2021 Volume 253 Issue 4 Pages 249-259
2021 Volume 253 Issue 4 Pages 249-259
Bacterial infection contributes to tumor development and malignant progression. Fusobacterium nucleatum (F. nucleatum) is reported to promote oral squamous cell carcinoma. However, molecular bases of F. nucleatum regulating oral cancer cells have not been fully elucidated. We report here that F. nucleatum down-regulates p53 and E-cadherin via the Wnt/NFAT pathway to promote cisplatin-resistance and migration in oral squamous carcinoma cells. We pretreated Cal-27 and HSC-3 cells with F. nucleatum and the survival rates against cysplatin (Cis-diamminedichloroplatinum, CDDP) were significantly higher in treated cells. The expressions of migration and apoptosis-related proteins like E-cadherin and p53 were lower in western blot analysis. We observed that F. nucleatum was an activator of the Wnt/NFAT pathway. The expression levels of the Wnt pathway gene wnt5a and Nuclear factors of activated T cells 3 (NFATc3) were notably higher in treated cells. With the inhibition effect of NFAT-inhibitory peptide VIVIT, the expressions of E-cadherin and p53 in response to F. nucleatum infection were up-regulated reversely. We concluded that F. nucleatum might promote cisplatin-resistance and migration of oral squamous cell carcinoma cells through the Wnt/NFAT pathway.
Oral squamous cell carcinoma (OSCC) is the most common oral and maxillofacial tumor worldwide (Chi et al. 2015). The five-year mortality rate of OSCC is high, in spite of the development of surgical and chemical therapies.
The studies of oral microbiota community and disease indicate that the abundance of some pathogenic bacteria is much higher in patients with oral diseases like periodontitis and tumor. Cysplatin (Cis-diamminedichloroplatinum, CDDP) (Dasari and Tchounwou 2014) is one of the most common chemotherapy drugs to treat OSCC by increasing p53 expression and inducing apoptosis. However, tumor recurrence is high in OSCC due to chemoresistance and metastasis (Kessler et al. 2008). Thus, it is essential to elucidate the mechanism of chemoresistance and metastasis.
Fusobacterium nucleatum (F. nucleatum) is known as periodontal pathogenic bacteria. Recently, the increased abundance of Fusobacteria is reported in patients with the OSCC progression from stage 1 to stage 4 (Yang et al. 2018). Moreover, F. nucleatum plays a role in promoting epithelial-mesenchymal transition (Abdulkareem et al. 2018) and proliferation (Geng et al. 2020) in OSCC cells in vitro. However, the potential effect and the molecular mechanisms of F. nucleatum on cisplatin-resistance and migration in oral cancer are still unknown.
The oral microbiota community is involved in the process of regulating the canonical Wnt signaling pathway in the OSCC mouse model (Stashenko et al. 2019). The expression of Wnt5a protein is significantly higher in OSCC cells than in non-cancerous regions without influencing the canonical WNT/β-catenin pathway (Prgomet et al. 2017). This indicates the activation of the non-canonical WNT/Ca2+ signaling pathway (Krishnamurthy and Kurzrock 2018).
Nuclear factor of activated T-cells (NFAT) is a family of five proteins that regulate the progression of various cancers (Qin et al. 2014; Gang et al. 2019). It is a downstream effector of the non-canonical WNT/Ca2+ signaling pathway. Recent evidence (Sengupta et al. 2013; Zheng et al. 2014; Peng et al. 2015) suggests that NFATc3 promotes the migration of tumor cells and restrains apoptosis (Ding et al. 2016).
This work aimed to examine the potential of F. nucleatum in cisplatin-resistance and migration of OSCC cells in vitro. We hypothesized that OSCC cells treated with F. nucleatum can acquire cisplatin-resistance and migration via the WNT signaling pathway.
The bacterium used in this study was Fusobacterium nucleatum strain ATCC 23726 (ATCC, Manassas, VA). F. nucleatum was cultured overnight at 37°C under anaerobic conditions (85% N2, 10% H2 and 5% CO2, Bugbox M, Bridgend, UK) in brain heart infusion (BHI) agar supplemented with 5 μg/ml hemin, 0.01% Vitamin K1 and 10% defibrinated sheep blood. We used McFarland method to count bacterial numbers.
Cell linesHuman oral squamous cell carcinoma lines Cal-27(ATCC® CRL-2095TM) and HSC-3 (JCRB0623) were maintained in Dulbecco’s Modified Eagle Medium-high glucose (DMEM) (GIBCO, Carlsbad, CA) and 10% fetal bovine serum (FBS) without antibiotics.
RNA extraction and qPCRWe used the TaKaRa MiniBEST Universal RNA Extraction Kit (Takara, Shiga, Japan) to extract total RNA from the OSCC cell lines. Total RNAs were reversely transcribed into cDNAs and the relative levels of mRNAs were detected by qPCR using the PrimeScriptTM RT-PCR Kit (Takara). The primers in the study were as follows: wnt5a forward, ATTCTTGGTGGTCGCTAGGTA, and reverse, CGCCTTCTCCGATGTACTGC; ACTB forward, CATGTACGTTGCTATCCAGGC, and reverse, CTCCTTAATGTCACGCACGAT; NFATc1 forward, CACCGCATCACAGGGAAGAC, and reverse, GCACAGTCAATGACGGCTC; NFATc2 forward, GAGCCGAATGCACATAAGGTC, and reverse, CCAGAGAGACTAGCAAGGGG; NFATc3 forward, GCTCGACTTCAAACTCGTCTT, and reverse, GATGCACAATCATCTGGCTCA; NFATc4 forward, CTTCTCCGATGCCTCTGACG, and reverse, CGGGGCTTGGACCATACAG (PrimerBank database). Results were analyzed using the comparative Ct method followed by unpaired t-tests.
Western blottingProteins were extracted from Cal-27 and HSC-3 cells by the Total protein extraction kit (KGP250; Keygentech, Jiangsu, China). Nuclear proteins and cytoplasmic proteins were separated by Nuclear Cytoplasmic Protein Extraction Kit (KGP150; Keygentech). The concentration of the protein samples was quantified by PierceTM BCA Protein Assay Kit (23227; Thermo ScientificTM, Carlsbad, USA). An equal amount of proteins (15-20 μg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblot analysis was carried out by using antibodies against p53 (2527T; CST, Massachusetts, USA), Bax (50599-2-lg; Proteintech, Rosemont, USA), Bcl-2 (12789-1-AP; Proteintech), caspase 3 (D3R6Y) (14220T; CST; Massachusetts, USA), Histone-H3 (4499T; CST), β-catenin (9582T; CST), E-cadherin (20874-1-AP; Proteintech), wnt5a (ab179824; Abcam; Cambridge, UK), NFATc3 (sc-8405; Santa Cruze; Texas, USA), β-actin (4970S; CST) and GAPDH (5174S; CST). Chemiluminescent HRP Substrate Western blotting detection system (P90720; Millipore Corporation; Massachusetts, USA) and autoradiography film (Tanon 5200; Bio-Equip, Shanghai, China) were utilized to visualize the protein strips. Bands were quantified using ImageJ (1.8.0; NIH, CA, USA) and normalized to Histone-H3, β-actin or GAPDH.
Cell counting kit-8 assayCell counting kit-8 assay (CCK-8) was used to evaluate proliferation and apoptosis levels. F. nucleatum was co-cultured with Cal-27 and HSC-3 cells. Since 10 μM (Johnsson et al. 1996) cisplatin is the maximum plasma level in clinical therapy, we chose 10 μM CDDP (P4394; MilliporeSigma, Darmstadt, Germany) in this study. Cells were divided into the control group [multiplicity of infection (MOI) = 0] and the experimental groups with the ascending amount of F. nucleatum (MOI = 100, 500, and 1,000). We cultivated 5,000 cells for proliferation assay and 104 cells for apoptosis assay per each well. Cell Counting Kit-8 assay kit (CCK-8; Dojindo, Kumamoto, Japan) was utilized to assess the different activities and survival rates among cells in 96-well plates for 5 days. We set four repeating wells for every group and repeated 3 times. Results were detected by the spectrophotometer at 450 nm.
JC-1 mitochondrial membrane potential assayProper MOI was selected based on the results of the CCK-8 assay. Cells were divided into four groups: positive control (CCCP), negative control (PBS), and two experimental groups (MOI = 0 and MOI = 500). We cultivated 5 × 105 cells per each well. We treated experimental groups with 10 μM CDDP for 24 h before we started to stain cells. We stained cells using the Mitochondrial membrane potential assay kit with JC-1 (tetraethylbenzimidazolylcarbocyanine iodide) (C0026; Beyotime Biotechnology, Shanghai, China) as recommended. Briefly, we added 2 μl 10 mM CCCP to positive control groups for 30 min before we started to collect cells. Cells of four groups were collected and washed with phosphate-buffered saline (PBS) three times. We incubated all the cells except for the negative control group with JC-1 dye and analyzed them with flow cytometry (Ex = 488 nm; Em = 530 nm). Positive and negative control groups were used to set gates. We set three repeating wells for every group and repeated 3 times.
Caspase 3/7 activity assayCaspase-Glo® 3/7 Assay kit (Promega, Wisconsin, USA) was utilized to detect Caspase-3 and -7 Activities in Cal-27 and HSC-3 cells at 24 h based on instructions. 104 cells were cultured in a well of 96-well plate. The control group (MOI = 0) and the experimental group (MOI = 500) were illustrated in Fig. 1. 100 μl of Caspase-Glo® 3/7 Reagent mixture was added to samples in 96-well plates. Cells were incubated for 1 h. The results were analyzed by the luminometer. We set four repeating wells for every group and repeated 3 times.

F. nucleatum inhibits CDDP-induced apoptosis of OSCC.
OD Value of CCK-8 assay in Cal-27 (A) and HSC-3 cells (B) at MOI = 0, MOI = 100, MOI = 500, MOI = 1,000. Results of CCK-8 assay in Cal-27 (C) and HSC-3 cells (D) at MOI = 0, MOI = 100, MOI = 500, MOI = 1,000 and LPS (1 μM) with CDDP (10 μM). The cell survival rates were evaluated by cell number counting after treatment at 1, 2, 3 and 5 days (*P < 0.05, ***P < 0.001, One-way ANOVA test). E (Cal-27) and F (HSC-3) were the results of the JC-1 assay at MOI = 0 and MOI = 500. The percentages of apoptotic cells detected by flow cytometry. G (Cal-27) and H (HSC-3) were outcomes of relative light units (RLU) of caspase 3/7 activities (*P < 0.05, ***P < 0.001, unpaired Student’s t-test).
Images of Cal-27 and HSC-3 cells treated with F. nucleatum (MOI = 500) were captured under a Leica inverted microscope. To conduct the wound healing assay, we implanted 106 Cal-27 and HSC-3 cells into 6-well plates and scratched the cell layer with a 1 ml pipette tip until cells were grown to confluency. F. nucleatum (MOI = 500) was added to the plates and both cell culture supernatant of the control group (MOI = 0) and the experimental group (MOI = 500) was replaced with 1%-serum medium simultaneously. The cells were photographed at 0 h, 6 h and 24 h. We set three repeating wells for every group and repeated 3 times.
Luciferase reporter gene assayMaximal transduction efficiency was obtained when 104 Cal-27 and HSC-3 cells co-transfected with 100 ng pGMNFAT-Luc (Yeasen, Shanghai, China) according to the protocol of Lipofectamine TM 3000 Reagent (Thermo Scientific). The pGMNFAT-Luc plasmid contained two tandem copies of the NFAT-consensus sequence upstream of the minimal TA promoter. 48 h after transfection, the cell viability was approximately the same as normal cells. The results of the control group (MOI = 0) and the experimental group (MOI = 500) were analyzed by the luminometer. We set four repeating wells for every group and repeated 3 times.
Validation by NFAT-inhibitory peptide VIVITTo validate the signal pathway reversely, we repeated the assays including CCK-8 and western blotting of E-cadherin and apoptosis-related proteins with NFAT-inhibitor VIVIT (HY-P1026; MedChemExpress, New Jersey, USA). It is a cell-permeable peptide inhibitor of NFAT that selectively inhibits calcineurin-mediated dephosphorylation of NFAT. The recommended working concentration of VIVIT is 10 μM (Aramburu et al. 1999; Ma et al. 2019) added at the same time as CDDP and we dissolved 1 mg VIVIT in 0.5931 mL DMEM to prepare 1 mM stock solution before we started.
Statistical analysisAll the results and figures obtained were analyzed by IBM SPSS Statistics 26.0 and GraphPad Prism 7.0 software. Statistical analyses including unpaired t-test and one-way ANOVA were performed in our study. All data were presented as mean ± SD in figures and recognized significant at p < 0.05.
We investigated the effect of F. nucleatum on the proliferation of OSCC cells by co-culturing different MOI of F. nucleatum with Cal-27 and HSC-3 cells. Cells were divided into the negative control group (MOI = 0), the experimental group (MOI = 100, 200, 500, and 1,000) and the positive control group (LPS). Lipopolysaccharide (LPS) is an agonist of toll-like receptor 4 (TLR4) and relative to the cisplatin-resistance (Sun et al. 2012) and epithelial-mesenchymal transition (EMT) (He et al. 2015) of OSCC. LPS is part of the cell wall of F. nucleatum and is released when F. nucleatum lyses. Therefore, 1 μg/ml LPS (Sun et al. 2012) was used as a positive control in this study. We observed that F. nucleatum had no significant influence on proliferation (Fig. 1A, B), and MOI = 500 group had the most remarkable effect on Cal-27 and HSC-3 cells of drug-resistance (Fig. 1C, D). Reduced CDDP-induced apoptosis was observed in all groups at the first 24 h. However, only the MOI = 500 group showed continued inhibition of apoptosis. The result was statistically significant.
We detected the value of mitochondrial membrane potential by JC-1 flow cytometry, which is related to the early stage of apoptosis (Fig. 1E, F). Forward scatter (FSC) signal was used for the discrimination of cells by size and side scatter (SSC) provides information about the internal complexity. We set fluorescence assisted cell sorting (FACS) gates according to the FSC/SCC scatter plot of two control groups. FL1 and FL2 represented fluorescein isothiocyanate (FITC) and propidium iodide (PI) channels separately. Normally cells should locate at the Q2 quadrant and apoptotic cells located at the Q3 quadrant. The values of the column were the percentages of apoptotic cells detected by flow cytometry (Q3, cells with decreased mitochondrial membrane potential). T-tests were used to analyze the relationship between the MOI = 0 group and the MOI = 500 group. The results indicated that the mitochondrial membrane potential dropped significantly lower in the experimental group.
Moreover, we chose the Caspase-Glo® 3/7 Assay kit to test the caspase 3/7 activity. The intensity of the bioluminescent signal was detected with a luminometer as RLU (relative luciferase unit). (Fig. 1G, H). The value of RLU in the control group was significantly higher than the experimental group (MOI = 500).
In summary, F. nucleatum could inhibit CDDP-induced apoptosis of Cal-27 and HSC-3 cells.
F. nucleatum promotes the migration of OSCCFig. 2A (Cal-27 cells) and Fig. 3A (HSC-3 cells) showed flat polygonal, round, irregular or fusiform-shaped cell morphology under the microscope, arranged in a paving stone-like structure. Cellular atypia was apparent while nuclear divisions were absent (magnification, × 200). The morphology of OSCC was changed by treating with F. nucleatum (MOI = 500). Cells had changed from a paving stone, sheet-like structure to a fibroblast-like spindle shape during 48 h. These changes indicated the possibility of EMT of Cal-27 and HSC-3 cell lines.
Fig. 2B (Cal-27 cells) and Fig. 3B (HSC-3 cells) showed the results of the scratch wound assay. The data were analyzed by Image J software. Quantitation of Cal-27 average migration areas were 3.207 ± 0.2094 (MOI = 0) and 4.399 ± 0.3659 (MOI = 500), and migration distances were 0.6346 ± 0.05779 (MOI = 0) and 0.9323 ± 0.07886 (MOI = 500) at 6 h, respectively (Fig. 2C). Quantitation of HSC-3 average migration areas were 1.441 ± 0.1067 (MOI = 0) and 2.307 ± 0.1815 (MOI = 500), migration distances were 0.11 ± 0.03333 (MOI = 0) and 0.5825 ± 0.04444 (MOI = 500) at 24 h, respectively (Fig. 3C). An unpaired t-test was performed to analyze the data. The differences between the control group and experimental group in average migration distance and area were statistically significant.
As previously described, F. nucleatum binds to E-cadherin on endothelial cells (Fardini et al. 2011). E-cadherin is a crucial protein related to migration. We have detected the level of E-cadherin. Western blotting results were shown in Fig. 2D (Cal-27 cells) and Fig. 3D (HSC-3 cells). E-cadherin was significantly down-regulated at 6 h (Cal-27 cells) and 24h (HSC-3 cells), and the differences were statistically significant.
In summary, the changed morphology and E-cadherin concentration indicated that F. nucleatum promoted the migration of Cal-27 and HSC-3 cells.

F. nucleatum promotes the migration of Cal-27.
(A) Cell morphology of Cal-27 with or without F. nucleatum (MOI = 500) at 6 h, 12 h, 24 h and 48 h (10 ×). (B) Wound healing assay of Cal-27 with F. nucleatum (MOI = 500) at 6 h (10 ×). (C) Average migration distance and average migration area (*P < 0.05, ***P < 0.001, unpaired Student’s t-test). (D) Expression of E-cadherin of Cal-27 (*P < 0.05, paired Student’s t-test).

F. nucleatum promotes the migration of HSC-3.
(A) Cell morphology of HSC-3 with or without F. nucleatum (MOI = 500) at 6 h, 12 h, 24 h and 48 h (10 ×). (B) Wound healing assay of HSC-3 with F. nucleatum (MOI = 500) at 6 h (10 ×). (C) Average migration distance (μm) and average migration area (μm2) (*P < 0.05, ***P < 0.001, unpaired Student’s t test). (D) Expression of E-cadherin of HSC-3 (*P < 0.05, paired Student’s t-test).
We detected the expressions of terminal apoptosis protein cleaved-caspase 3 and endogenous apoptosis-related proteins Bax, Bcl-2 and p53 for further exploration. As shown in Fig. 4A (Cal-27 cells) and Fig. 4B (HSC-3 cells), the results of western blotting demonstrated that the expressions of cleaved-caspase 3, Bax/Bcl-2 and p53 were decreased significantly in experimental groups (MOI = 500) under the stimulation of 10μM CDDP for 24 h. Considering that CDDP causes tumor cell apoptosis by up-regulating the expression of p53 in OSCC cells, we could find out from the protein bands that F.nucleatum down-regulated p53 expression through the endogenous apoptotic pathway, thus promoted the drug-resistance in Cal-27 and HSC-3 of CDDP.
We suspected that F. nucleatum might regulate p53 and E-cadherin through the Wnt non-canonical pathway. To verify this, we conducted the qPCR assay and comparative Ct method to determine relative mRNA levels. Cal-27 and HSC-3 cells were treated with F. nucleatum (MOI = 500) at 0 min, 40 min, 80 min and 2 h. Fig. 4C (Cal-27 cells) and Fig. 4E (HSC-3 cells) manifested transcription levels of critical factors of the Wnt pathway. Among all of the Wnt mRNA, wnt5a increased most significantly at 40 min (Cal-27 cells) and 80 min (HSC-3 cells). In comparison, β-catenin was significantly reduced on the contrary. The western blotting showed that Wnt5a was up-regulated within 80 min, and then down-regulated in both Cal-27 (Fig. 4G) and HSC-3 cells (Fig. 4H). The expression of β-catenin was down-regulated at 80 min significantly. These indicated that F. nucleatum might activate the non-canonical Wnt pathway to down-regulate apoptosis-related proteins and migration-related proteins.
We made a surmise that NFAT might be involved in the regulation of F. nucleatum. To test this, we transfected luciferase report plasmid pGMNFAT-Luc into OSCC cells and detected luciferase activity by microplate spectrophotometer. In Cal-27 (Fig. 4D) and HSC-3 cells (Fig. 4F) co-cultured with F. nucleatum (MOI = 500) respectively, the value of relative luminescence units (RLU) was increased significantly. The peak value occurred at 80 minutes in Cal-27, while the value of RLU in HSC-3 kept growing within 2 h. Since NFAT is a family of five proteins, we next conducted the qPCR and western blotting to filter out relative protein. Fig. 4D (Cal-27 cells) and Fig. 4F (HSC-3 cells) showed that NFATc3 was expressed much higher than others in the NFAT family within 2 h. Besides, NFATc3 was up-regulated significantly at 40 min in Cal-27 (Fig. 4G) and 80 min in HSC-3 cells (Fig. 4H).
The survival rate of Cal-27 (Fig. 5A) and HSC-3 cells (Fig. 5B) under 10 μM CDDP were quantified by CCK-8 assay. The survival rates of the inhibitor groups were significantly lower than that of the F. nucleatum-treatment groups, and the differences were statistically significant. Fig. 5C, E (Cal-27 cells) and Fig. 5D, F (HSC-3 cells) demonstrated the expression of terminal apoptosis protein cleaved-caspase 3, endogenous apoptosis proteins Bax, Bcl-2, p53 and migration key protein E-cadherin. The results showed that the expressions of apoptosis-related proteins and E-cadherin in the inhibitor group were significantly up-regulated, indicating that the inhibitor VIVIT inhibited the promotion effect of F. nucleatum to drug resistance and migration.
In summary, F. nucleatum may down-regulate p53 and E-cadherin through the Wnt/NFAT pathway, and promote migration and cisplatin-resistance.

F. nucleatum activates Wnt5a/NFAT pathway.
Western blotting in Cal-27 (A) and HSC-3 (B) at MOI = 0 and MOI = 500 of the relative expression of caspase 3, cleaved-caspase 3, Bax, Bcl-2 and p53. Results of qPCR (C) and western blotting (G) in Cal-27 about key factors on the Wnt signal pathway. Results of qPCR (E) and western blotting (H) in HSC-3 about key factors on the Wnt signal pathway. D (Cal-27) and F (HSC-3) were outcomes of relative light units (RLU) of NFAT activities and qPCR of NFAT family (*P < 0.05, ***P < 0.001, unpaired Student’s t-test).
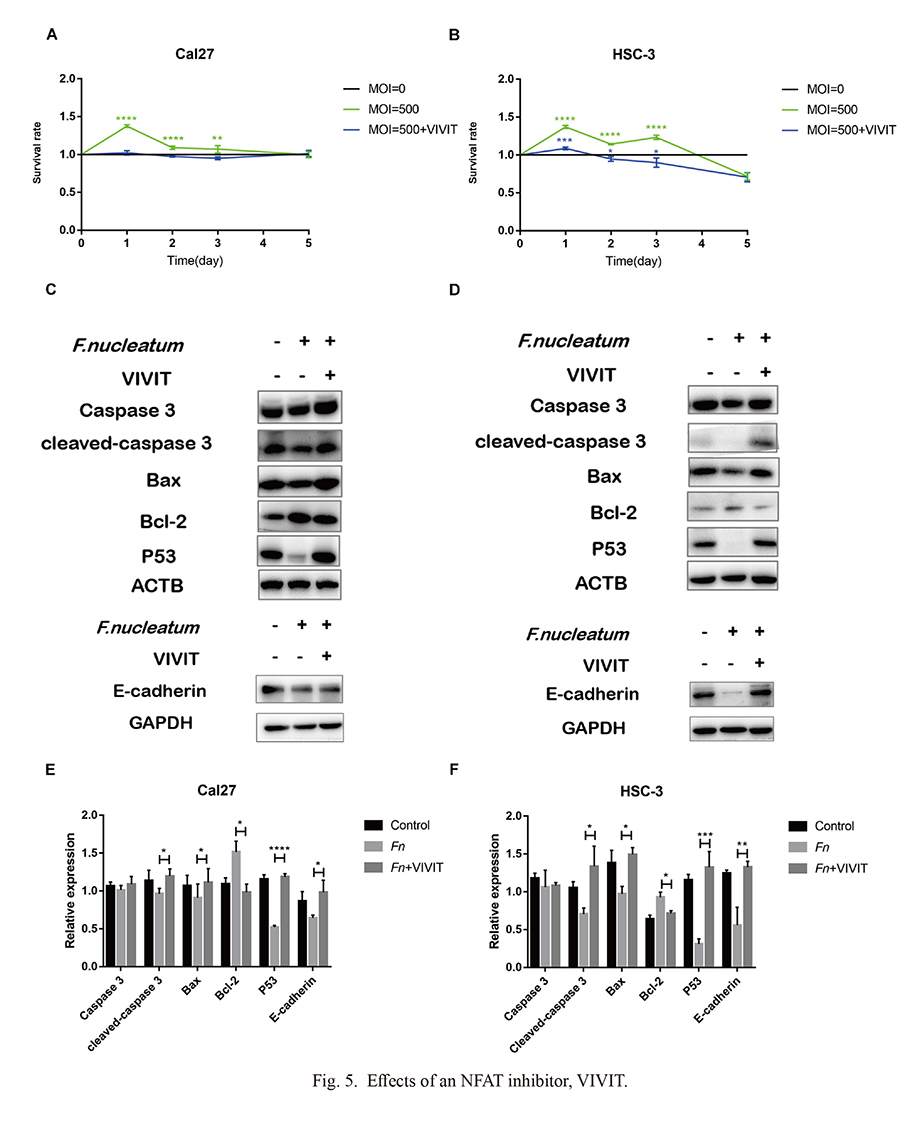
Effects of an NFAT inhibitor, VIVIT.
CCK-8 assay in Cal-27 (A) and HSC-3 (B). Groups were divided into the control group, experimental group (F. nucleatum) and inhibitor group (F. nucleatum +VIVIT) with CDDP (10 μM). The cell survival rates were evaluated at 1, 2, 3 and 5 days (*P < 0.05, ***P < 0.001, One-way ANOVA test). The relative expressions of Caspase 3, cleaved-caspase 3, Bax, Bcl-2, p53 and E-cadherin of Cal-27 (C, E) and HSC-3 (D, F) were examined by western blotting (*P < 0.05, ***P < 0.001, paired Student’s t-test).
OSCC is the sixth most common cancer worldwide, accounting for 90% of malignant oral tumors. Increasing evidence supports a connection between the oral microbiota community and various human malignant lesions (Plottel and Blaser 2011). Previous studies have largely focused on microbial involvement of F. nucleatum in the OSCC progression (Abdulkareem et al. 2018; Geng et al. 2020; Zhang et al. 2020).
In agreement with previous studies (Abdulkareem et al. 2018; Zhang et al. 2020), we confirmed that F. nucleatum promoted the migration of OSCC cells. The mechanism of this effect is still not clear. Zhang et al. (2020) believes that FadA, a surface adhesin expressed by F. nucleatum, is the crucial factor to activate the EMT process. Another study (Rubinstein et al. 2019) illustrates that FadA can activate the Wnt/β-catenin pathway through E-cadherin in colorectal carcinoma cells, which is in contrast with our results. Prgomet et al. (2017) examines 21 OSCC patients to explore the pattern of expressions of Wnt5a and β-catenin by immunohistochemistry in diagnostic biopsies. Along with their other research (Prgomet et al. 2015) in vitro, they confirm that there is no correlation between the expressions of Wnt5a and β-catenin. Shaw et al. (1988) obtained the similar conclusion with us that Wnt5a activated the non-canonical Wnt/Ca²⁺ pathway to advance the migration of OSCC cells.
Most OSCC are naturally resistant to anti-cancer drugs, and drug-resistance is a vital factor influencing the prognosis of OSCC patients (Xiong et al. 2011). HSC-3 is relatively more sensitive to CDDP treatment than Cal-27, while it presents a lower band density of cleaved-caspase 3. F. nucleatum is proved to affect the chemoresistance of various tumor cells. Unfortunately, to date, the correlation between F. nucleatum and chemoresistance of OSCC cells remains unknown. The effect of F. nucleatum on proliferation is controversial. Geng et al. (2020) reports that F. nucleatum could promote the proliferation of an OSCC cell line, Tca8113, but this does not happen in human immortalized oral epithelial cells (HIOEC cell), SCC-9 and HSC-4 (OSCC cells) (Zhang et al. 2020). To confirm the effect of F. nucleatum on proliferation, we conducted the CCK-8 assay. Similar results were obtained in Cal-27 and HSC-3 cell lines with HIOECs. Meanwhile, we also observed the down-regulation of p53, similarly to the results in Tca8113. Although there ought to have more experiments to confirm the result, F. nucleatum is more unlikely to influence the proliferation of OSCC cells so far. Consequently, we believe that F. nucleatum could accelerate tumor progress by suppressing drug-induced apoptosis, rather than promoting proliferation.
NFAT is reported to be a downstream target of the non-canonical Wnt pathway, which down-regulates p53 and E-cadherin at the same time. As mentioned above, F. nucleatum may promote the migration of OSCC cells through the non-canonical Wnt/Ca2+ pathway. The NFAT family is first described in T lymphocytes with five members (Shaw et al. 1988; Mancini and Toker 2009). NFATc1-4 are ubiquitously expressed in both immune and non-immune cells. In resting cells, NFAT exists in the cytoplasm as a phosphorylated protein, with a low affinity for DNA binding. With an increased level of calcium, NFAT is dephosphorylated to transport into the nucleus and up-regulate transcription of NFAT-downstream genes. Previously published data display important roles of NFATs in modulating tumor progression. NFATc2 is reported to enhance cell death and repress cell cycle. NFATc1 up-regulates cell proliferation and transformation, while it suppresses cell death reversely (Robbs et al. 2008). NFATc3 plays a crucial role in EMT (Sengupta et al. 2013; Peng et al. 2015; Zheng et al. 2014) and is a negative regulator of p53 (Ding et al. 2016).
In our study, the qPCR and western blotting analysis showed that NFATc3 was increased remarkably to down-regulate E-cadherin and p53 in both Cal-27 and HSC-3 cells. Our basic findings were consistent with the researches mentioned above (Sengupta et al. 2013; Peng et al. 2015; Ding et al. 2016). We discovered that the increase of NFATc3 was accompanied by a decrease of NFATc2 in Cal-27 cells. In particular, the expression of NFATc3 mRNA dropped slightly at the first 40min, and then rose rapidly in HSC-3 cells, which was just opposite to the expression trends of NFATc2 mRNA. According to the study conducted by Kar and Parekh (2015), NFATc2 and NFATc3 require distinct subcellular membrane receptors and Ca2+ signals for activation. NFATc2 is modulated by sub-plasmalemmal Ca2+ domains, while NFATc3 requires Ca2+ stimulated by nuclear inositol 1, 4, 5-trisphosphate (InsP3) receptors additionally. Moreover, based on the previous report by Imamura et al. (1998), the C-terminus motif of NFATs is divided into two sub-motifs. NFATc2-C contains both two sub-motifs, while NFATc3 only has the first sub-motif. The different C-terminal domain of NFATs is responsible for their opposite pro-apoptotic characteristic (Mognol et al. 2016).
All these above suggest two possible explanations. One is that the target of F. nucleatum might be proteins like InsP3 receptors (Mognol et al. 2016) on the endoplasmic reticulum and nuclear envelope, which could stimulate small calcium signals within the nucleus, independently of cytoplasmic Ca2+. Another is that NFATc3 is exported out of the nucleus much faster than NFATc2 (Echevarría et al. 2003), which is rephosphorylated within the nucleus much quicker (Kar et al. 2016). Therefore, we could presume that the stimulation of F. nucleatum may be short and mild, which is not enough to activate NFATc2. Conversely, this stimulation could activate NFATc3 at a relatively shorter period of time and promote rephosphorylation for the next time.
FadA was reported to induce Annexin A1 in colorectal cancer (Rubinstein et al. 2019). OSCC patients with lower expression of Annexin A1 were found to benefit from cisplatin induction (Zhu et al. 2013) and TGFβ1/EGF-induced EMT was reversed by Annexin A1 in OSCC (Wan et al. 2017). Besides, Annexin A1 was relative to the activity of NFAT in RAW 264.7 (Kao et al. 2014). All these studies indicated that FadA might be the key factor to induce NFAT to down-regulate E-cadherin and p53.
Fap2, another outer membrane autotransporter adhesin of F. nucleatum, activated TIGIT inhibitory receptor of T cells to protect colorectal cancer (Gur et al. 2015). TIGIT was highly expressed on T cells from peripheral blood mononuclear cells and tumor‐infiltrating lymphocytes collected from OSCC patients (Liu et al. 2020). Fap2 might be a cause of chemoresistance of OSCC cells via tumor immune escape.
In general, our study aimed to explore the possible signaling pathway to elucidate the association between F. nucleatum and OSCC cells. As shown in Fig. 6, we demonstrate for the first time that F. nucleatum could down-regulate p53 and E-cadherin through the Wnt5a/NFATc3 pathway to promote cisplatin-resistance and migration of OSCC cells (Cal-27 and HSC-3). A combination of an NFAT inhibitor VIVIT can overcome cisplatin-resistance and holds promise for future CDDP treatment.

Signal pathway diagram in this study.
F. nucleatum could down-regulate p53 and E-cadherin through the Wnt5a/NFATc3 pathway to promote cisplatin-resistance and migration of OSCC cells.
This study was supported by a grant from the National Natural Science Foundation of China (81771074) and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD, 2018-87).
The authors declare no conflict of interest.