including glucocorticoids and immunosuppressants can be effective for promoting resolution of this condition (Bala et al. 2001).
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening syndrome characterized by intense immune activation (George 2014). Patients with HLH typically present with clinical signs and symptoms that include fever, cytopenia, hepatosplenomegaly, and hyperferritinemia (La Rosée et al. 2019). Infections, autoinflammatory and autoimmune diseases, as well as malignancies and acquired immune deficiency syndrome have all been associated with the development of HLH (La Rosée et al. 2019). Interestingly, HLH itself rarely results from infections other than those caused by viruses.
Disseminated nontuberculous mycobacteria (NTM) infections may develop in severely immunocompromised patients (Saleeb and Olivier 2010). While disseminated NTM infections were previously common among patients infected with human immunodeficiency virus (HIV), the incidence of these infections has recently increased among patients undergoing therapeutic immunosuppression (Skogberg et al. 1995). Because of a rare condition, only a few reports documenting associations between NTM infection and HLH have been described. Both HLH and disseminated NTM infections can be fatal, prompt diagnosis is mandatory for improving patient’s outcome, especially those are endogenously or therapeutically immunosuppressed (Shi and Jiao 2019). Of note, there is currently no documented relationship between MP and HLH, although HLH can develop in association with panniculitis characteristic of Weber-Christian disease (WCD) (Schettert et al. 1997). WCD is a systemic inflammatory disease accompanied by relapsing panniculitis, fever, lipoatrophy, and lipophagia (White and Winkelmann 1998), which in part resembles with MP manifestations. Here, we report a case of MP complicated with disseminated NTM with HLH.
Case Presentation
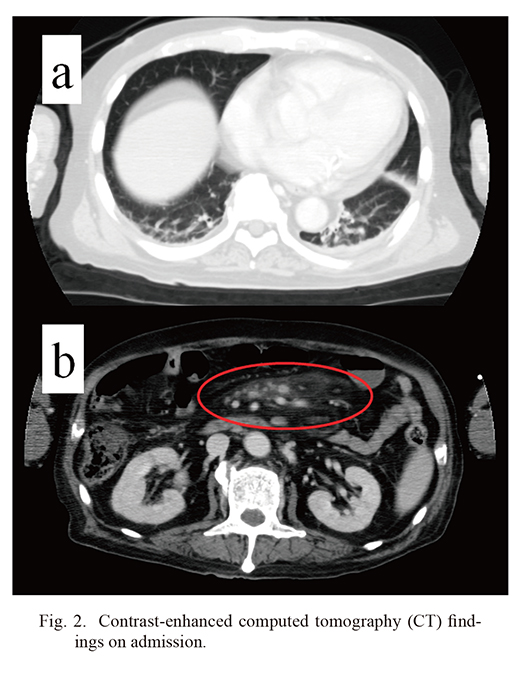
A 71-year-old Japanese man previously diagnosed with MP was referred to our hospital for further examination of back pain. The patient was diagnosed with MP one year prior to this admission. The first manifestations of this disorder were fever, nausea and abdominal pain. A computed tomography (CT) scan revealed intraabdominal lymphadenopathy with increased density of the mesenteric fat. MP was diagnosed based on the pathological findings that included intraabdominal lymph node and mesenteric adipose tissue, which showed panniculitis with histocytes and lymphocytes infiltration in the absence of neoplastic lymphocytes with nuclear hyperchromasia or karyorrhexis (Fig. 1a, b). Plasma cell infiltration was not remarkable and hyalinized vessels were not observed. Immunohistochemically, lymphocytes were mainly stained for CD3, CD8, granzyme B; in contrast, they were not stained for CD4, CD20 and T-cell intracellular antigen 1 (TIA-1). A few lymphocytes were stained for both CD3 and CD4. CD68-positive histiocytes were also scattered as shown in Fig. 1c. Although Castleman’s disease was considered because of lymphadenopathy, the specific histopathological findings seen in Castleman’s disease such as hyaline-vascular type and plasma cell type were not observed (Yu et al. 2017). Therapy was initiated with oral prednisolone (PSL) at 30 mg/day. Although findings on abdominal CT did not resolve completely, his overall condition improved by immunosuppressive therapy. One year later, he was admitted to a local hospital for management of back pain related to lumbar spinal canal stenosis. At presentation, he received oral PSL (15 mg/day) and cyclosporin A (CyA; 150 mg/day) for two months. Shortly after admission, he developed fever and neutropenia. Antibiotics were administered and CyA was discontinued; however, these strategies proved to be ineffective. The patient was transferred to our hospital for further investigation. In addition to the one-year history of MP, his past medical history was notable for steroid-induced diabetes mellitus. On admission, physical examination revealed a body temperature of 39.0°C, blood pressure at 99/67 mmHg, and a pulse rate of 86/min. Chest auscultation revealed neither heart murmurs nor crackles. Physical examination revealed back pain only. Results of peripheral blood cell counts and serum biochemistries are described in Table 1. They include reduced white blood cell count of 1,700/μL (reference range, 3,300-8,600/μL) (75% neutrophils, 17% lymphocytes, 5% monocytes, 0% eosinophils, and 0% basophils), hemoglobin (7.3 g/dL; reference range, 13.7-16.8 g/dL), and platelet count (98,000/μL; reference range, 158,000-348,000/μL), elevated serum C-reactive protein (5.48 mg/dL; reference range, < 0.3 mg/dL) and erythrocyte sedimentation rate (39 mm/h; reference range, < 10 mm/h), respectively. Lactate dehydrogenase (183 IU/L; reference range, 124-222 IU/L), aspartate aminotransferase (14 IU/L; reference range, 13-30 IU/L), alanine aminotransferase (12 IU/L; reference range, 7-23 IU/L), alkaline phosphatase (194 IU/L; reference range, 106-322 IU/L). creatine kinase (5 IU/L; reference range, 41-153 IU/L), were normal. Serum aldolase (56.4 IU/L; reference range, 2.1-6.1 IU/L), blood urea nitrogen (34 mg/dL; reference range, 8.0-22.0 mg/dL), serum creatinine (0.81 mg/dL; reference range, 0.47-0.79 mg/dL), and soluble interleukin-2 receptor (sIL-2R) (5,760 IU/L; reference range, 122-496 IU/mL) levels were also increased. Additional tests revealed high levels of serum ferritin (1121 ng/mL; reference range 12-60 ng/mL); a cytomegalovirus antigenemia test (C7-HRP) was negative. A full evaluation of connective tissue-associated pathology was also negative. Chest and abdominal contrast-enhanced CT revealed linear and reticular shadows bilaterally in the lower lung fields and increased density of the mesenteric fat (Fig. 2). Blood cultures performed at this time were negative for bacteria. As laboratory tests revealed pancytopenia together with elevated levels of serum sIL-2R and ferritin, we performed bone marrow aspiration. Bone marrow was notable for hypoplastic marrow and prominent infiltration with hemophagocytes (Fig. 3a). The diagnosis of HLH is based on the presence of at least five of eight clinical signs, including fever, splenomegaly, pancytopenia, hypertriglyceridemia, hemophagocytosis, low/absent NK cell activity, hyperferritinemia, and high serum levels of sIL-2R (Henter et al. 2007). This case fulfilled five of these diagnostic criteria, therefore a diagnosis of HLH was established.
We administered increased dose of PSL to 30 mg/day and resumed treatment with CyA at 100 mg/day. For his moderate grade of neutropenia, prophylactic antibiotics were also administered. In spite of improvement of his clinical symptoms, his cognitive function gradually declined; as such, an evaluation of his cerebrospinal fluid (CSF) test was performed. Ziehl-Neelsen staining revealed the presence of mycobacteria in the CSF; further testing with polymerase chain reaction (PCR) identified the microorganism of NTM, M. intracellulare. Further testing resulted in positive for Ziehl-Neelsen staining of microorganisms in blood, urine, bone marrow, and gastric fluid. PCR testing of these samples documented infection with M. intracellulare (Fig. 3b). The patient was diagnosed with disseminated NTM (M. intracellulare) infection. Later, mycobacterial blood cultures were noted to be positive for M. intracellulare. Therefore, anti-mycobacterial chemotherapy was initiated at 30 days after admission; the drug regimen included clarithromycin (800 mg/day), rifampicin (450 mg/day), ethambutol (750 mg/day) and together with streptomycin (750 mg two times per week). PSL doses were increased up to 60 mg/day. Although abdominal CT findings of MP was not completely improved, all his symptoms such as fever and pancytopenia gradually disappeared after treatment. During PSL tapering, he developed bacterial pneumonia (Escherichia coli) and received antibiotics for treatment (Fig. 4). At 130 days after admission, he was transferred to another hospital to continue long-term antimicrobial treatment.
Informed consent was obtained from the patient.
Discussion
HLH is associated with infections, malignancy, autoimmune and inflammatory disorders (Larroche and Mouthon 2004) (Emile et al. 2016). Although the most predisposing disease of HLH is T-cell lymphoma and B-cell lymphoma, other important predisposing diseases are systemic lupus erythematosus, adult-onset Still’s disease (AOSD), and viral infections (Ramos-Casals et al. 2014; Rivière et al. 2014). Currently, there are only a few reports of MP associated with HLH; as such, there is no consensus with respect to appropriate management of these patients. Although infections are believed to be among the most common causes of this disorder (George 2014), NTM is a rare cause of secondary HLH (Yang et al. 2003; Shi and Jiao 2019). In our case, his condition resolved in response to treatment with antibiotics and immunosuppressive agents. Epstein-Barr virus and CMV are the pathogens most frequently associated with HLH (Fisman 2000; Rouphael et al. 2007), nevertheless, our findings reveal that NTM infections may elicit similar pathogenesis. As shown here, disseminated infection with M. intracellulare may also result in HLH, particularly in patients undergoing immunosuppressive therapy. Disseminated NTM infections develop primarily in severely immunocompromised patients (Tortoli 2009). Although HIV-infection and HIV-associated immunodeficiencies are the best known risk factors associated with disseminated NTM infections, the frequency of disseminated NTM infections is currently on the rise among patients who are therapeutically immunosuppressed (Skogberg et al. 1995). Patients with disseminated NTM infections typically present with fever as an initial symptom; the overall mortality rates from this infection are approximately 30% (Chou et al. 2011). In our case, M. intracellulare was detected in gastric fluid and sputum cultures in addition to CSF and blood. NTM infections in immunocompetent patients are typically detected as localized pulmonary infections and develop in those with underlying pulmonary diseases. A chest CT performed on our patient revealed fine reticular shadows. M. intracellulare most likely invades via the respiratory tract and ultimately results in disseminated infection (Torriani et al. 1994). By contrast, the entry of MAC to blood via lymph node through gastrointestinal tract was suggested by autopsy findings in acquired immune deficiency syndrome (AIDS) patients (Torriani et al. 1996). We have additionally performed Ziehl-Neelsen staining of lymph node for diagnosis of MP, but the result was negative. Mechanisms proposed to explain central nervous system (CNS) involvement include dissemination through bloodstream rather than direct extension from the respiratory tract or gastrointestinal tract to the CNS (Torriani et al. 1994, 1996). Given the comparatively slow growth of these microorganisms, positive CSF and blood cultures might take time to develop. Therefore, acid-fast staining of bone marrow biopsy might be used as an early indicator of disseminated NTM.
NTM-associated HLH has been reported in patients with disseminated or extra-pulmonary NTM infections; these conditions are associated with 27% mortality (Shi and Jiao 2019). As such, a multidisciplinary team including microbiologists and infectious disease specialists should be consulted with the goal of identifying optimal management strategies for these conditions (Akram and Attia 2020). While infections have been implicated as triggers of this disorder, the incidence of NTM-associated HLH appears to be relatively uncommon. Although disseminated NTM may contribute to the development of HLH, the syndrome primarily involves dysregulation of innate immune cells, notably macrophages (Holland 1996), which then results in generalized increased production of proinflammatory cytokines. As such, endogenous factors could be related to the development of HLH, notably in patients with connective tissue disorders. Previous studies have revealed that systemic lupus erythematosus and systemic juvenile idiopathic arthritis predominate among the rheumatic diseases that may be complicated with HLH (Fukaya et al. 2008). Likewise, MP, a syndrome that features proliferation of histiocytes and lymphocytes in the mesentery, has often been associated with systemic macrophage activation syndrome (Tavares Pereira et al. 2016). It is certainly possible that the immunological features characteristic of MP may also promote the development of HLH, in addition to factors associated with immunosuppression-related disseminated NTM infection.
MP, also known as sclerosing mesenteritis, belongs to a spectrum of diseases that target the fatty tissue of mesentery (Araújo-Filho et al. 2016). MP is characterized by chronic inflammation of the adipose tissue in the mesentery. There is a significant clinical overlap between this disease and entities that include the mesenteric manifestations of cytophagic histiocytosis (Cheah et al. 1992).
MP can be associated with T-cell lymphoma, gastric carcinoma, WCD and rheumatic disease such as systemic lupus erythematosus (Dor et al. 1982; Issa and Baydoun 2009). In our case, the classification criteria for rheumatic diseases were not fulfilled and the gastric cancer was denied in the previous hospital. The most important differential diagnosis of the present case is panniculitis-like T-cell lymphoma and WCD since these diseases cause MP as well as HLH (White and Winkelmann 1989; Wang et al. 2013; Hrudka et al. 2019). Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is an unusual type of non-Hodgkin lymphoma that usually develops the nodules within subcutaneous adipose tissue (Lester et al. 2015) and SPTCL is sometimes cause mesenteric mass and HLH (Lester et al. 2015; Hrudka et al. 2019). Histopathological findings typically reveal subcutaneous infiltrates simulating a panniculitis showing small, medium-sized, or sometimes large pleomorphic T cells with hyperchromatic nuclei and often with many macrophages (Willemze et al. 2005). Necrosis, karyorrhexis, and hemophagocytosis are commonly observed (Willemze et al. 2005). Immunohistochemically, these lymphomas show a α/β+, CD3+, CD4-, CD8+ T-cell phenotype, with expression of cytotoxic proteins (Willemze et al. 2005). However, SPTCL was unlikely in our case because there were no skin symptoms and no specific pathological findings of SPTCL were observed, such as a pattern of isolated adipocytes surrounding by a rim of abnormal lymphocytes with hyperchromatic irregular nuclei (“rimming”) in the fat tissues. Furthermore, this case was successfully treated with anti-mycobacterial treatment without deterioration of symptoms. On the other hand, WCD is an inflammatory disease involved in panniculitis, idiopathic nodular panniculitis, which is characterized by subcutaneous nodules, inflammatory cells in the fat nodules, and systemic symptoms including inflammation of mesentery fatty tissue (White and Winkelmann 1998). WCD can be accompanied by cytophagic histiocytic panniculitis and HLH (White and Winkelmann 1989). While our patient manifested no skin lesions suggesting WCD, the diagnosis of cytophagic histiocytic panniculitis due to WCD may be challenging, even though biopsies of the mesenteric lymphoid tissue revealed predominant histiocyte infiltration in addition to panniculitis. Likewise, the development of NTM-associated hemophagocytosis is also rare; the underlying biological features such as the activated innate immunity can contribute to the emergence of this rare complication and to the clinical course observed here. Cytophagic histiocytosis with mesenteric involvement had been reported in patients with HLH (Behrens et al. 2006); as such, HLH may develop in a subset of patients presenting with MP and associated disorders. HLH observed in this case may be derived from a combination of immune dysregulation together with responses toward systemic NTM infection.
In conclusion, we experienced a rare case of MP associated with disseminated NTM-associated HLH. To the best of our knowledge, this is the first report of this extremely rare combination, especially, MP that develops as a complication of HLH with disseminated NTM. Further accumulation of such cases is needed to clarify the pathogenesis of MP with HLH under NTM infection.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors are grateful to Enago (http://www.enago.jp) for the English language review.
References
-
Akram,
S.M. &
Attia,
F.N.
(2020) Mycobacterium avium intracellulare. In StatPearls, Treasure Island (FL).
-
Araújo-Filho,
I.,
Pinheiro,
F. &
Meneses Rêgo,
A.C.
(2016) Mesenteric panniculitis in the elderly: update on diagnostic and therapeutic approach. Int. J. Surg. Med., 2.
-
Bala,
A.,
Coderre,
S.P.,
Johnson,
D.R. &
Nayak,
V.
(2001) Treatment of sclerosing mesenteritis with corticosteroids and azathioprine. Can. J. Gastroenterol., 15, 533-535.
-
Behrens,
E.M.,
Kreiger,
P.A.,
Cherian,
S. &
Cron,
R.Q.
(2006) Interleukin 1 receptor antagonist to treat cytophagic histiocytic panniculitis with secondary hemophagocytic lymphohistiocytosis. J. Rheumatol., 33, 2081-2084.
-
Cheah,
P.L.,
Looi,
L.M.,
Tan,
P.E.,
Bosco,
J. &
Kuperan,
P.
(1992) Cytophagic histiocytic panniculitis: a diagnostic dilemma. Hematol. Oncol., 10, 331-337.
-
Chou,
C.H.,
Chen,
H.Y.,
Chen,
C.Y.,
Huang,
C.T.,
Lai,
C.C. &
Hsueh,
P.R.
(2011) Clinical features and outcomes of disseminated infections caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 2004-2008. Scand. J. Infect. Dis., 43, 8-14.
-
Dor,
A.M.,
Kohler,
J.L.,
Aubrespy,
P.,
Scheiner,
C.,
Pizzi,
M. &
Lebreuil,
G.
(1982) Pseudo-tumorous panniculitis of the mesentery. An unusual initial stage of acute lupus erythematosus in a 10-year-old girl. Sem. Hop., 58, 2847-2850.
-
Emile,
J.F.,
Abla,
O.,
Fraitag,
S.,
Horne,
A.,
Haroche,
J.,
Donadieu,
J.,
Requena-Caballero,
L.,
Jordan,
M.B.,
Abdel-Wahab,
O.,
Allen,
C.E.,
Charlotte,
F.,
Diamond,
E.L.,
Egeler,
R.M.,
Fischer,
A.,
Herrera,
J.G., et al.
(2016) Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood, 127, 2672-2681.
-
Fisman,
D.N.
(2000) Hemophagocytic syndromes and infection. Emerg. Infect. Dis., 6, 601-608.
-
Fukaya,
S.,
Yasuda,
S.,
Hashimoto,
T.,
Oku,
K.,
Kataoka,
H.,
Horita,
T.,
Atsumi,
T. &
Koike,
T.
(2008) Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: analysis of 30 cases. Rheumatology (Oxford), 47, 1686-1691.
-
George,
M.R.
(2014) Hemophagocytic lymphohistiocytosis: review of etiologies and management. J. Blood Med., 5, 69-86.
-
Henter,
J.I.,
Horne,
A.,
Aricó,
M.,
Egeler,
R.M.,
Filipovich,
A.H.,
Imashuku,
S.,
Ladisch,
S.,
McClain,
K.,
Webb,
D.,
Winiarski,
J. &
Janka,
G.
(2007) HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer, 48, 124-131.
-
Holland,
S.M.
(1996) Host defense against nontuberculous mycobacterial infections. Semin. Respir. Infect., 11, 217-230.
-
Hrudka,
J.,
Eis,
V.,
Heřman,
J.,
Prouzová,
Z.,
Rosenwald,
A. &
Duška,
F.
(2019) Panniculitis-like T-cell-lymphoma in the mesentery associated with hemophagocytic syndrome: autopsy case report. Diagn. Pathol., 14, 80.
-
Issa,
I. &
Baydoun,
H.
(2009) Mesenteric panniculitis: various presentations and treatment regimens. World J. Gastroenterol., 15, 3827-3830.
-
La Rosée,
P.,
Horne,
A.,
Hines,
M.,
von Bahr Greenwood,
T.,
Machowicz,
R.,
Berliner,
N.,
Birndt,
S.,
Gil-Herrera,
J.,
Girschikofsky,
M.,
Jordan,
M.B.,
Kumar,
A.,
van Laar,
J.A.M.,
Lachmann,
G.,
Nichols,
K.E.,
Ramanan,
A.V., et al.
(2019) Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood, 133, 2465-2477.
-
Larroche,
C. &
Mouthon,
L.
(2004) Pathogenesis of hemophagocytic syndrome (HPS). Autoimmun. Rev., 3, 69-75.
-
Lester,
L.,
Ewalt,
M.,
Warnke,
R. &
Kim,
J.
(2015) Systemic panniculitis-like T-cell lymphoma with involvement of mesenteric fat and subcutis. J. Cutan. Pathol., 42, 46-49.
-
Ramos-Casals,
M.,
Brito-Zerón,
P.,
López-Guillermo,
A.,
Khamashta,
M.A. &
Bosch,
X.
(2014) Adult haemophagocytic syndrome. Lancet, 383, 1503-1516.
-
Rivière,
S.,
Galicier,
L.,
Coppo,
P.,
Marzac,
C.,
Aumont,
C.,
Lambotte,
O. &
Fardet,
L.
(2014) Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am. J. Med., 127, 1118-1125.
-
Rouphael,
N.G.,
Talati,
N.J.,
Vaughan,
C.,
Cunningham,
K.,
Moreira,
R. &
Gould,
C.
(2007) Infections associated with haemophagocytic syndrome. Lancet. Infect. Dis., 7, 814-822.
-
Saleeb,
P. &
Olivier,
K.N.
(2010) Pulmonary nontuberculous mycobacterial disease: new insights into risk factors for susceptibility, epidemiology, and approaches to management in immunocompetent and immunocompromised patients. Curr. Infect. Dis. Rep., 12, 198-203.
-
Schettert,
I.T.,
Cardinalli,
I.A.,
Ozello,
M.C.,
Vassallo,
J.,
Lorand-Metze,
I. &
de Souza,
C.A.
(1997) Hemophagocytic syndrome: pitfalls in its diagnosis. Sao Paulo Med. J., 115, 1548-1552.
-
Shi,
W. &
Jiao,
Y.
(2019) Nontuberculous Mycobacterium infection complicated with Haemophagocytic syndrome: a case report and literature review. BMC Infect. Dis., 19, 399.
-
Skogberg,
K.,
Ruutu,
P.,
Tukiainen,
P. &
Valtonen,
V.V.
(1995) Nontuberculous mycobacterial infection in HIV-negative patients receiving immunosuppressive therapy. Eur. J. Clin. Microbiol. Infect. Dis., 14, 755-763.
-
Tavares Pereira,
J.P.,
Romão,
V.,
Eulálio,
M.,
Jorge,
R.,
Breda,
F.,
Calretas,
S.,
Leitão,
S.,
Eugénio,
G.,
Santos,
R. &
Carvalho,
A.
(2016) Sclerosing mesenteritis and disturbance of glucose metabolism: a new relationship? A case series. Am. J. Case Rep., 17, 55-59.
-
Torriani,
F.J.,
Behling,
C.A.,
McCutchan,
J.A.,
Haubrich,
R.H. &
Havlir,
D.V.
(1996) Disseminated Mycobacterium avium complex: correlation between blood and tissue burden. J. Infect. Dis., 173, 942-949.
-
Torriani,
F.J.,
McCutchan,
J.A.,
Bozzette,
S.A.,
Grafe,
M.R. &
Havlir,
D.V.
(1994) Autopsy findings in AIDS patients with Mycobacterium avium complex bacteremia. J. Infect .Dis., 170, 1601-1605.
-
Tortoli,
E.
(2009) Clinical manifestations of nontuberculous mycobacteria infections. Clin. Microbiol. Infect., 15, 906-910.
-
Wang,
W.,
Pardee,
T.S. &
Beaty,
M.W.
(2013) Subcutaneous panniculitis-like T cell lymphoma with mesenteric involvement. J. Hematop., 6, 155-159.
-
White,
J.W. Jr. &
Winkelmann,
R.K.
(1989) Cytophagic histiocytic panniculitis is not always fatal. J. Cutan. Pathol., 16, 137-144.
-
White,
J.W. Jr. &
Winkelmann,
R.K.
(1998) Weber-Christian panniculitis: a review of 30 cases with this diagnosis. J. Am. Acad. Dermatol., 39, 56-62.
-
Willemze,
R.,
Jaffe,
E.S.,
Burg,
G.,
Cerroni,
L.,
Berti,
E.,
Swerdlow,
S.H.,
Ralfkiaer,
E.,
Chimenti,
S.,
Diaz-Perez,
J.L.,
Duncan,
L.M.,
Grange,
F.,
Harris,
N.L.,
Kempf,
W.,
Kerl,
H.,
Kurrer,
M., et al.
(2005) WHO-EORTC classification for cutaneous lymphomas. Blood, 105, 3768-3785.
-
Yang,
W.K.,
Fu,
L.S.,
Lan,
J.L.,
Shen,
G.H.,
Chou,
G.,
Tseng,
C.F. &
Chi,
C.S.
(2003) Mycobacterium avium complex-associated hemophagocytic syndrome in systemic lupus erythematosus patient: report of one case. Lupus, 12, 312-316.
-
Yu,
L.,
Tu,
M.,
Cortes,
J.,
Xu-Monette,
Z.Y.,
Miranda,
R.N.,
Zhang,
J.,
Orlowski,
R.Z.,
Neelapu,
S.,
Boddu,
P.C.,
Akosile,
M.A.,
Uldrick,
T.S.,
Yarchoan,
R.,
Medeiros,
L.J.,
Li,
Y.,
Fajgenbaum,
D.C., et al.
(2017) Clinical and pathological characteristics of HIV-and HHV-8-negative Castleman disease. Blood, 129, 1658-1668.