2022 Volume 256 Issue 4 Pages 327-336
2022 Volume 256 Issue 4 Pages 327-336
Urinary exosomal miRNA is an ideal non-invasive biomarker of renal disease, but little is known about its ability to diagnose idiopathic membranous nephropathy (IMN). The purpose of this study was to explore the clinical value of urinary exosomal miRNAs in IMN. Urine samples were collected from 36 IMN patients and 36 healthy subjects. Some samples were used to analyze the miRNA profiles of urinary exosomes by high-throughput sequencing. The remaining cases were verified by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Additionally, the serum of the patients and healthy people was collected, and the clinical parameters were detected. Through high-throughput sequencing of samples, it was found that 20 miRNAs were markedly down-regulated. MiR-9-5p and miR-30b-5p were selected for verification, and the results were consistent with those of high-throughput sequencing. MiR-9-5p was correlated with the level of triglyceride and estimated glomerular filtration rate. MiR-30b-5p was related to the levels of anti-phospholipase A2 receptor antibody, serum albumin, β 2-microglobulin and the ratio of global sclerosis/observed glomeruli number. The analysis of Receiver Operating Characteristic curves revealed that miR-30b-5p and miR-9-5p showed a potential diagnostic value for IMN. This study showed that there were significant differences in urinary exosome miRNA profiles between IMN patients and healthy persons. MiR-30b-5p and miR-9-5p may become new non-invasive biomarkers of IMN.
Membranous nephropathy (MN) is a group of diseases that was characterized by the deposition of immune complexes under glomerular basement membrane epithelial cells with basement membrane thickening. It can be divided into idiopathic membranous nephropathy (IMN) and secondary membranous nephropathy (SMN) in terms of the etiology, in which the unknown etiology is called IMN. IMN is the most common disease of idiopathic nephrotic syndrome in the middle-aged and elderly, accounting for up to 6.0% ~ 28.8% of primary glomerular diseases (Keri et al. 2019). The natural course of MN is variable, with approximately one-third of patients having spontaneous remission, whereas still 30% to 40% of patients do not present good efficacy, and a part of them progress to end-stage renal disease within 5-10 years (Lai et al. 2015). IMN is a histopathological diagnosis, and renal biopsy remains the gold standard. However, renal biopsy is invasive, and improper tissue sampling may lead to misdiagnosis. Therefore, new and reliable biomarkers are required for the non-invasive diagnosis, prognosis and monitoring circumstances of IMN.
It is believed that exosomes are released through the fusion of multivesicular bodies and plasma membranes. Their diameter is about 40-100 nm (Johnstone et al. 1987), containing nucleic acids (including miRNA, IncRNAs, mRNAs and DNA), lipids, proteins and so on. As vesicles carry biological information, exosomes can be extracted from all kinds of body fluids, such as blood (Li et al. 2018), urine, saliva (Berckmans et al. 2011), seminal fluid, amniotic fluid (Dixon et al. 2018), cerebrospinal fluid (Kong et al. 2018), bile (Li et al. 2016a), pericardial fluid (Beltrami et al. 2017), ascites (Hu et al. 2019) and pleural effusion (Bard et al. 2004). The exosomes extracted from the urine can reflect the functional changes of the urinary system such as kidney, prostate and bladder (Gonzales et al. 2009; Wang et al. 2020). In IgA nephropathy, Min et al. (2018) found that miR-29c, miR-146a and miR-205 were significantly different in urinary exosomal miRNA profiles between patients with IgA nephropathy and healthy controls. In the urine exosomes of type 1 diabetes patients with microalbuminuria, Barutta et al. (2013) found that miR-130a and miR-145 were enriched, and miR-155 and miR-424 decreased. In vitro experiments and animal models, it was found that miR-145 was overexpressed in the glomeruli of diabetic mice, and the level of miR-145 in urine exosomes increased after glomerular mesangial cells were exposed to high glucose (Barutta et al. 2013). The above miRNAs may be new non-invasive biomarkers of IgA nephropathy and diabetic nephropathy of type 1 diabetes. In addition, lupus nephritis, focal segmental glomerulosclerosis and diabetic nephropathy of type 2 diabetes have also been in related reports. Therefore, based on these studies, it can be hypothesized that there is also a diagnostic value of miRNA in the urine exosomes of IMN patients. This paper will explore the expression profile of urinary exosome miRNA in IMN and search for miRNA with diagnostic value.
The study protocols and consent forms were approved by the Shanxi Provincial People’s Hospital (protocol code 2019LL205 and date of approval 2020.1) and adhered to the Helsinki Declaration guidelines on ethical principles for medical research involving subjects. Written informed consent was obtained from all participants. 36 IMN patients who had never been treated with glucocorticoids or other immunosuppressive drugs were recruited for this study. In addition, we chose 36 healthy subjects as controls (Table 1). There was no significant difference in age and sex among the groups.
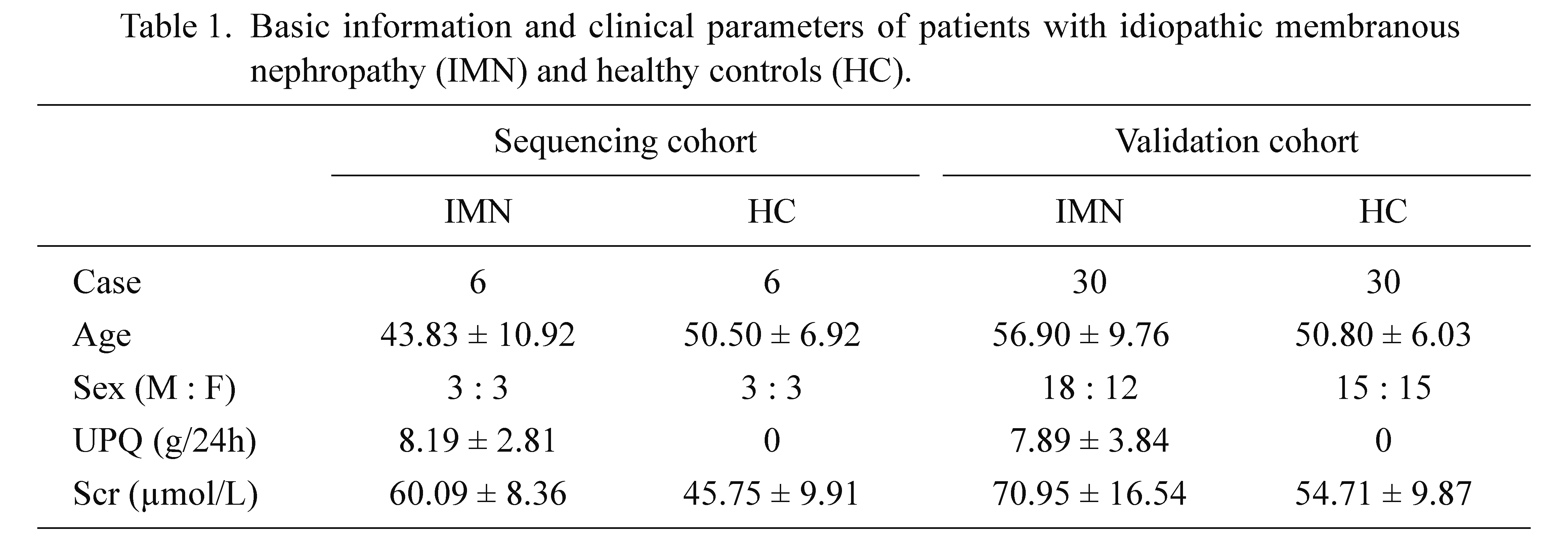
Basic information and clinical parameters of patients with idiopathic membranous nephropathy (IMN) and healthy controls (HC).
M, male; F, female; URE, urinary protein excretion; Scr, serum creatinine.
The inclusion criteria were as follows: IMN patients were hospitalized at Shanxi Provincial People’s Hospital nephrology department from January 2020 to January 2021. Renal biopsy confirmed that their pathological type was IMN and they had not received glucocorticoid or immunosuppressive therapy. Their renal function was normal before admission.
The exclusion criteria were as follows: 1) Secondary membranous nephropathy patients, such as malignant tumor-related nephropathy, hepatitis-related nephropathy, drug-related and toxicant-related nephropathy and others; 2) Patients with acute (severe) infection, severe cardiopulmonary disease, severe cerebrovascular disease (or sequelae), severe liver diseases, major surgery, malignant tumor, acute poisoning and other major diseases that may affect kidney function within the latest 3 months; 3) renal pathology results confirm membranous nephropathy, but the patient has co-occurrence of other diseases that can cause renal damage, such as diabetes, hypertension, IgA nephropathy, systemic lupus erythematosus, and so on.
Collection of serum and urine specimensAll patients met the inclusion criteria, and they were prohibited from eating or drinking from 8:00 p.m. the night before specimens were collected. Venous blood was collected the next morning from elbow vein blood and then centrifuged at 3,000 × g at 25°C for 10 min. Serum was separated. 2 ml of serum was collected and stored at −80°C for later use. At the same time, the patient’s first-morning urine (approximately 15 ml) was collected into a centrifuge tube. Then these samples were centrifuged at room temperature at 1,500 × g for 5 min within 2 hours after collection, and the supernatant was collected in a new sterile centrifuge tube and stored at −80°C for later use.
Clinical parameters measurement and collectionThe Beckman AU5800 automatic biochemical analyzer (Beckman Coulter Corporation, Tokyo, Japan) was used to measure the serum concentrations of albumin (ALB), triglyceride (TG), creatinine, β2-microglobulin (β2-MG), Cystatin C (CysC). The estimated glomerular filtration rate calculation was based on the Modification of Diet in Renal Disease (MDRD) deformation formula published in 2006 by the Journal of the Nephropathy Association. Twenty-four-hour urine protein quantification (UPQ) was detected by Biosystems BA400 (Biostec, Chongqing, China). The serum concentrations of anti-phospholipase A2 receptor antibody (anti-PLA2R) were measured by AFS2000 (Vazyme, Nanjing, China). Pathological reports of patients were collected, and pathological staging (PTNM) and the ratio of global sclerosis/observed glomeruli number (GS/GN) data were extracted.
Urinary exosome isolationTo obtain more exosomes, we use exosomes extraction kit for urine (Baiaolaibo, Beijing, China). Firstly, the urine samples of IMN patients were thawed at 2-8°C. After thawing, 2 samples were mixed (about 20 ml) and centrifuged at 3,000 ×g for 15 min at 4°C. The precipitation was discarded and the supernatant was collected. The supernatant was carefully moved into another clean centrifuge tube. Secondly, the supernatant was centrifuged at 10,000 × g at 4°C for 20 min. The precipitation was discarded again and the supernatant was collected into another clean centrifuge tube as described above. The 5 ml exosome extract were added to the supernatant of 20 ml, fasten down the centrifugal tube tightly and mixed upside down for about 1 min to make the liquid mix well. It was put in a refrigerator at 4°C overnight (keep it for not less than 10 hs). Finally, the mixture was taken out and centrifuged 10,000 × g for 60 min at 4°C. The supernatant was removed and the precipitation was collected. The precipitate was exosome, which was stored at −80°C until downstream applications.
Transmission electron microscopy (TEM) and western blottingThe exosomes were separated from the mixed urine sample by the above method and then suspended in 1 × phosphate-buffered saline (PBS). Then exosomes were placed on a carbon-coated a 200 mesh copper grid for 20 min. Excess liquid was removed from the edge with filter paper. Then, 2% phosphotungstic acid solution (HT152250ML, Sigma, Germany) was added and negatively stained for 10 min at room temperature. After removing unnecessary liquid again with filter paper, the copper mesh was dried with an incandescent lamp. The micrographs were obtained using a JEM-1011 transmission electron microscope (JEOL, Tokyo, Japan). Total proteins of the isolated exosomes were extracted using a protein extraction kit (Applygen Technologies Inc., Beijing, China) following the manufacturer’s protocols. Concentrations were determined using a BCA protein assay kit. Then, the proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. We purchased antibodies as follows: anti-CD63 (Absin, Shanghai, China), and CD9 (Absin, Shanghai, China). After blocking with 5% non-fat milk buffer for 1 h, the membranes were incubated with primary antibody (CD63, 1:1,000; CD9, 1:1,000) overnight at 4°C. After washing with 1 × TBST for 4 times, 15 min each time, secondary antibody incubation at dilution of 1:10,000 was performed for 1 h at room temperature. The membranes were washed again. Targeted proteins were detected on a gel imaging system using ECL western blotting substrate (Thermo Fisher Scientific, Waltham, MA, USA) and the band density was analyzed with Image software.
Illumina sequencing by synthesis (SBS) technology and quantification of miRNAs by reverse-transcription quantitative polymerase chain reaction (RT-qPCR) analysisIn this experiment, the single-ended 50 bp sequencing mode of Illumina Hiseq3000 sequencing platform was used for high-throughput sequencing of samples. The original data need to be removed by primers and adaptor sequences, and after the quality test and length screening of the sequencing fragments, the reliable sequencing fragments are selected finally. Then the type and quantity of small (sRNA) were counted, and the length distribution of small RNAs was calculated. Generally speaking, the length range of small RNA is 18 to 30 nucleotides (nt), and the peak of length distribution can help us to judge the types of small RNAs, such as miRNA concentrated in 21 or 22 nt in 24 nt, piwi-interacting RNAs concentrated in 30 nt.
To validate the results from high-throughput sequencing, miR-9-5p and miR-30b-5p were used for RT-qPCR analysis based on expression level and biological significance. Primers for candidate miRNAs were designed and synthesized by Wuhan Servicebio Technology Co., Ltd. MiRNA was extracted using a miRcute miRNA Isolation Kit (Tiangen Biotech, Beijing, China). The quantity (ng/ml) and purity of the obtained RNA were measured by NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) (the absorbance ratio of RNA isolate at 260 nm and 280 nm [A260/280]). Reverse transcription was conducted using amiRcute miRNA First Strand cDNA Synthesis Kit (Tiangen Biotech, Beijing, China), according to the manufacturer’s instructions. RT- qPCR was performed on an Applied Biosystems 7500 Fast Dx Real-Time PCR Instrument (Applied Biosystems, Carlsbad, CA, USA) using MiRNA fluorescence quantitative detection kit (catalog number FP411 Tiangen Biotech, Beijing, China). All PCR reactions were carried out in triplicate. A melt curve was generated and used to validate the specificity and identity of the PCR reactions. RNU6 served as the endogenous reference control. The relative expression levels of miRNAs in IMN patients and control groups were obtained following the 2-△△CT method.
Statistical analysisStatistical analysis was carried out with GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA) and SPSS 26.0 (IBM, Armonk, NY, USA). We chose the urine of two IMN patients with the same pathological stage and a similar amount of urinary protein and anti-PLA2R to mix. The mixed sample is counted as one sample. Their clinical indicators are expressed as averages. DESeq software was used to analyze the differential expression of cases between the control and IMN groups. RT-qPCR data were compared using the Mann-Whitney U test. To analyze the correlation between miR-9-5p/miR-30b-5p and clinical indexes, Pearson correlation coefficient was used when the normal distribution was satisfied, and Spearman correlation coefficient was used when the normal distribution was not satisfied. Receiver operating characteristic (ROC) curves were constructed to evaluate the diagnostic value of exosomal miRNAs in IMN patients. P value < 0.05 was considered statistically significant.
Exosomes were identified by TEM and western blot. Irregular spherical exosomes can be observed under TEM between 40 and 100 nm in diameter (Fig. 1). Western blotting demonstrated that the exosomes were enriched with typical exosomal markers, CD 63, and CD 9 (Fig. 2). These results indicate that the urine-derived particles isolated from healthy subjects and IMN patients are exosomes.
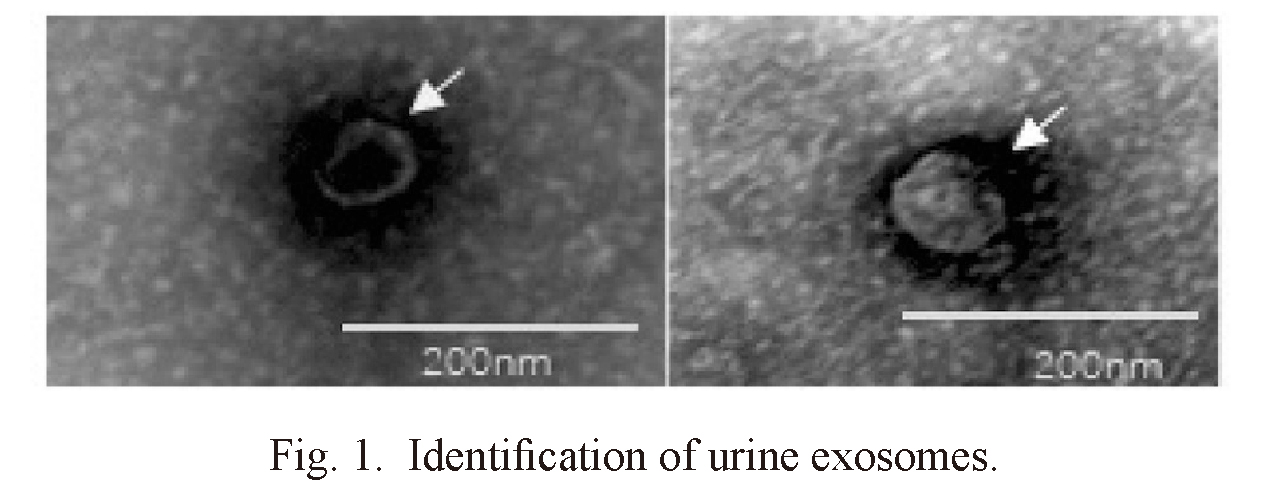
Identification of urine exosomes.
Transmission electron microscopy (TEM) images indicate exosome morphology (arrows). Bars = 200 nm.
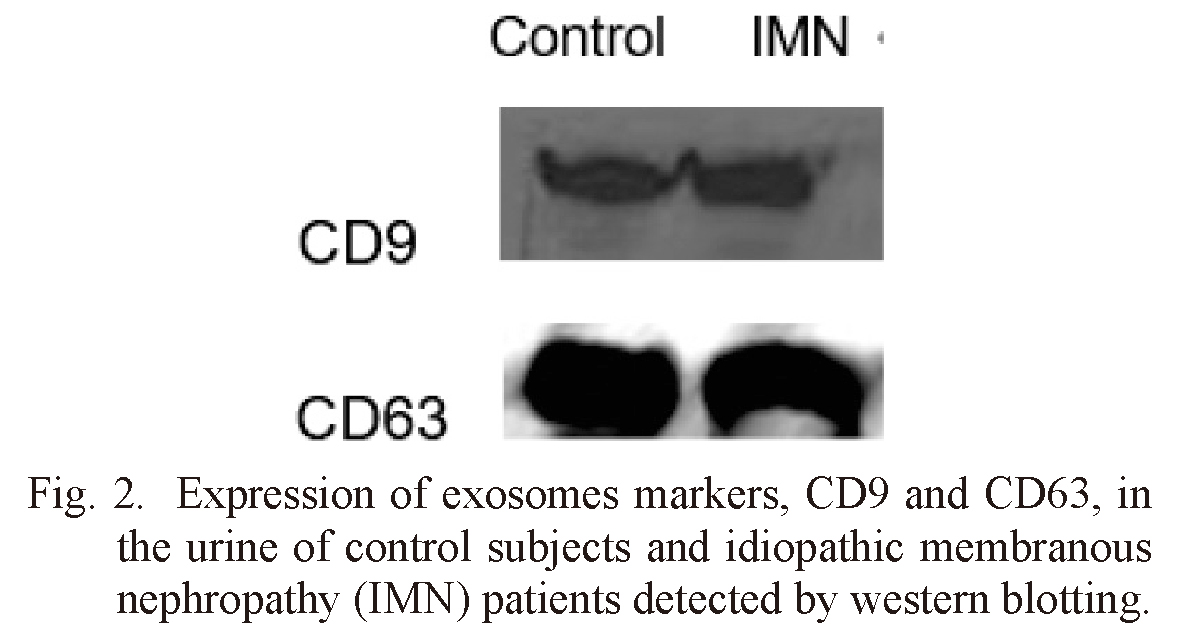
Expression of exosomes markers, CD9 and CD63, in the urine of control subjects and idiopathic membranous nephropathy (IMN) patients detected by western blotting.
The exosomal miRNA was detected by Illumina SBS technique. On average, about 1,800 miRNAs were detected per sample (Table 1). The volcano map showed significant differences in urinary exosomal miRNAs (Fig. 3). We used log2 (fold change) to screen differential genes. When the gene satisfies p value ≤ 0.05 and | log2 (fold change) | ≥ 1, we think that there are significant differences between IMN groups and healthy control groups. A total of 43 miRNAs were significantly changed, 25 of these miRNAs can be retrieved in the miRNA database (Table 2). The heat map showed the above 25 miRNAs (Fig. 4). Compared with the healthy control groups, 20 miRNAs were down-regulated and 5 miRNAs (including miR-186-5p, miR-429, miR-451a, miR-486-5p and let-7d-3p) were up-regulated. By searching the miRNA database (TargetScanHuman7.2), we selected 2 miRNAs (hsa-miR-30b-5p and hsa-miR-9-5p) that may be related to the pathogenesis of IMN from the down-regulated miRNAs for subsequent validation.
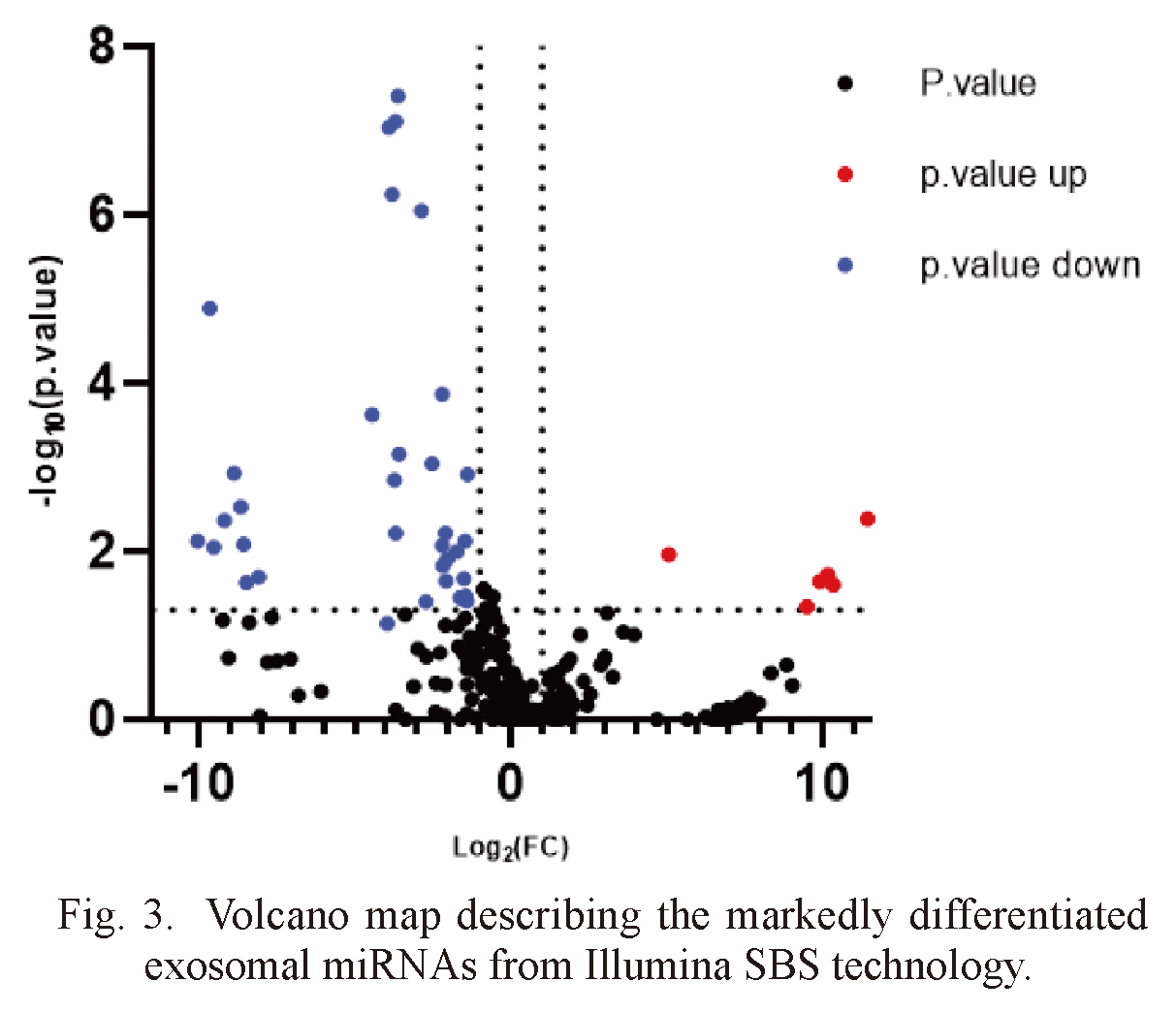
Volcano map describing the markedly differentiated exosomal miRNAs from Illumina SBS technology.

There are markedly differentiated urinary exosomal miRNA profiles.
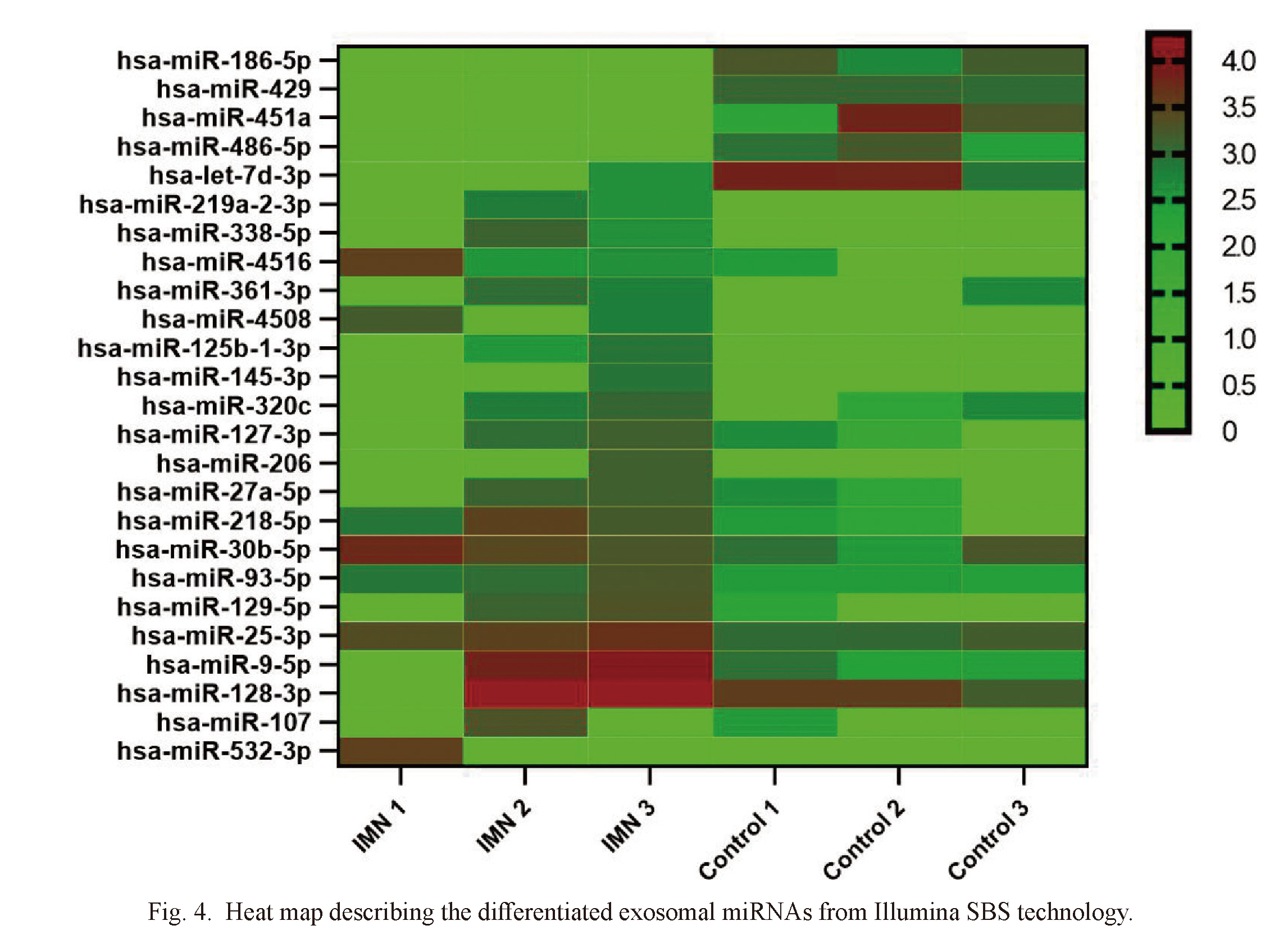
Heat map describing the differentiated exosomal miRNAs from Illumina SBS technology.
IMN, idiopathic membranous nephropathy.
For the purpose of better understanding the functional information of differentially expressed miRNA in IMN, three groups of ontologies of differentially expressed miRNAs were analyzed by GO: biological process (GO-BP), cellular component (GO-CC) and molecular function (GO-MF). The top 10 differentially enrichments are shown in Fig. 5 with p values indicating statistical significance. GO analysis revealed that differentially expressed miRNAs were mainly involved in localization, metabolism, cellular component organization, catabolic, nucleoside and ribonucleoside binding. The differentially expressed miRNAs were enriched in intracellular parts, organelles, cytoplasm and membrane-bounded organelle. Between the IMN and healthy control groups, KEGG pathway analysis screened out 20 paths with the most difference (Fig. 6). Among them, MAPK signaling pathway (Yang et al. 2021), fatty acid biosynthesis (Fujita et al. 2006), ubiquitin mediated proteolysis (Kitzler et al. 2012), autophagy (Yang et al. 2021), chagas disease (Xavier-Júnior et al. 2015), endocytosis (Sasaki et al. 2017) may be related to the pathological mechanism of IMN. Endocytosis and SNARE interactions may be related to the release and uptake of exosomes.
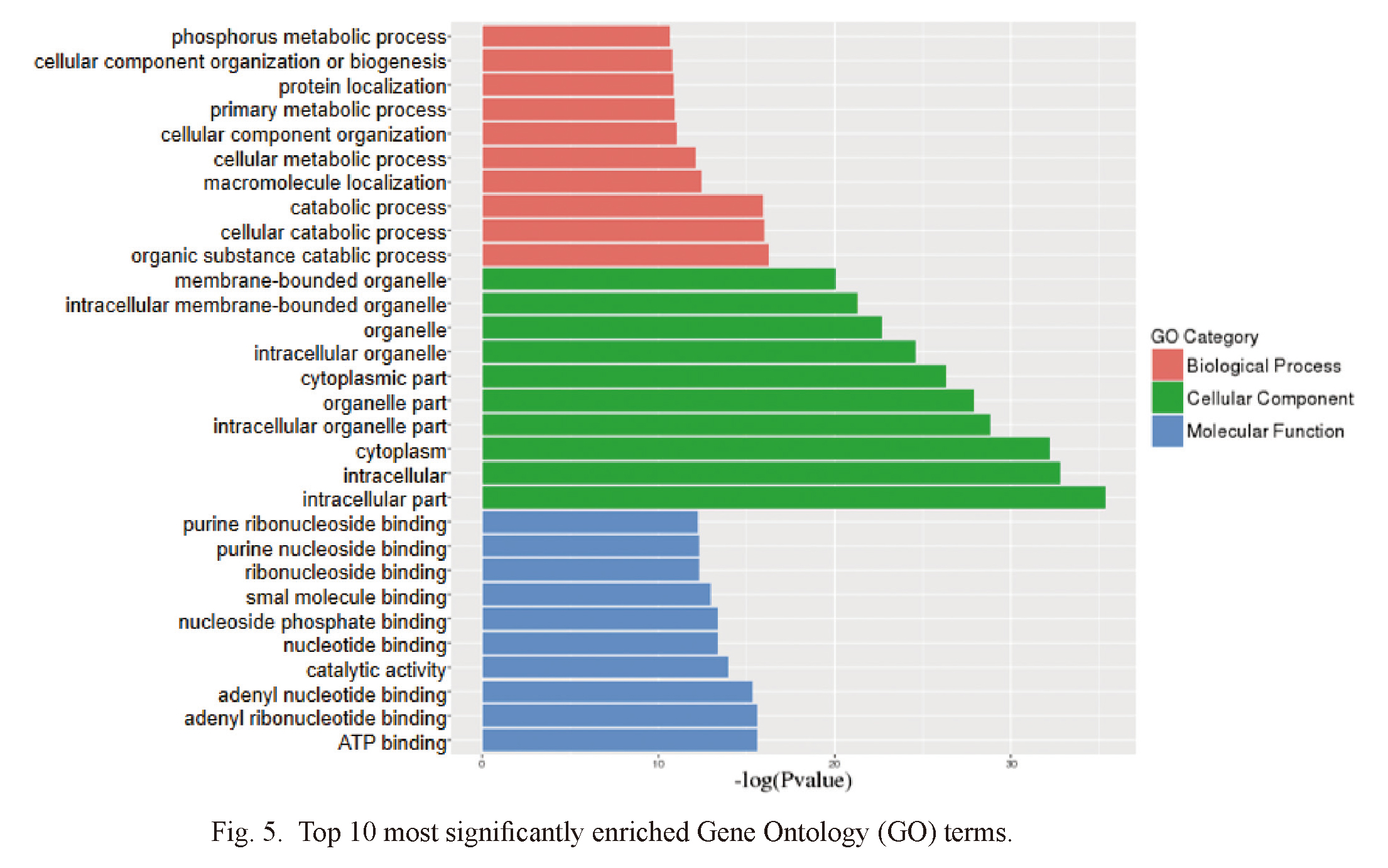
Top 10 most significantly enriched Gene Ontology (GO) terms.
Horizontal axis represents the -log (P value). Vertical axis represents GO terms.
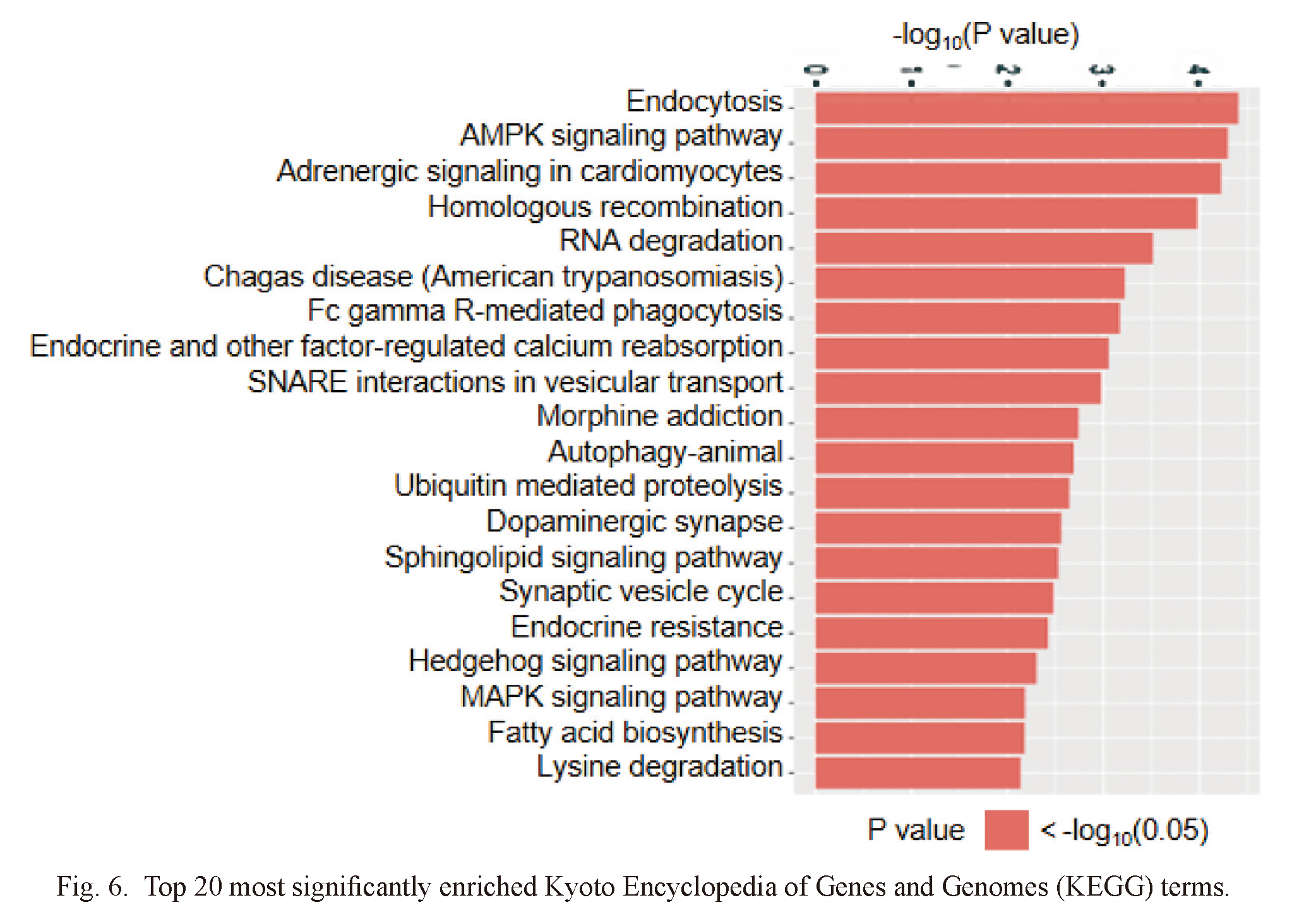
Top 20 most significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) terms.
Horizontal axis represents -log10 (P value). Vertical axis represents KEGG pathway name.
We performed RT-qPCR testing in two separate cohorts (15 samples from 30 IMN patients and 15 samples from 30 healthy control subjects) to confirm the results of Illumina SBS technology. We selected two differentially expressed miRNAs (including hsa-miR-30b-5p and hsa-miR-9-5p) for RT-qPCR. MiRNAs were regarded as a significant difference if they met the following criteria: a p value < 0.05, a mean > 2.0-fold increase for comparison of the controls and IMN. Two miRNAs were significantly downregulated in urine exosomes of IMN patients compared with those of the healthy control group, confirming the reliability of the miRNA-seq data (Fig. 7).
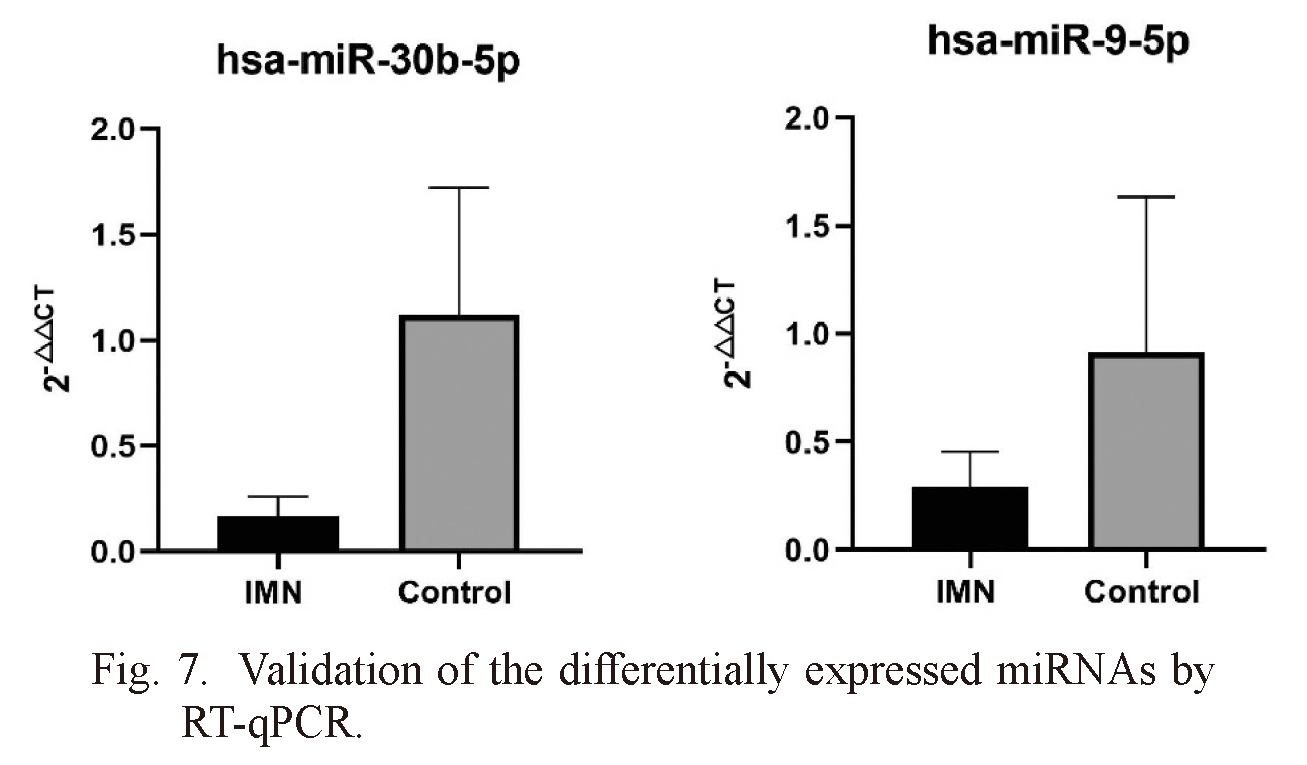
Validation of the differentially expressed miRNAs by RT-qPCR.
IMN, idiopathic membranous nephropathy. Two downregulated miRNAs were selected. Bars represent the mean ± standard deviation (n = 15). p < 0.05.
To further study the relationship between urinary exosomal miRNAs and IMN, Spearman and Pearson correlation coefficient was used to evaluate whether these miRNAs were related to the relevant clinical parameters in patients. As shown in Table 3, miR-30b-5p were significantly related to the anti-PLA2R (r = −0.618, p = 0.016), ALB (r = −0.686, p = 0.005) and β2-MG (r = −0.521, p = 0.047) in the serum. And miR-30b-5p was correlated with GS/GN (r = 0.733, p = 0.004). When the significant level was 0.05, no clinical indicators were found to be statistically related to miR-9-5p. When the test level was 0.10, two indicators were found to be statistically related to the second miRNA, namely eGFR (r = 0.494, p = 0.061) and TG (r = 0.441, p = 0.100). These results demonstrate that in the two urinary exosomal miRNAs, miR-30b-5p is closely associated with anti-PLA2R, ALB, β2-MG and GS/GN and miR-9-5p are associated with eGFR and TG. They may be used as auxiliary indicators for the diagnosis of IMN.
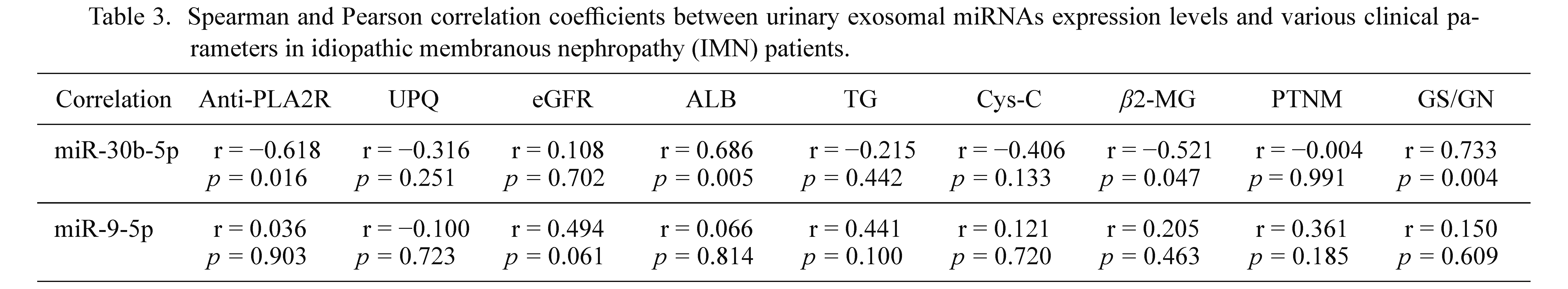
Spearman and Pearson correlation coefficients between urinary exosomal miRNAs expression levels and various clinical parameters in idiopathic membranous nephropathy (IMN) patients.
Anti-PLA2R, anti-phospholipase A2 receptor antibody; UPQ, twenty-four-hour urine protein quantification; eGFR, estimated glomerular filtration rate; ALB, serum albumin; TG, serum triglyceride; Cys-C, cystatin C; β2-MG, β2-microglobulin; PTNM, pathological staging; GS/GN, the ratio of global sclerosis/observed glomeruli number.
ROC curves were constructed to evaluate the diagnostic value of exosomal miRNAs in IMN patients. The difference between the control group and IMN group was statistically significant, with an area under the curve (AUC) of 0.867 [95% confidence interval (CI): 0.724 to 1.01, p = 0.0006] for exosomal miR-30b-5p, and 0.724 (95% CI: 0.537 to 0.912, p = 0.0363) for exosomal miR-9-5p (Fig. 8). These results suggested that miR-30b-5p and miR-9-5p can be used as potential biomarkers of IMN.
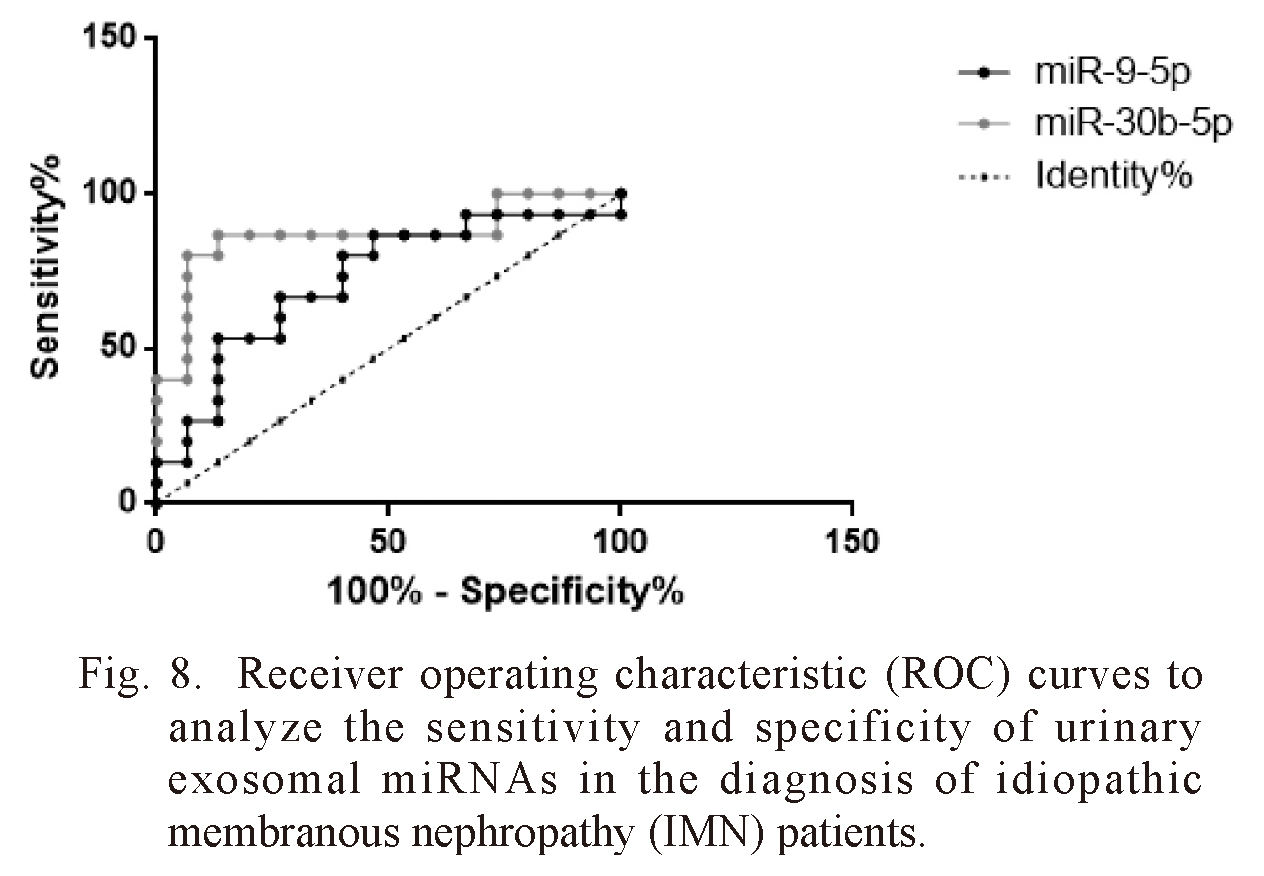
Receiver operating characteristic (ROC) curves to analyze the sensitivity and specificity of urinary exosomal miRNAs in the diagnosis of idiopathic membranous nephropathy (IMN) patients.
The AUC and p-values of the miRNAs were as follows: miR-30b-5p (AUC = 0.867, p = 0.0006), miR-9-5p (AUC = 0.724, p < 0.036).
Urine has the advantages of easy access and large quantity and can accumulate the changes of the body. So, it is an ideal source of non-invasive biomarkers for diagnosis. The urinary exosomes are secreted by urinary cells such as the glomerulus, renal tubule, collecting duct, ureter, and bladder (Pisitkun et al. 2004). Previous studies have confirmed that the exosomes isolated from human urine have a complete RNA profile similar to that of the kidney (Miranda et al. 2010). Exosomes, as an extracellular vesicle, can protect the internal RNA from degradation, which provides the possibility for obtaining physiological and pathological information of the kidney. Therefore, urinary exosomal miRNAs have application value. At present, the research on urinary exosomes has been involved in a variety of renal diseases, such as diabetic nephropathy, IgA nephropathy, lupus nephritis, polycystic kidney disease, renal transplantation and so on. But there are few studies on idiopathic membranous nephropathy.
In this study, the urine of patients with IMN and healthy subjects was collected, the exosomes were extracted, and the miRNAs were sequenced by Illumina SBS technology. The miRNA expression profile of urinary exosomes of IMN was obtained. By comparing with the healthy control group, we found that there were significant differences in the expression of 25 miRNAs in the urinary exosomal miRNA between IMN patients and healthy control subjects. To verify the sequencing results, we selected miR-9-5p and miR-30b-5p for qPCR verification. The results showed that they were significantly down-regulated, consistent with the sequencing results. MiR-30b-5p had good correlation with anti-PLA2R, ALB, β2-MG and GS/GN. The correlation of miR-9-5p was general, which may be related to the small sample size. Therefore, miR-9-5p and miR-30b-5p may become biomarkers of IMN, but more sample size verification is needed.
PLA2R1 and HLA-DQA1 had been proven to be risk alleles in idiopathic membranous nephropathy (Pandey 2011). For now, anti-PLA2R is the most significant index used to diagnose IMN besides renal biopsy. About 70% of IMN patients have anti-PLA2R. By searching the miRNA database (TargetScanHuman7.2),miR-30b-5p and miR-9-5p may be involved in the regulation of PLA2R1. Other miR-30 family (miR-30s) members were related with HLA-DQA1. Therefore, we used anti-PLA2R to evaluate the diagnostic value of miR-9-5p and miR-30b-5p. Through Spearman correlation analysis, it was found that there was a significant negative correlation between miR-30b-5p and anti-PLA2R.
MiR-30b-5p and miR-9-5p may play a role in the pathogenesis of IMN. As we know, MN and diabetic nephropathy have varying degrees of excessive accumulation of extracellular matrix, then gradually appear glomerulosclerosis leading to renal fibrosis. Some studies have found that miR-30b-5p and miR-9-5p may be involved in the process of renal fibrosis. In diabetic nephropathy mouse model and human kidney tissue, miR-30b-5p was significantly down-regulated, which promoted epithelial-to-mesenchymal transition (EMT) in diabetic nephropathy. This effect may be achieved by targeting SNAI1 (a main activator of EMT). While the overexpression of miR-30b-5p can reduce EMT induced by high glucose (Wang et al. 2021). MiR-9-5p has been found to protect against renal fibrosis by preventing the downregulation of genes related to key metabolic pathways, including mitochondrial function, oxidative phosphorylation, fatty acid oxidation, and glycolysis in unilateral ureteral obstruction mice (Fierro-Fernández et al. 2020). MiR-9-5p is down-regulated in urinary exosome miRNAs of IMN patients, which may reflect the active metabolism of renal fibrosis-related pathways in IMN patients. Therefore, miR-30b-5p and miR-9-5p may also be involved in the process of renal fibrosis in IMN.
The main pathological change of IMN is glomerular podocyte injury caused by immune complex deposition. MiRNA is necessary for podocytes to maintain homeostasis. The miR-9-5p and miR-30s may be involved in the maintenance of podocyte homeostasis. Especially in this experiment, miR-30b-5p and GS/GN were strong correlation. Some studies have confirmed that all members of the miR-30s in the glomeruli of focal segmental glomerulosclerosis patients are significantly lower than those of the control group. In the rat model, it was further confirmed that miR-30 plays a protective role by directly inhibiting Notch1 and p53, which mediate podocyte injury. Down-regulation of miR-30 induces proteinuria and podocyte injury (Wu et al. 2014). In addition, recent studies have found that miR-30s may block uPAR-ITGB3 (Urokinase plasminogen activator receptor-injury through integrin β3) signal transduction by regulating calcium/calcineurin signaling, and improving podocytes injury and proteinuria in mice (Lang et al. 2019). Both corticosteroid and triptolide can protect podocytes by maintaining miR-30s expression and reducing proteinuria (Wu et al. 2014; Yang et al. 2017). It has been also found that miR-9-5p can alleviate podocyte injury by being regulated by cancer susceptibility candidate 2 (CASC2) and targeting PPARγ (Li et al. 2020). Therefore, miR-30s and miR-9-5p can not only be used as biomarkers of podocytes injury but also play a therapeutic role by regulating their expression.
In addition to the two miRNAs mentioned above, by reviewing the literature, we found that miR-532-3p (Barbagallo et al. 2019), miR-429 (Li et al. 2016b), miR-129-5p (Huang et al. 2020a), miR-107 (Barbagallo et al. 2019), miR-25-3p (Huang et al. 2020b), and miR-206 (Ding et al. 2015; Guo et al. 2016) in the differential expression profile of urine exosomes were all reported to be involved in glomerular podocyte injury, in which miR-532 and miR-107 were confirmed to be involved in glomerular podocyte injury in MN. What was more surprising was that miR-532-3p (Jiang et al. 2019), miR-9-5p (Majd et al. 2018), miR-30b-5p (Chen et al. 2014), miR-129-5p (Chen et al. 2014), miR-125b (Wang et al. 2018; Zheng et al. 2020), and miR-338-5p (Holla et al. 2016) were found to be involved in the regulation of regulatory T cells (Tregs) in the differentially expressed miRNAs, in which four miRNAs, miR-532-3p, miR-9-5p, miR-30b-5p, and miR-129-5p all played roles in podocyte injury and Tregs regulation. Therefore, we infer that the IMN patients’ Tregs levels may have been changed by exosomes from the kidney. To verify this conjecture, another study is ongoing. We detected serum Foxp3 levels in 40 IMN patients and 40 controls and found that Foxp3 levels decreased significantly in the IMN group (data not published). This discovery is consistent with a recent report (Motavalli et al. 2021). They discovered that the serum Tregs in IMN patients decreased significantly, the ratio of Th17/Treg increased, and the immune balance was disrupted. Therefore, the downregulation of Tregs and the disorder of immune balance may be involved in the pathogenesis of IMN. Based on the above findings, we believe that exosomes from the kidney may be involved in the regulation of Tregs, leading to immune disorder, and an excessive immune response may lead to kidney damage. The above inference needs to be confirmed by further experiments.
In summary, through the analysis of urine exosomal differential expression profiles in IMN patients, we proposed that miR-9-5p and miR-30b-5p may become a biomarker of IMN. In the differential expression profile, miR-9-5p, miR-30b-5p, miR-532-3p, miR-429, miR-129-5p, miR-107, miR-25-3p, and miR-206 were involved in podocytes injury. MiR-532-3p, miR-9-5p, miR-30b-5p, miR-129-5p, miR-125b, and miR-338-5p may be related in the regulation of Tregs. Above miRNAs may play a different but important role in the pathogenesis of IMN.
In conclusion, this study showed that there were significant differences in urinary exosomal miRNA profiles between IMN patients and healthy control subjects. Among them, miR-9-5p showed a correlation with the levels of TG and eGFR. MiR-30b-5p was related to the levels of anti-PLA2R, ALB, β2-MG and GS/GN. The analysis of ROC curves revealed that the expression of miR-30b-5p and miR-9-5p showed a potential diagnostic value for IMN. Therefore, miR-9-5p and miR-30b-5p may become new non-invasive biomarkers of IMN.
Thank you for the financial support of 136 Xing Medical Project in the Department of Nephrology, Shanxi Provincial People’s Hospital. This research was funded by National Natural Science Foundation of China, grant number 81450033 and Shanxi provincial special supporting fund for project 136 of Shanxi Provincial People’s Hospital (xy2018005).
Mr. Hao and Dr. Guo completed the experiment, drawing and writing paper. Miss Li completed part of the experiments. Miss Zhang is responsible for the collection and handling of specimens. Pro. Li completed the experimental design and worked out the experimental plan. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.