2022 Volume 256 Issue 4 Pages 283-290
2022 Volume 256 Issue 4 Pages 283-290
Aging affects various sensory functions of the body. However, the effect on the oral mucosal nociception has remain unclear, so this elucidation is very important. Therefore, this study aimed to evaluate the effect of age-related changes in transient receptor potential vanilloid 1 (TRPV1) and TRPV2 expression in the trigeminal ganglion (TG) neurons on intraoral mucosal heat sensitivity in the senescence-accelerated mouse prone 8 (SAMP8) model. We used 23-week-old (aged) and 7-week-old (young) SAMP8 mice. Heat stimulation was applied to the palatal mucosa under light anesthesia; moreover, the heat head withdrawal threshold (HHWT) was measured. We counted the number of TRPV1-immunoreactive (IR) and TRPV2-IR TG neurons innervating the palatal mucosa. Additionally, we investigated changes in HHWT when TRPV1 or TRPV2 antagonists (SB366791 or Tranilast) were administered to the palatal mucosa. Aged SAMP8 mice showed a higher HHWT than young SAMP8 mice. Compared with the aged SAMP8 mice, young SAMP8 mice showed a larger number of TRPV1-IR small-diameter neurons and a smaller number of TRPV2-IR medium-sized neurons innervating the palatal mucosa. SB366791 administration increased the HHWT in young, but not aged SAMP8 mice. Contrastingly, Tranilast administration increased the HHWT in aged, but not young SAMP8 mice. These results suggest that the modulation of heat pain sensitivity in the oral mucosa due to aging is dependent on changes in the TRPV1 and TRPV2 expression patterns in the TG neurons innervating the palatal mucosa.
Aging is known to affect heat nociception (Chao et al. 2007; Daguet et al. 2020). Many studies have reported that the pain threshold increases with aging when contact heat stimulation or radiant heat stimulation is applied to the skin of limbs (Gibson and Farrell 2004; Lautenbacher et al. 2005, 2017). The oral cavity is routinely exposed to various thermal, chemical, and mechanical stimuli. Sensing a noxious temperature in the intraoral mucosa is considered a crucial function of the body’s defense mechanisms; furthermore, heat nociception abnormalities can be a factor for diseases, including aspiration pneumonia, in the elderly (Ebihara et al. 2011). In the clinical setting, it has been reported that heat nociception in the intraoral mucosa is affected by aging; however, the underlying mechanism remains unclear (Kaplan et al. 2011).
Polymodal receptors, which are widely distributed in tissues, express transient receptor potential (TRP) channels that serve as transducers of heat, mechanical, chemical, and noxious stimuli (Ferrandiz-Huertas et al. 2014; McEntire et al. 2016; Mickle et al. 2016; Shibasaki 2016). Among the TRP channels, TRP vanilloid 1 (TRPV1) and TRPV2 are activated by heat stimulation at temperatures of > 43°C and > 52°C, respectively (Caterina et al. 1999; Tominaga and Caterina 2004). TRPV1 and TRPV2 are expressed in nociceptive neurons that innervate intraoral tissues and are involved in intraoral noxious heat sensing (Kido et al. 2003; Wang et al. 2011; Maruno et al. 2017). We previously reported that an increase in TRPV1-and TRPV2-expressing trigeminal ganglion (TG) neurons, which innervate the buccal mucosa, was involved in enhanced heat hyperalgesia due to buccal mucosal incision (Urata et al. 2015, 2018). However, the aging effects on TRPV1 and TRPV2 expression in the TG neurons and intraoral heat nociception remain unclear. Recent studies have used senescence-accelerated mouse prone 8 (SAMP8) mice to examine the age-related pathogenesis associated with behavioral disorders and cognitive abnormalities (Takeda et al. 1994; Takeda 2009; Liu et al. 2020). SAMP8 mice resent with a rapid increase in the aging degree gauge, including the incidence of learning/memory impairment, decreased immune function, and aging amyloidosis, in an age-dependent manner (Takeda 2009; Fernandez-Gomez et al. 2010). SAMP inbred strains include SAMP1, SAMP2, SAMP3, SAMP6, SAMP7, SAMP8, SAMP9, SAMP10, and SAMP11. Compared to the other SAMP mice, SAMP8 mice show similar physiological and morphological changes to the aged human brain (Akiguchi et al. 2017). Therefore, to investigate the effects of aging in a model more similar to humans, SAMP8 mice were selected to investigate intraoral heat nociception and TRPV1 and TRPV2 expression in the TG neurons. This study aimed to examine age-related changes in TRPV1 and TRPV2 expression in the TG neurons innervating the palatal mucosa, and the effect of these changes on palatal mucosal heat nociception in SAMP8 mice.
We used male 23-week-old and 7-week-old SAMP8 mice (n = 25 per age group, weighing 20-30 g, Japan SLC, Shizuoka, Japan) as aged and young SAMP8 mice, respectively. Old SAMP8 mice, whose age was found to be related to altered oral pain susceptibility in our previous studies, were used as a model for old age (Ikutame et al. 2020). In addition, young SAMP8 mice, which have been reported in other previous studies to show no remarkable neurological changes due to aging, were used as a model for young age (Fujibayashi et al. 1994). All mice were maintained in a controlled temperature (23°C) environment under a 12-h light/dark cycle with free access to food and water. All experiments were conducted following the guidelines of the International Association for the Study of Pain (Zimmermann 1983) and approved by the Animal Experimentation Committee at Nihon University School of Dentistry (AP18DEN017, approval date: June 29, 2018). We used the minimum number of mice required for statistical analysis. All animals underwent a one-week habituation period before the experiments.
Measurement of heat sensitivityAs previously described, heat stimulation was conducted after confirming that the mice were maintained at a defined depth of anesthesia. Briefly, mice were anesthetized with 2% isoflurane (Mylan, Canonsburg, PA, USA). After discontinuing the supply of isoflurane, we checked whether an identical hind limb withdrawal reflex was induced by an identical noxious pinch stimulation to the hind paw, as well as whether the breathing and cardiac rhythm of the mice were appropriate. The mouth was kept open using a mouth opener under a constant adjusted anesthesia depth. Subsequently, heat stimulation was applied at 2 mm inside from the left upper molar using a contact heat probe (25 mm2; Intercross, Tokyo, Japan). Since the palatal mucosa is immobile, it is easy to apply heat stimulus to the same place repeatedly. Therefore, the palatal mucosa was selected as the place to stimulate. The heat stimulation intensity was gradually increased at a specified rate (35-60°C, 1°C/s, cutoff: 60°C); moreover, the lowest intensity of heat stimulation required to induce the head withdrawal reflex was defined as the heat head withdrawal threshold (HHWT). Stimuli were administered at 3-min intervals, and the average of three measurements was defined as the HHWT for each mouse. HHWT measurements were performed under blinded conditions.
Immunohistochemistry in the TGTG neurons innervating the palatal mucosa were identified using retrograde labeling techniques with 4% hydroxystilbamidine [3 µL, Fluoro Gold (FG); Fluorochrome, Denver, CO, USA], dissolved in saline. To label the TG neurons innervating the palatal mucosa, we subcutaneously injected FG into the left palatal mucosa using a 27-gauge needle. On day 7 after the FG injection, mice were deeply anesthetized using an intraperitoneal injection of a mixture of butorphanol (2.5 mg/kg; Meiji Seika Pharma, Tokyo, Japan), midazolam (2.0 mg/kg; Sandoz, Tokyo, Japan), and medetomidine (0.15 mg/kg; Zenoaq, Fukushima, Japan). Subsequently, the mice were transcardially perfused with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Next, the TG neurons were removed and fixed in 4% paraformaldehyde for 24 h at 4°C and placed in 0.01 M phosphate-buffered saline (PBS) containing 20% sucrose for 6 h for cryoprotection. After embedding in TissueTek (Sakura Finetek, Tokyo, Japan), the TG slices were made in the horizontal plane at a 10-µm thickness along the long axis of the ganglion. Five sections per TG (at 100-µm intervals) were collected and mounted onto MAS-coated Superfrost Plus microscope slides (Matsunami, Tokyo, Japan). The TG sections were incubated with rabbit anti-TRPV1 polyclonal antibody (1:500 diluted in 0.01 M PBS with 4% normal goat serum, Cat # ACC-030; Alomone, Jerusalem, Israel) or rabbit anti-TRPV2 polyclonal antiserum (1:200 diluted in 0.01 M PBS with 4% normal goat serum, Cat# PAB14319; Abnova, Taipei, Taiwan) at 4°C for 12 h. Next, the TG sections were incubated with Alexa Fluor 488 conjugated goat anti-rabbit IgG (1:200 diluted in 0.01 M PBS; Thermo Fisher Scientific, Tokyo, Japan) for 2 h at room temperature. Sections were coverslipped using a mounting medium (Thermo Fisher Scientific, Tokyo, Japan). FG-labeled TRPV1-immunoreactive (IR) and TRPV2-IR TG neurons were identified and analyzed using a fluorescence microscope (BZ-9000 system; Keyence, Tokyo, Japan). TG neurons showing fluorescence intensity more than twice the average background level were considered IR neurons. The ratio of FG-labeled TRPV1-IR or TRPV2-IR TG neurons was calculated using the following equation: 100 × total number of FG-labeled TRPV1-IR or TRPV2-IR TG neurons / total number of FG-labeled TG neurons.
Peripheral administration of TRPV1 and TRPV2 antagonistsWe prepared the following in advance: N-(3-methoxyphenyl)-4-chlorocinnamide (SB366791; 6 μg/μl dissolved in 20% dimethylsulfoxide in saline, Sigma-Aldrich, St. Louis, MO, USA) as a selective TRPV1 antagonist and 2-[[3-(3,4-dimethoxyphenyl)-1-oxo-2-propen-1-yl] amino] benzoic acid (Tranilast; 1.2 μg/μl dissolved in 20% dimethylsulfoxide in saline, Cayman, MI, USA) as a TRPV2 antagonist. We submucosally administered 3 µL of SB366791 (18 µg), Tranilast (3.6 µg), or vehicle into the left palatal mucosa in young and aged SAMP8 mice using a 27-gauge needle. These doses of SB366791 and Tranilast were determined as previously described (Barabas and Stucky 2013; Urata et al. 2015, 2018). The HHWTs were measured before and at 30, 60, 90, and 120 min after antagonist administration, as described above. All behavioral tests were conducted under blinded conditions.
Statistical analysisData are expressed as the mean ± standard error of the mean. Statistical analyses were performed using the Student’s t-test or two-way repeated measures analysis of variance followed by Sidak’s multiple comparison tests, where appropriate. Statistical significance was set at p < 0.05.
Aged SAMP8 mice had a significantly higher HHWT in the left palatal mucosa compared to the young SAMP8 mice (young SAMP8 mice: 46.5 ± 0.6°C, aged SAMP8 mice: 50.2 ± 0.7°C, p < 0.05) (Fig. 1).
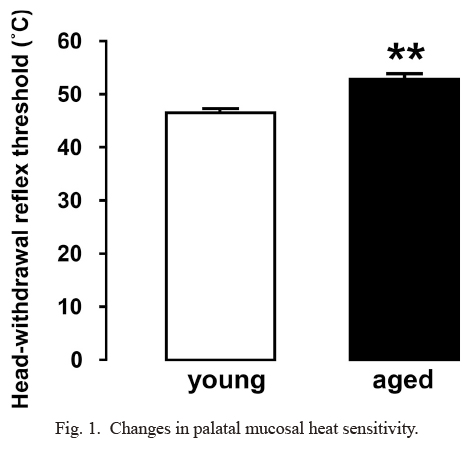
Changes in palatal mucosal heat sensitivity.
Heat head withdrawal threshold in the 7-week-old (young) and 23-week-old (aged) senescence-accelerated mouse prone 8 (SAMP8) mice. Data are represented as mean ± standard error of the mean. (n = 5 in each; Student’s t-test) *p < 0.05, ***p < 0.001.
There was no significant difference in the total number of FG-labeled TG neurons innervating the left palatal mucosa between the young and aged SAMP8 mice (Table 1A).
Compared with young SAMP8 mice, aged SAMP8 mice had a significantly smaller number of TRPV1-IR TG neurons, especially small-diameter neurons (Table 1B, Fig. 2A-C). On the other hand, aged SAMP8 mice had a significantly larger number of TRPV2-IR TG neurons, especially medium-diameter neurons, compared with young SAMP8 mice (Table 1B, Fig. 2D-F).

Incidence of Fluoro Gold (FG)-immunoreactive (IR), transient receptor potential vanilloid 1 (TRPV1)-IR and TRPV2-IR trigeminal ganglion (TG) neurons innervating palatal mucosa.
Percentages of FG-IR (A), and FG-labeled TRPV1 and TRPV2-IR TG neurons (B) were presented in 7-week-old (young) and 23-week-old (aged) senescence-accelerated mice prone 8 (SAMP8) mice. Data represent mean ± standard error of the mean (SEM) (n = 5 in each; Student’s t-test). *p < 0.05 (young vs. aged).
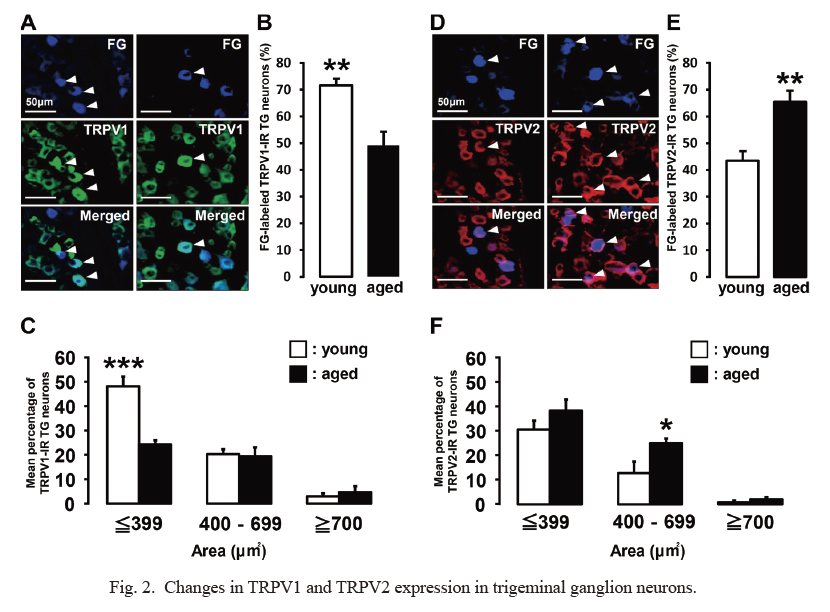
Changes in TRPV1 and TRPV2 expression in trigeminal ganglion neurons.
(A) Fluoro Gold (FG)-labeled transient receptor potential vanilloid 1 (TRPV1)-immunoreactive (IR) trigeminal ganglion (TG) neurons in 7-week-old (young) and 23-week-old (aged) senescence-accelerated mice prone 8 (SAMP8) mice. The arrowheads indicate FG-labeled TRPV1-IR TG neurons. Scale bars, 50 μm. (B) Percentages of FG-labeled TRPV1-IR TG neurons. Data represent mean ± standard error of the mean (SEM). (n = 5 in each; Student’s t-test). (C) Size distribution of FG-labeled TRPV1-IR TG neurons. Data represent mean ± SEM. (n = 5 in each; Student’s t-test). (D) FG-labeled transient receptor potential vanilloid 2 (TRPV2)-IR TG neurons in 7-week-old and 23-week-old SAMP8 mice. The arrowheads indicate FG-labeled TRPV2-IR TG neurons. Scale bars, 50 μm. (E) Percentages of FG-labeled TRPV2-IR TG neurons. Data represent mean ± SEM. (n = 5 in each; Student’s t-test). (F) Size distribution of FG-labeled TRPV2-IR TG neurons. Data represent mean ± SEM. (n = 5 in each; Student’s t-test) *p < 0.05, **p < 0.001.
In young SAMP8 mice, SB366791 palatal mucosal administration induced a significant increase in the HHWT, which peaked 30 min after administration, while Tranilast and vehicle palatal mucosal administration did not alter the HHWT (Figs. 3A and 4A). In contrast, in aged SAMP8 mice, Tranilast palatal mucosal administration induced a significant increase in the HHWT, which peaked 30 min after administration, while SB366791 and vehicle palatal mucosal administration did not alter the HHWT (Figs. 3B and 4B).

Changes in palatal mucosal heat sensitivity following SB366791 administration.
Time course of heat head withdrawal threshold (HHWT) after administration with SB366791 to 7-week-old (young) (A) and 23-week-old (aged) senescence-accelerated mice prone 8 (SAMP8) mice (B) (n = 5 in each; two-way repeated-measures analysis of variance followed by Sidak’s multiple-comparison test). **p < 0.01.

Changes in palatal mucosal heat sensitivity following Tranilast administration.
Time course of heat head withdrawal threshold (HHWT) after administration with Tranilast to 7-week-old (young) (A) and 23-week-old (aged) senescence-accelerated mice prone 8 (SAMP8) mice (B) (n = 5 in each; two-way repeated-measures analysis of variance followed by Sidak’s multiple-comparison test). *p < 0.05.
Aging is associated with various impaired physiological functions, including nociception. Many previous studies have reported that the skin pain threshold induced by heat stimulation increases with aging (Gibson and Farrell 2004; Lautenbacher et al. 2005, 2017). Myelinated Aδ-fiber nociceptors and C-fiber polymodal nociceptors are involved in pain signal transmission from the peripheral nervous system to the central nervous system (Kunimoto 2012; Baron et al. 2013; Iyengar et al. 2017). Myelinated Aδ-fiber nociceptors and C-fiber polymodal nociceptors possess TRPV1 and TRPV2, whose thresholds of channel opening are 43°C and 52°C, respectively and operate as heat-sensitive nociceptors (Treede et al. 1995). However, it remains unclear whether orofacial heat pain sensation changes with aging, even though it has been established that aging alters the excitability of nociceptive neurons innervating the extremities (Chakour et al. 1996; Namer 2010; Taguchi et al. 2010). In this study, the palatal mucosal HHWT in aged SAMP8 mice was significantly higher than that in young SAMP8 mice, which is consistent with previous clinical reports that the heat threshold increases with advancing age (Lautenbacher et al. 2005). Taken together, these results indicate that SAMP8 mice are a suitable animal model for investigating age-related changes in intraoral heat sensitivity.
Age-related decline in nociceptive neuronal excitability may be dependent on changes in the expression and function of nociceptive ion channels, including TRP channels (Wang and Albers 2009). Additionally, the soma diameter of primary sensory neurons and the thickness of their axons are correlated. Small-diameter and medium-sized primary sensory neurons mainly contain unmyelinated C fiber axons and myelinated Aδ fiber axons, respectively (Basbaum et al. 2009; Vang et al. 2012). TRPV1 is mainly expressed in the small-diameter TG and dorsal root ganglion (DRG) neurons with unmyelinated C fibers (Tominaga et al. 1998; Caterina et al. 1999). However, TRPV2 is highly expressed in the medium- to large-sized TG and DRG neurons with Aδ fibers (Benham et al. 2003). TRPV2 is activated by strong noxious heat stimulation (> 52°C) and is involved in heat pain reception (Caterina et al. 1999; Tominaga and Caterina 2004). Therefore, we examined differences in the number of TRPV1-IR and TRPV2-IR TG neurons innervating the palatal mucosa in young and aged SAMP8 mice. Additionally, changes in the HHWT after administering SB366791 or Tranilast to the left palatal mucosa were measured to clarify the involvement of age-related changes in TRPV1 and TRPV2 expression as well as heat sensitivity in the palatal mucosa.
Young SAMP8 mice showed a larger number of small-diameter TRPV1-IR TG neurons than aged SAMP8 mice. SB366791 administration to the left palatal mucosa increased HHWT in young, but not aged SAMP8 mice. Previous studies have reported decreased TRPV1 expression in neurons in aged mice (Wang and Albers 2009; Mitsuoka et al. 2018). Heat pain sensitivity in the hind paw was attenuated compared with that in young mice due to an age-related decrease in TRPV1 expression in the DRG neurons (Wang and Albers 2009). Therefore, the difference in TRPV1 expression in sensory neurons is correlated with changes in heat pain sensitivity. Taken together, these findings suggest that the palatal mucosal heat pain sensation in young SAMP8 mice depends on TRPV1 expression in the small-diameter TG neurons innervating the palatal mucosa. It has been reported that TRPV1 expression in the skin itself increases with aging (Lee et al. 2012). In many previous studies, the noxious heat threshold of the skin increased with aging (Gibson and Farrell 2004; Lautenbacher et al. 2005, 2017). The cause for this contradiction is not clear. Additionally, the effect of aging on TRPV1 expression on the mucosal epithelium itself remains unclear. However, since the acceptance of noxious heat stimuli occurs at nerve endings (Barkai et al. 2020), a decrease in TRPV1 expression at nerve endings may be strongly involved in decline the noxious heat threshold on oral mucosa.
Aged SAMP8 mice showed a larger number of medium-diameter TRPV2-IR neurons than young SAMP8 mice. Tranilast administration to the left palatal mucosa increased the HHWT values in aged, but not young SAMP8 mice. Few studies have investigated the involvement of age-related changes in TRPV2 expression in primary sensory neurons and pain sensitivity. Previous studies on TRPV2 expression in non-neuronal cells have reported an increased TRPV2 expression with advancing age (Iwata et al. 2013; Eubler et al. 2021); however, age-related changes in TRPV2 expression in primary sensory neurons remain unclear. We previously reported a correlation between TRPV2 expression in the TG neurons innervating the orofacial region and orofacial perception of noxious stimuli (Urata et al. 2018). Trigeminal nerve injury and tissue inflammation increased the number of TRPV2-positive TG neurons and induced orofacial pain hypersensitivity, which was suppressed by TRPV2 antagonism (Urata et al. 2018; Sugawara et al. 2019). TRPV2 upregulation in primary nociceptive neurons induced by peripheral inflammation contributes to peripheral sensitization, which results in pain hypersensitivity to noxious heat stimulation (Shimosato et al. 2005). On the other hand, β-amyloid-treated animals present with upregulated TRPV2 mRNA and protein expression in the hippocampus (Thapak et al. 2021). SAMP8 mice show age-dependent neuronal histopathological changes, including amyloid deposits in the neurons (del Valle et al. 2011). Therefore, the age-related neurological changes in our study may have resulted from changes in TRPV2 expression in the central nervous system. Our findings suggest that TRPV2 expression in the medium-diameter TG neurons plays a key role in the palatal mucosal heat pain sensation in aged SAMP8 mice. However, other studies have reported decreased heat sensitivity of Aδ fibers in the extremities with aging (Kemp et al. 2014). This discrepancy may be attributed to differences in the stimulation properties of the stimulated site. Further studies are required to elucidate the aging effects on changes in the excitability of nociceptive neurons, including the involvement of TRPV1 and TRPV2. In addition, it has been reported that immunomodulatory dysfunction is prominent in 40-week-old SAMP8 mice (Fernandez et al. 2021). Furthermore, TRPV1 and TRPV2 expression has also been reported to be associated with changes in macrophage polarity (Eubler et al. 2021; Lv et al. 2021) . Therefore, this study using SAMP8 may also reflect the influence of age-related changes on immune function.
Given the aging population, it is important to elucidate the relationship between aging and pain, which involves complex factors. Our findings could facilitate the elucidation of the mechanisms underlying age-related pain regulation. In conclusion, SAMP8 mice are suitable for investigating age-related changes in oral mucosal heat sensitivity. Heat nociception is TRPV1-dependent at a young age; however, it becomes TRPV2-dependent with aging, which results in concomitant changes in heat sensitivity in the oral mucosa.
This study was supported in part by grants from the Sato Fund, Uemura Fund, Dental Research Center in Nihon University School of Dentistry, KAKENHI (18K17157, 20K18619, 20K09896, 19K10049), and Nihon University Multidisciplinary Research program (21-1301). We would like to thank all the members of the Department of Complete Denture Prosthodontics and the Department of Physiology of Nihon University School of Dentistry. We also thank Editage (https://www.editage.jp) for the English language editing.
The authors declare no conflict of interest.