2021 年 255 巻 3 号 p. 229-237
2021 年 255 巻 3 号 p. 229-237
De novo aortic insufficiency is often documented during long-term left ventricular assist device (LVAD) support, despite the absence of aortic insufficiency at the time of LVAD implantation. However, whether aortic insufficiency affects long-term mortality and symptomatic heart failure in LVAD-supported patients remains controversial. We aimed to examine whether aortic insufficiency development influenced mortality and symptomatic heart failure following LVAD implantation. Fifty-three patients who underwent durable LVAD implantation between January 1, 2008 and April 31, 2017 were retrospectively examined in a single center institute. After discharge, we performed the echocardiographic examination in accordance with the Japanese registry for the mechanically assisted circulatory support protocol. Aortic insufficiency was graded on an interval scale (severe = 4, moderate = 3, mild = 2, trivial or none = 1). Kaplan-Meier estimates for long-term mortality at the follow-up were generated. We used a logistic regression model to identify risk factors for symptomatic heart failure. The overall median duration of LVAD support was 856.3 ± 430.8 days (range, 12-1,744 days). We did not observe a significant difference in long-term mortality in patients with aortic insufficiency ≥ 3 grade compared with patients with aortic insufficiency < 3 grade (P = 0.767; log-rank). Aortic insufficiency was associated with an increased risk for heart failure event after discharge (odds ratio, 4.12; confidence interval, 1.48-16.93; P = 0.005). Aortic insufficiency was an independent risk factor for symptomatic heart failure and was not associated with long-term mortality. Aortic insufficiency progression was associated with symptomatic heart failure.
Left ventricular assist device (LVAD) is a standard treatment for patients with end-stage heart failure as a bridge to transplantation or destination therapy. As the outcome of LVAD support improves and/or destination therapy increases, a longer period of LVAD support is needed (Kormos et al. 2019).
Previous studies have focused on the development of de novo aortic insufficiency and its progression after LVAD implantation, as the persistent elevation of aortic root pressure may lead to altered valve and aortic geometry and, consequently, aortic root enlargement and commissural fusion of the aortic valve (Pak et al. 2010). However, the mechanism of aortic insufficiency has not been completely elucidated and is multifactorial. Aortic insufficiency in 25%-30% of patients with LVAD support worsens within 1-year after LVAD implantation (Cowger et al. 2010, 2015; Jorde et al. 2014; Patil et al. 2014). In terms of hemodynamics, the progression of aortic insufficiency can lead to an ineffective LVAD output and end-organ low output due to the adverse vicious cycle of regurgitant blood from the outflow graft in the aorta back into the left ventricular inflow cannula. Theoretically, the closed loop circuit leads to an increased risk of heart failure and, consequently, an increase in symptomatic heart failure (Bouabdallaoui et al. 2018). However, the effect of aortic insufficiency on clinical outcomes is unknown.
The present study aimed to examine the long-term outcome of aortic insufficiency in LVAD-supported patients and to evaluate the risk factors for symptomatic heart failure during LVAD follow-up.
A total of 53 patients who underwent LVAD implantation between January 2008 and April 2017 at Tohoku University Hospital were retrospectively reviewed. The study protocol was reviewed and approved by our institutional review board (Ethics Committee of Tohoku University Graduate School of Medicine, approval number; 2019-1-421). The patients provided informed consent.
We considered pre-, intra-, and post-operative data. Hemodynamic parameters were recorded, including central venous pressure, pulmonary arterial pressure, pulmonary capillary wedge pressure, and the cardiac index. Preoperative echocardiographic examinations performed in all patients included in this study. All evaluations were performed within 1 month before surgery. We graded aortic insufficiency, mitral regurgitation, and tricuspid regurgitation severity quantitatively using a conventional 4-point scale (severe = 4, moderate = 3, mild = 2, trivial or none = 1). With regard to post-LVAD aortic insufficiency, we evaluated the degree of aortic insufficiency based on multi-parametric approach including a regurgitant-jet/left ventricular outflow tract (LVOT) height ratio and peak systolic-to-diastolic velocity ratio of the outflow cannula in addition to traditional quantitative transthoracic echocardiography (TTE) parameters (pressure half-time, vena contracta, proximal isovelocity surface area). When the sonographic degree of aortic insufficiency was deemed to be significant, aortography and right heart catheterization were preformed to assure the angiographic degree of aortic insufficiency.
If patients had moderate or severe aortic insufficiency at the time of LVAD implantation, aortic valve surgery was performed, which included aortic valve replacement, approximation stitches as described by Park et al. (2004), or aortic closure. We performed mitral valve repair in patients with significantly high pulmonary artery wedge pressure. Ultimately, the decision to perform tricuspid valve repair was considered based on the severity of tricuspid regurgitation by intraoperative transesophageal echocardiography.
After durable LVAD implantation, all patients were treated with standardized heart failure medical care. Anticoagulation and antiplatelet therapies with warfarin and aspirin were administered. After discharge, we checked all patients regularly by echocardiographic examination in accordance with the Japanese registry for mechanically assisted circulatory support (J-MACS) protocol. The presence of valve insufficiency was determined until the time of the final follow-up or censoring events, such as symptomatic heart failure, death, and heart transplantation. Common symptomatic heart failure manifestations include the followings; exercise intolerance due to dyspnea or fatigue, increased body weight, an increased need for diuretics, orthopnea, paroxysmal nocturnal dyspnea, increased jugular venous pressure, pulmonary rales, cardiomegaly, pulmonary vascular engorgement, and pulmonary edema. Heart failure event defined as readmission for symptomatic heart failure, urgent emergency department visit, and unscheduled outpatient visit for heart failure symptom.
All statistical analyses were performed with JMP Pro 13.0.0 software (SAS Institute, Inc., Cary, NC, USA). Categorical variables are presented as frequencies (%), and continuous variables are summarized as mean ± standard deviation. The chi-square test or Fisher’s exact test were used to compare categorical variables. The Student’s t-test was used for continuous variables, as appropriate. Univariate and multivariate logistic regression analyses were performed to identify the independent risk factors for heart failure event. Survival was estimated using Kaplan-Meier curves, and the log-rank test was used to evaluate the impact of aortic insufficiency on survival. Two group comparisons were analyzed based on the 2-way repeated-measures analysis of variance between patients who were censored heart failure event and those who were not. Two group comparisons were also analyzed by 2-way repeated-measures analysis of variance between patients who had aortic insufficiency ≥ 3 grade and those with aortic insufficiency < 3 grade. A P-value less than 0.05 was considered statistically significant.
Thirteen patients were censored for heart failure event after discharge (heart failure group). Table 1 shows the preoperative baseline characteristics, and Table 2 shows the hemodynamic and echocardiographic data. We showed overall patient data, and compared the patients who were censored for heart failure event (heart failure group) with those who were not (non-heart failure group) during the follow-up period. Patients in our cohort were younger than those in the Society of Thoracic Surgeons Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) database reports (Kormos et al. 2019); however, they were comparable to those in the J-MACS reports (Nakatani et al. 2017). Almost all preoperative hemodynamic and echocardiographic data were not significantly different between the non-heart failure and heart failure groups. Table 3 illustrates the intraoperative characteristics. Most patients (n = 28, 52.8%) received the Heart Mate II (Abbott Medical, Abbott Park, IL, USA). Other LVAD implants included Jarvik2000 (Jarvik Heart, New York, NY, USA) (n = 9, 17.0%), DuraHeart (Terumo Heart, Ann Arbor, MI, USA) (n = 9, 17.0%), EVAHEART (Sun Medical Technology Research Corp., Suwa, Japan) (n = 6, 11.3%), and HeartWare HVAD (HeartWare Inc., Framingham, MA, USA) (n = 1, 1.9%).

Preoperative patient characteristics.
Continuous variables are shown as mean ± standard deviation, and categorical variables are reported as percentages and counts.
CRTD, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter defibrillator; PMI, pacemaker implantation.

Preoperative hemodynamic and echocardiographic data.
Continuous variables are shown as mean ± standard deviation, and categorical variables are reported as percentages and counts.

Intraoperative outcome.
Continuous variables are shown as mean ± standard deviation, and categorical variables are reported as percentages and counts.
AVR/AVP, aortic valve replacement/plasty; CPB, cardiopulmonary bypass; LVAD, left ventricular assist device; MVR/MVP, mitral valve replacement/plasty; TVR/TVP, tricuspid valve replacement/plasty; RVAD, right ventricular assist device.
Table 4 shows the postoperative outcomes. The overall mean duration of LVAD support was 856.3 ± 430.8 days (range, 12-1,744 days). There was no significant difference in early and late outcomes between groups, except for redo surgery for aortic insufficiency. The overall in-hospital mortality was 11.3%. Two patients in the heart failure group needed to undergo aortic valve replacement for more than moderate aortic valve insufficiency.

Early and late mortality and morbidity.
Continuous variables are shown as mean ± standard deviation, and categorical variables are reported as percentages and counts.
ICU, intensive care unit; LVAD, left ventricular assist device.
Fig. 1 depicts the Kaplan-Meier survival curve of the overall cohort. The estimate showed that the 1-year survival was 86.8% and 3-year survival was 67.6%. Ten patients died after discharge. The most common cause of death was infection-related complications (n = 4, 40.0%), followed by device malfunction and stroke (n = 2, 20.0%), respectively. Fig. 2 depicts the Kaplan-Meier survival curve and freedom from heart failure event between aortic insufficiency ≥ 3 grade and aortic insufficiency < 3 grade. There was no significant difference in survival between the 2 groups (P = 0.767; log-rank) (Fig. 2A), whereas there was a significant difference in freedom from heart failure event between the 2 groups (P = 0.019; log-rank) (Fig. 2B).
Fig. 3 depicts the difference in tendency of aortic insufficiency (Fig. 3A), mitral regurgitation (Fig. 3B), tricuspid regurgitation (Fig. 3C), tricuspid regurgitation pressure gradient (TR-PG) (Fig. 3D), left ventricular end-diastolic diameter (LVDd) (Fig. 3E), and right ventricular fractional area change (RV-FAC) (Fig. 3F), independent of heart failure event during LVAD support. The aortic insufficiency grade gradually increased over time in both groups (Fig. 3A). Furthermore, the aortic insufficiency grade was significantly increased in heart failure event group compared with non-heart failure event group (P = 0.002). TR-PG was significantly higher in heart failure event group than non-heart failure group (P = 0.025) (Fig. 3D). The mitral regurgitation grade (Fig. 3B), tricuspid regurgitation grade (Fig. 3C), and LVDd (Fig. 3E) gradually increased in both groups, but there was no significant difference between the 2 groups (P = 0.086, 0.069, and 0.434, respectively). RV-FAC tended to decrease in heart failure event group compared with non-heart failure group; however, there was no significant difference between the 2 groups (P = 0.086) (Fig. 3F).

Kaplan-Meier survival curves after left ventricular assist device (LVAD) implantation.
Data represents survival in patients with LVAD
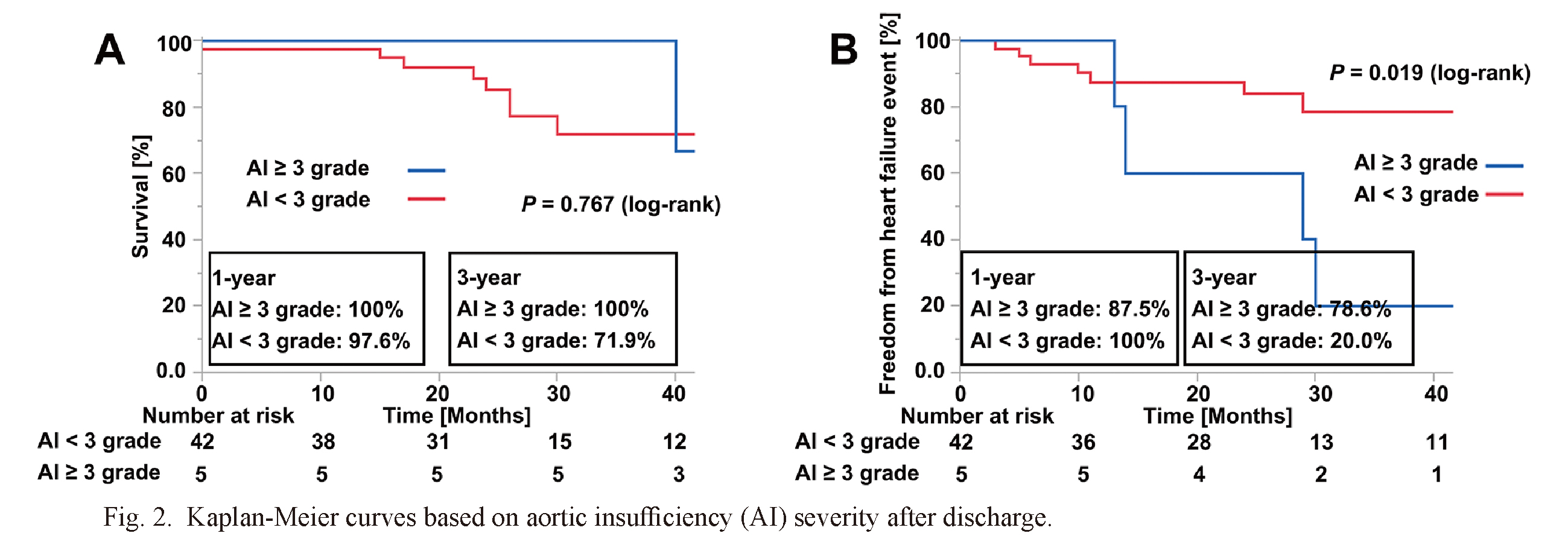
Kaplan-Meier curves based on aortic insufficiency (AI) severity after discharge.
(A) Survival rate is depicted for patients with AI ≥ 3 and AI < 3. (B) Freedom from heart failure event is depicted for patients with AI ≥ 3 and AI < 3.

Serial changes during post-LVAD implantation in A) aortic insufficiency, B) mitral regurgitation, C) tricuspid regurgitation, D) tricuspid regurgitation pressure gradient, E) left ventricular diastolic diameter and F) right ventricular fractional area change.
Two group comparisons were performed between the patients who were censored with developing heart failure event and those who were not.
Fig. 4 shows the difference in tendency in mitral regurgitation (Fig. 4A), tricuspid regurgitation (Fig. 4B), TR-PG (Fig. 4C), LVDd (Fig. 4D), and RV-FAC (Fig. 4E) between aortic insufficiency ≥ 3 grade and aortic insufficiency < 3 grade. The mitral regurgitation grade (Fig. 4A), tricuspid regurgitation grade (Fig. 4B) and LVDd (Fig. 4D) tended to increase in aortic insufficiency ≥ 3 grade compared with aortic insufficiency < 3 grade, whereas there was not significant difference between 2 groups (P = 0.104, 0.512 and 0.165). Table 5 depicts the aortic valve openings at one month, 6 months, 1 year and the last follow-up point after LVAD implantation and the relationship between aortic valve openings and progression of aortic insufficiency. The frequency of aortic valve openings in patients with aortic insufficiency ≥ 2 grade was significantly lower than that in patients with aortic insufficiency < 2 grade at 1 month after LVAD implantation and at the last follow-up point (P = 0.022 and 0.012, respectively).

Serial changes during post-LVAD implantation in A) mitral regurgitation, B) tricuspid regurgitation, C) tricuspid regurgitation pressure gradient, D) left ventricular diastolic diameter and E) right ventricular fractional area change.
Two group comparisons were performed between the patients who developed aortic insufficiency with a grade ≥ 3 and those < 3.

Proportion of patients exhibiting aortic valve openings stratified by the degree of aortic insufficiency at the time of most recent follow-up.
AI, aortic insufficiency; AV, aortic valve.
Risk factors for heart failure event after LVAD implantation are summarized in Table 6. Multivariable logistic regression analysis showed that only aortic insufficiency grade was significantly associated with heart failure event, with odds ratios of 4.12 (95% confidence interval, 1.48-16.93; P = 0.005) in multivariate analysis.
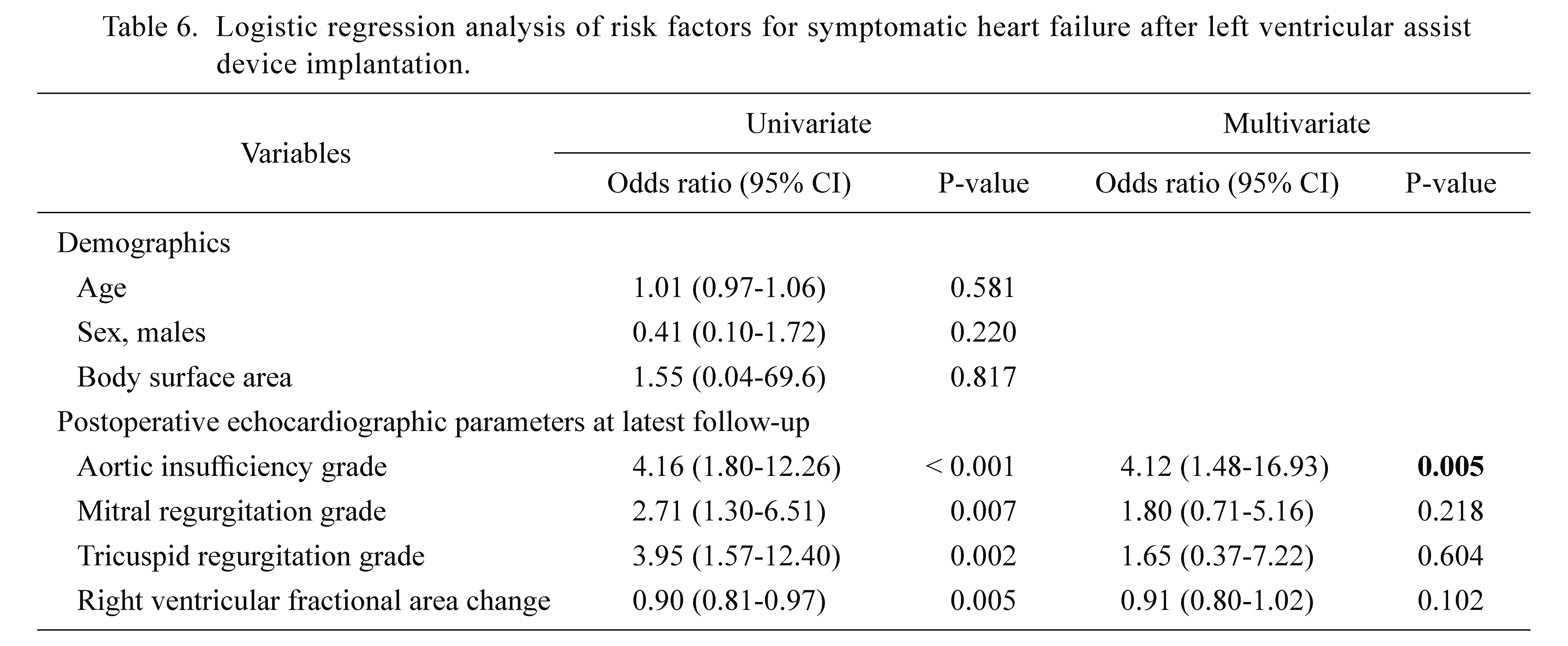
Logistic regression analysis of risk factors for symptomatic heart failure after left ventricular assist device implantation.
CI, confidence interval.
This study aimed to evaluate the effect of aortic insufficiency on long-term mortality and heart failure event during the follow-up period. The major findings of our study were that aortic insufficiency did not appear to affect long-term mortality and that aortic insufficiency was an independent predictive risk factor for heart failure event.
Aortic insufficiency is considered a major complication of LVAD support. A previous observational study revealed that aortic insufficiency gradually increased despite no aortic insufficiency at the time of LVAD implantation. The one-year prevalence of a greater than mild grade of de novo aortic insufficiency was between 25% and 30% of patients with LVAD (Cowger et al. 2010, 2015; Jorde et al. 2014). In our study, we detected 17% of patients with a greater than mild grade of de novo aortic insufficiency, and this data was comparable to that in previous reports. The management of LVAD in patients with progressive aortic insufficiency has been documented, such as decreasing the pump speed and allowing for at least intermittent aortic valve opening (Bouabdallaoui et al. 2018), as several studies clearly demonstrate that patients with intermittent aortic valve opening during LVAD support are less likely to develop aortic insufficiency (Pak et al. 2010; Cowger et al. 2010, 2014; Jorde et al. 2014). In our present study, the aortic valve was found to be closed at the latest follow-up point more frequently in patients with aortic insufficiency ≥ 2 grade compared to the patients with aortic insufficiency < 2 grade, and we considered that aortic valve closing was associated with the progression of aortic insufficiency. However, there is no clear evidence regarding device management to reduce aortic insufficiency. In other words, it may be that progressive aortic insufficiency is unavoidable to some extent despite the documented management. Some reports demonstrated that aortic insufficiency was not associated with long-term mortality (Patil et al. 2014; Cowger et al. 2014; Holley et al. 2017), whereas others have reported that aortic insufficiency worsens long-term mortality (Toda et al. 2011; Auvil et al. 2020). The cause of death due to aortic insufficiency is hemorrhagic cerebral infarction, sepsis, and multiorgan failure related to congestive heart failure. For these reasons, congestive heart failure is considered to be associated with aortic insufficiency. However, it is difficult to elucidate the correlation between death and aortic insufficiency as many confounding factors might contribute to congestive heart failure and clinical events. In our study, there was no significant difference in long-term mortality between aortic insufficiency ≥ 3 grade and aortic insufficiency < 3 grade. Infection and cerebral events were mainly related to the cause of death, and it was not likely to be associated with heart failure in our study. The grade of aortic insufficiency among the patients with in-hospital mortality was none or trivial. One patient with in-hospital mortality needed aortic valve intervention, and postoperative course was uneventful. However, the patient died by subarachnoid hemorrhage, which was not related to the aortic valve intervention or heart failure. Redo surgery for aortic insufficiency may be attributed to long-term mortality; however, in our experience, 3 patients needed aortic valve replacement for de novo aortic insufficiency, and we performed a successful surgery on all 3 patients. Redo aortic valve repair or replacement is a high-risk surgery; however, regular follow-up and adequate consideration of aortic valve repair or replacement for aortic insufficiency could prevent adverse events. Consequently, long-term mortality could be comparable to that in patients without aortic insufficiency.
Aortic insufficiency progression is expected to cause excessive left ventricular loss of unloading and inadequate peripheral perfusion and, consequently, cause symptomatic heart failure. However, it was often multifactorial in patients supported by LVAD. Theoretically, aortic insufficiency increases LVDd and mitral regurgitation grade, and the increased mitral regurgitation caused the elevation of pulmonary artery wedge pressure and pulmonary artery hypertension. Consequently, right ventricular dysfunction may persist. In addition, right heart failure after LVAD implantation reportedly increases mortality and complications (Dang et al. 2006). Based on echocardiographic parameters of right heart failure, some previous reports suggested that tricuspid regurgitation was associated with right heart failure and mortality in patients with LVAD support in the long-term follow-up and recommended the use of concomitant tricuspid valve repair to prevent tricuspid regurgitation progression (Piacentino et al. 2011; Maltais et al. 2012; Han et al. 2016; Nakanishi et al. 2018). However, Cowger et al. (2014) suggested that patients with moderate or worse aortic insufficiency was more likely to have right ventricular hypokinesis than those with less aortic insufficiency whereas they found no significant impact of aortic insufficiency development on right ventricular dysfunction. In our study, aortic insufficiency was an independent predictive risk factor for heart failure event, whereas mitral regurgitation, tricuspid regurgitation, and RV-FAC were not independent risk factors for heart failure event. Based on these results, we considered that clinical symptom was mainly associated with left ventricular heart failure, which was attributed to decreased effective forward flow due to aortic insufficiency progression. As tricuspid regurgitation and TR-PG increased and RV-FAC decreased in patients with aortic insufficiency ≥ 3 grade at the time of the last echocardiogram, we could speculate that right ventricular dysfunction was also partially correlated with heart failure event. Thus, we should not exclude left ventricular heart failure caused by aortic insufficiency progression in patients with LVAD and should evaluate whether effective systemic perfusion is maintained in patients with LVAD suffering from systemic heart failure. The most reliable method to evaluate the systemic perfusion would be cardiac catheterization, which we performed rather aggressively to elucidate the condition of systemic prefusion. We believe that we should lower the threshold to proceed in the evaluation of hemodynamics by cardiac catheterization specifically in LVAD-supported patients.
Recently, patients have had to receive LVAD support for a longer period due to a severe donor shortage and an increased number of patients for destination therapy worldwide. J-MACS revealed that the likelihood of bridge-to-transplant patients undergoing heart transplantation was 1.0% within 360 days, 7.8% within 720 days, and 38.8% within 1,080 days in patients with durable LVAD (Nakatani et al. 2017). Therefore, symptomatic heart failure due to aortic insufficiency is an unavoidable event, and we should pay attention not only to right heart hemodynamics, but also aortic insufficiency progression during the management of patients with LVAD.
There were some limitations to our present study. First, this was a retrospective study conducted in a single center. Second, the sample size was small. Furthermore, right heart catheterizations were not regularly performed for the confirmation of echocardiography. It is possible that other factors were considered in readmission for symptomatic heart failure. Further prospective studies are needed to elucidate the impact of aortic insufficiency and predictive risk factors for readmission for symptomatic heart failure.
In conclusion, we demonstrated that aortic insufficiency did not increase the incidence of early-mortality and late-mortality. Aortic insufficiency was an independent risk factor for heart failure event.
We would like to thank Editage (https://www.editage.jp) for English language editing.
The authors declare no conflict of interest.